ABSTRACT
Soils in areas of underground natural gas storage facilities (UGS) can become contaminated with hydrocarbons, which impacts negatively on soil properties and functions of soil biocenosis. The purpose of this study was to biodiagnose soil samples from the territory of an UGS (Stepnoye district, Saratov region) using bioassay. For microbiological analysis of the soil samples, the following parameters were selected: The total number of heterotrophic microorganisms, the content of hydrocarbon-oxidizing and methylotrophic bacteria, as well as the number of iron-oxidizing bacteria given that in the area of this natural gas storage facility clusters of bacteriomorphic magnetite may have been formed. Dehydrogenase, catalase and β-fructofuranosidase activities were studied as informative and sensitive indicators. The biodiagnostic results showed reduced levels of heterotrophic microorganisms in the soil samples from the area of this UGS compared to the background samples obtained outside the UGS. A high content of indicator organisms: Hydrocarbon-oxidizing and methylotrophic bacteria, not only facultative, but obligate was observed in some soil samples which suggests the emission of methane into the upper layers of the soil. The presence of iron-oxidizing microorganisms in the soil samples was confirmed by an increased activity of dehydrogenases and catalases. The observed correlations between the studied biological and physico-chemical parameters of soil confirmed the presence of hydrocarbon pollution.
Key words: Underground gas storage, bioassay, soil microorganisms, soil enzymes, magnetic susceptibility.
Soil above underground gas storage (UGS) can undergo pollution with hydrocarbons (Cao and Staszewska, 2011; Gennadiev et al., 2015) and other toxic compounds which negatively affects the functioning of soil biocenosis. Qualitative and quantitative changes occur in the soil microflora both at the population level and the cellular microbial level (Guo et al., 2012). Gas migrations to soil surface has been stated as one of the basic reasons of pollution and degradation of soil in districts with UGS and this is connected to horizontal and vertical zones formed on fractured grounds of sedimentary cover in platform structures used for constructing natural gas storages (Mozharova and Kulachkova, 2008; Peterson et al., 2015). Imperfections during implementation of series of technological operations contained in plans of ecological protection and destruction of rules for protecting the environment also lead to pollution in territories of UGS.
Formation of gaseous bitumen and bacterial anomalies has been observed due to leakage of gas around artificial storages (
Mozharova, 2010;
Sharafutdinov et al., 2015). Methane has been observed as the gas prevalent in water-bearing horizons, rocks and soils of UGS. A decrease in the concentration of hydrocarbon gases has been observed in the summer period due to the proliferation of hydrocarbon-oxidizing microflora (methane-oxidizing bacteria) (Ho et al., 2013; Kizilova et al., 2013). While a rapid accumulation of methane was observed during autumn, which can result in a negative press firstly, on the organic horizons and which can further cause complete damage to soil properties and functions (Cao and Staszewska, 2011). Hence, the need to conduct regular soil ecological monitoring in districts of UGS, to help assess the quality of the environment.
In our view, an analysis of indicative microbiological and biochemical indices, serves as an important component for ecological monitoring of soil in districts of UGS. The advantage of using soil microorganisms as indicators is that they are highly sensitive to changes in the external environment and their ability to respond in various ways, and also their high rate of reproduction enables us to detect in a short time the changes that occur under the influence of ecological factors (Sumampouw and Risjani, 2014). An indication of the enzyme activity of soils provides information about biochemical processes taking place in soil and partly helps to confirm the state of microbial communities during anthropogenic disturbances (Utobo and Tewari, 2015).
The microbiological analysis has a peculiar significance in areas with low hydrocarbon concentration (low-yielding oil wells, coal-mines and territories of UGS), and this is determined by the high sensitivity of microorganisms in comparison with the sensitivity of existing gas-analyzing methods. In connection with the aforementioned, this research aims at bio-assaying soil of UGS with the help of microbiological and biochemical analysis. This work analyzes the total number of heterotrophic microorganisms, the amount of hydrocarbon-oxidizing, iron-oxidizing and methylotrophic bacteria, and also the activities of dehydrogenases, catalases and β-fructofuranosidases in the soil samples. It also aims to solve the question of a correlation between these physico-chemical indications, the number of the researched physiological groups of microorganisms and the activity of enzymes in soil samples obtained from the underground gas storage facility.
Study area and soil sampling
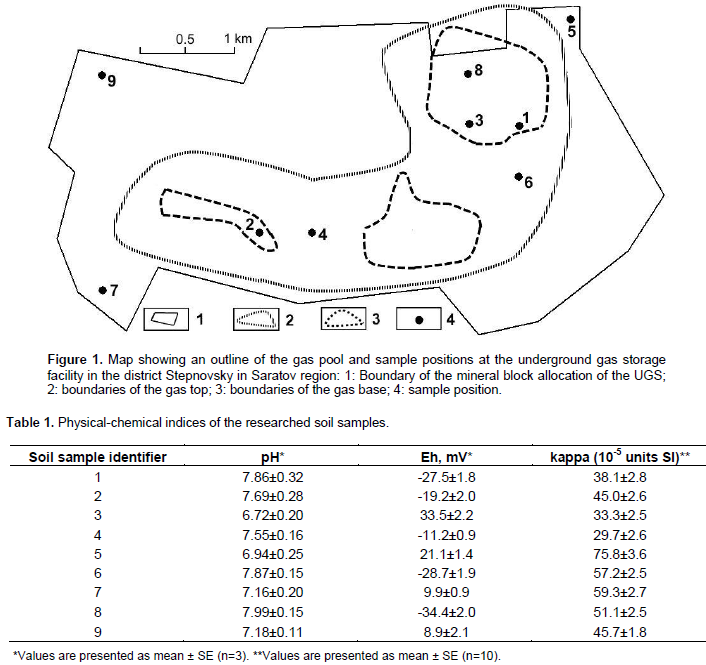
The objects of this study were dark-brown soil samples from the territory of Stepnovsky UGS in Saratov region (Russia). Figure 1 represents a map which shows the sampled territory with points where the soil samples were obtained. The soils were sampled at a depth of 5 to 10 cm using the «envelope method». One sample obtained from the center and 4 samples from the corners of the sample point and also from 2 to 3 samples points around the top of the envelope. The weight of the combined samples varied in a scale of about 0.5 to 1.0 kg. Control (background) soil samples were obtained from approximately 5 to 6 km away from the zone of influence of the UGS. Sample 10-Сtrl in the diagrams and tables represents an average value of 5 measured control soil samples. The following were determined in the soil samples: hydrogen indication, redox potential and magnetic susceptibility (Table 1). Magnetic susceptibility of the soil samples was determined under laboratory conditions using a serial susceptimeter KT-10 (Magiera and Strzyszcz, 2000). A ten-fold measurement of magnetic susceptibility was carried out on each soil sample, and an arithmetic mean average of the ten-fold measurement served as the final value.
Enumeration of microorganisms
The total number of culturable heterotrophic microorganisms (HMs) in the soil (1 g, wet weight) was determined by plate dilution technique using beef-extract agar medium. The number of hydrocarbon-oxidizing microorganisms (HOMs) was evaluated on modified mineral agar medium, g l-1: Na2HPO4 - 6.0; КÐ2РО4 - 3.0; NaCl - 0.5; NH4Cl - 1.0 with vaseline oil (1%) as a sole carbon and energy source (Mills et al., 1978). Methylotrophic microorganisms (MMs) were enumerated on mineral Hirsch medium, g l-1: КÐ2РО4 - 1.36; Na2HPO4×7Ð2О - 2.13; (NH4)2SO4 - 0.5; MgSO4×7H2O - 0.2; CaCl2×2H2O - 0.1; FeSO4×7H2O - 0.005; ÐœnSO4×5H2O - 0.0025; Na2MoO4×2H2O - 0.0025; рР7.0 with methanol (0.4%) as the only source of carbon and energy (Hirsch and Conti, 1964).
An account of the number of neutrophilic iron-oxidizing microorganisms was carried out on a selective agar medium with the following composition, g l-1: FeSO4×7H2O - 5.9; (NH4)2SO4 - 0.5; NaNO3 - 0.5; K2HPO4 - 0.5; MgSO4×7H2О - 0.5; lemon acid - 10.0, sucrose - 2.0, peptone - 1.0, pH 7.0 (Granina et al., 2003). Plating on beef-extract agar and on selective media for an account of the number of hydrocarbon-oxidizing, methylotrophic and iron-oxidizing bacteria was carried out from dilutions 10-2, 10-3, 10-4, and 10-5 in replicates. The plates with inoculants were incubated at 28 to 30°C for 2 to 5 days at a temperature of 28 to 30°Ð¡ in an incubator and colony-forming units (CFU) were counted and calculated per gram of dry soil (Foght and Aislabie, 2005).
Analysis of soil enzyme activity
Dehydrogenase activity (DHA) in the soil samples was determined by a modified spectrophotometric method according to Öhlinger (1996). This method involves colorimetric determination of 2.3.5-triphenylformazan produced after the reduction of 2.3.5-triphenyltetrazolium chloride by soil microorganisms. The absorbance value obtained photometrically using a microplate photometer iMARK was converted to 2.3.5-triphenyl formazan using its standard curve. The activity of dehydrogenase was expressed as µL H2 g-1 of dry soil day-1. Catalase activity (CA) in the soil samples was measured using titration method (Johnson and Temple, 1964), which is based on the measurement of the dissociation rate of hydrogen peroxide during its interaction with soil and the amount of undissociated peroxide determined by titrating with potassium permanganate. The activity of catalase was expressed as mL of 0.1 N КMnO4 g-1 of dry soil h-1.
The activity of β-fructofuranosidase (β-FFA) was determined using colorimetric method: substrate 5% solution of sucrose, incubation time of 3 h, incubation temperature of 30°Ð¡, reduced sugars in the filtration appear with the help of 0.2% ferricyanide solution, its content is calculated on a standard scale composed for glucose (Shi et al., 2008). The β-FFase activity was expressed as mg of glucose g-1 of dry soil h-1. All measurements were conducted in triplicates replicates per soil sample.
Statistical analysis
Data were analyzed statistically using the software (Microsoft Excel 2010). The differences in probable errors р<0.05 and р<0.01 was considered the confidence. The interrelationships between the physico-chemical and biological indices of soil were evaluated using pair correlation coefficients for p<0.05.
In the evaluated soil samples, the total number of culturable heterotrophic microorganisms varied differently (Figure 2A). In samples (1, 2, 3, 4, 5 and 7), the amount of heterotrophic microorganisms comprised about 6 to 26×105 CFU g-1 of dry soil. In samples 6 and 9, it was much higher, 48×105 CFU g-1 of soil and 75×105 CFU g-1 of dry soil, respectively. The number of heterotrophic microorganisms in sample 8 was highest, 685×105 CFU g-1 of dry soil, this could be due to a high level of soil pollution with organic compounds in that sampled area. An increase in the amount of hydrocarbon gases and in the number of hydrocarbon-oxidizing microorganisms has been observed in earlier explored gas and oil sites and also a reduction in the value of redox potential in the soil profile as compared to background soils (Mozharova and Kulachkova, 2008; Mozharova, 2010).
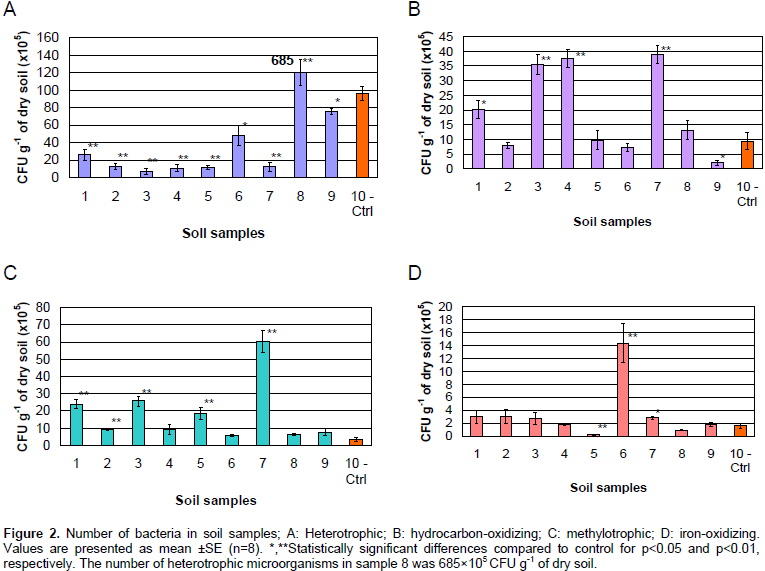
Gaseous and bacterial anomalies have also been found in soils above underground gas storages due to emission of methane into the atmosphere, increase in the activity of bacteria methane oxidation, decrease and wide variation in redox potential. In soils of gas bearing territories, hydrocarbon gases and products of their microbiological transformations promote an increase in the biomass of methylotrophic microorganisms and the formation of organic carbon and nitrogen. In connection with the peculiarity of this territory, the number of cultivable aerobic hydrocarbon-oxidizing and methylotrophic microorganisms was evaluated. The difference in the number of hydrocarbon-oxidizing bacteria was low; it was at an average of 2 to 39×105 CFU g-1 of dry soil (Figure 2B). The methylotrophic microbial content varied in different soil samples from 1 to 60×105 CFU g-1 of dry soil (Figure 2C).
In recent years, within the confines of soil-ecological monitoring of commercial and industrial landscapes, researchers now conduct a magnetic measurement of soils which is an accessible express method of analysis for preliminary examination of urban territories (Jordanova et al., 2003; Zhang et al., 2011). One of the important magnetic characteristics of soils is its magnetic susceptibility; this is a physical quantity which reflects the ability of soil substances to change magnetic moments when exposed to an external magnetic field due to the presence of magnetite contributing a great deal to the magnetic properties of soil (Dearing et al., 1996). Natural soils above artificial underground gas deposits in zones dispersed and dominated by the influence of hydrocarbon gases have been known to possess anthropogenic properties such as new formations of micro-dispersed bacteriomorphic magnetite and iron organic complexes formed during the synthesis of magnetite and bacteria plays an active role in its decomposition giving rise to a breakdown of complex organic substances which is accompanied by the release of energy (Mozharova and Kulachkova, 2008; Mozharova, 2010; Sabrina et al., 2011).
Magnetic susceptibility was determined in the soil samples above the underground natural gas storage (Table 1). These indications corresponded to normal values for dark-brown soils, showing the absence of an expressed anthropogenic transformation, and at the same time not excluding the existence of an increased iron content synthesized via a biochemical path in soils with higher values of magnetic susceptibility. Based on this, an enumeration of the number of iron-oxidizing bacteria was evaluated in the soil samples above the underground natural gas storage (Figure 2D). The amount of iron-oxidizing bacteria in most of the soil samples was at a range of 1 to 3×105 CFU g-1 of dry soil. Sample 5 was identified with a low iron oxidizing bacterial content (0.2×105 CFU g-1 of dry soil), whose sampling was at the borders of the researched territory (Figure 1). A high iron-oxidizing bacterial content was observed in sample 6 - 14.4×105 CFU g-1 of dry soil. Dehydrogenase activity in the researched soil samples was at an average of 0.211 to 0.637 µL Ð2 g-1 of dry soil day-1 (Table 2). The low significance of dehydrogenase activity indicates the indigent levels of this enzyme in the soil, which justifies the existence of soil chemical agents inhibiting this enzyme.
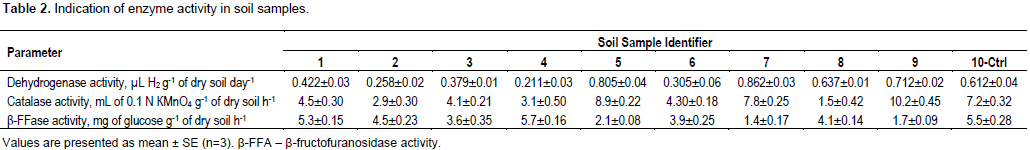
Soil dehydrogenases as one of the most sensitive enzymes to hydrocarbon pollution is to a high degree inhibited not by hydrocarbons, but by products of their degradation which can when accumulated in soil for a long time possess toxic effects (Sumampouw and Risjani, 2014; Utobo and Tewari, 2015). Minimal values were observed in samples 2 and 4 (0.211 and 0.258 µL Ð2 g -1 of dry soil day-1), while samples 5, 7 and 9 showed maximal activity ranging from 0.712 to 0.862 µL Ð2 g-1 of dry soil day-1. Soil samples with maximal values for dehydrogenase activity also showed maximal values for catalase activity ranging from 7.8 to 10.2 mL of 0.1 N КMnO4 g-1 of dry soil h-1 (Table 2). Catalase activity in other samples was low ranging from 1.5 to 4.5 mL of 0.1 N КMnO4 g-1 of dry soil h-1.β-FFase activity in soil samples 5, 7 and 9 was lower than in other samples, comprising of 2.1, 1.4 and 1.7 mg of glucose g-1 of dry soil h-1, respectively. In other samples the activity of β-FFase was at a range of 3.6 to 5.7 mg of glucose g-1 of dry soil h-1 (Table 2).
Various methodological approaches for evaluating the ecological state of soils exist, but the microbiological method in our opinion serves as the most sensitive of them all. Multifunctional microbiota participating in opposite reactions performs the function of stabilizing the metabolic balance of nature. Once in soil, hydrocarbons are known to react with soil microorganisms, leading to changes in species composition, strength, and efficiency of microbial biomass (Guo et al., 2012). Contamination by hydrocarbons leads to a deterioration in the agro-chemical and agro-physical characteristics of the soil, and an increase in phytotoxicity. Petroleum hydrocarbons appreciably influence the biochemical processes in the soil (Wyszkowska and Wyszkowski, 2010; Achuba and Okoh, 2014).
Comparing the amount of heterotrophic and hydrocarbon-oxidizing bacteria in the samples, it is observed that in three of the samples (3, 4 and 7) the amount of hydrocarbon-oxidizing bacteria was higher than heterotrophic bacteria. Such an increase in the content of hydrocarbon-oxidizing bacteria can be associated with a selective impact of corresponding substrates. Therefore, the identified characteristics may indirectly indicate the presence of hydrocarbons in the soil. Comparing the amount of heterotrophic and methylotrophic bacteria in the samples, it was discovered that, in three samples (3, 5 and 7) the content of methylotrophic bacteria was higher than heterotrophic. Samples 3 and 7 also were characterized by a high number of hydrocarbon-oxidizing bacteria. The correlation coefficient between the number of methylotrophic and hydrocarbon-oxidizing bacteria in the soil samples was 0.637 (p<0.05) (Table 3).
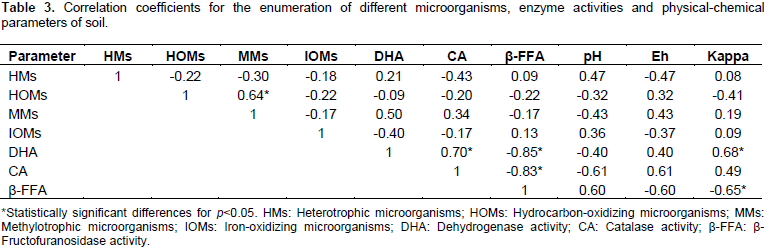
Attention should be drawn to the fact that in the soil samples 5, 7 and 9, the number of methylotrophic bacteria is higher than hydrocarbon-oxidizing, which shows the proliferation of not only facultative methylotrophs but obligate methylotrophs. This serves as an indirect proof of the existence of methane in upper horizons of soil above the underground natural gas storage. Soil sample 6 with a high content of iron-oxidizing bacteria was sampled directly above the main trunk of the gas well near the gas distribution station. It is possible that a cluster of bacteriomorphic magnetite had been formed in that area. Significant correlation were no found between the values of soil pH, redox potential and the content of the microbial physiological groups in the soil samples studied. The highest values of correlation coefficients observed were between the number of heterotrophic bacteria and the soil pH values (r=0.471) (p<0.05) and also the number of heterotrophic bacteria and the values of redox potential (r=-0.466) (p<0.05) (Table 3).
In all the soil samples from the UGS territory (sample 8 as an exception), total number of heterotrophic microorganisms was lower than in the control samples (as indicated in the section «study area and soil sampling») which were obtained from the boundaries of the UGS (Figure 2A), and this points to an inhibitory effect of the proposed pollutants. Number of hydrocarbon-oxidizing (Figure 2B) and methylotrophic (Figure 2C) microorganisms in a series of samples was higher than in control samples. Based on the results of the microbiological analysis, five out of the nine studied soil samples (3, 4, 5, 7 and 9) contained a high amount of methylotrophic, hydrocarbon-oxidizing bacteria or both, and the other mentioned microorganisms simultaneously. The number of iron-oxidizing bacteria in the soil samples from the UGS storage facility did not differ from the values in the control samples (with sample 6 as an exception) (Figure 2D).
It is known that none of the biological processes in soil occurs without dehydrogenase, whose activity directly indicates the intensity of hydrocarbon degradation in the soil, depending on the environmental conditions (Pascual et al., 2000; Makoi and Ndakidemi, 2008; Kumar et al., 2013). Catalase activity is also an informative indicator of soil biological activity in conditions of hydrocarbon contamination (Maila and Cloete, 2005). In soil samples obtained within internal and external contours of the gas bearing territory (Figure 1), where supposedly the gas flow in the soil was high, the dehydrogenase and catalase activities were high. This is possibly connected with an inhibition of soil oxido-reductases by hydrocarbons as a result of their recent entry in soil. Low activity of these enzymes may also be due to a chronic impact of hydrocarbons or its oxidized products. On the contrary, in zone 1 (boundary of the mineral block allocation of the UGS) with low gas torrent, activities of dehydrogenases and catalases in soil (sample 5, 7 and 9) were higher than in other samples.
The correlation coefficient between the number of methylotrophic bacteria and dehydrogenase activity in the soil samples was 0.502 (p<0.05). Samples 5, 7 and 9, in which the values of dehydrogenase and catalase activity of soil were high, showed a significant content of obligate methylotrophic bacteria. Given that microorganisms serve as the main source of dehydrogenases and catalases in soil, increased levels of these researched oxido-reductases in these soil samples is probably due to the proliferation of specialized soil microflora in the microbocenosis. The presence of hydrocarbon substrate in the soil must have possibly led to an increased dehydrogenase activity, as well as various peroxides (catalase substrates) formed as a result of response to stress by the soil community due to pollutants.
β-FFase enzyme activity reflects the intensity of hydrolytic processes in soil, determining the level of fertility and soil biological activity (Shi et al., 2008). In addition, β-FFase is highly sensitive to the impact of negative factors. In soil samples 5, 7 and 9, the activity of invertases was at its maximum and in combination with an increased content of obligate methylotrophic microorganisms points to the presence of hydrocarbon pollutants in soil.A direct correlation between catalase activity in soil samples and dehydrogenase activity was observed (r=0.702; p<0.05); an inverse correlation between dehydrogenase activity and β-FFase (r=-0.852; p<0.05), and an inverse correlation between the activity of catalase and β-FFase (r=-0.827; p<0.05) (Table 3). Given the aforementioned, the result of this enzymatic indication of soils indicates the presence of hydrocarbon contamination in a number of soil samples.
A direct correlation was also found between the magnetic susceptibility of soils and dehydrogenase activity (r=0.677; p<0.05), magnetic susceptibility and catalase activity (r=0.492; p<0.05) and an inverse correlation was shown between the magnetic susceptibility and β-FFase activity (r=-0.650; p<0.05) (Table 3). The relationship identified can be a reflection of the reactions of soil microorganisms to changing environmental conditions. The results of this microbiological and biochemical indication of soils confirmed the presence of hydrocarbon gases in the areas of natural gas storage. In our view, suitable assessment of the ecological state of soil in districts of gas storages can help not to only discover fresh entry of methane gas in soil but also identify changes in soil during chronic impact of hydrocarbons. Issues of increased authentic control of bedded gas losses and impermeability of UGS has an enormous significance in the ecology of many districts. The results of this research bring in considerable contribution in assessing the sanitary-epidemiological safety of soil in territories of Saratov region.
The penetration of hydrocarbon gases from the lower layers and soil formations to upper layers and strata is accompanied by the formation of gas and bacterial anomalies, which are positioned one above the other. Formation of bacterial abnormalities occurs in a relatively short time after the appearance of the gas coating in the earth formation, promoting a sharp decrease in the content of methane and heavy hydrocarbons in the thick deposits. In some cases, bacterial anomalies are detected and fixed earlier than gaseous defects and this helps detect zones with accumulated stray gases. Differences were observed in the microbiological and biochemical soil indicators on the territory of the UGS in comparison with the background (control) samples, obtained outside the boundaries of the UGS. In all of the gas-bearing zones was observed: a reduced number of heterotrophic microorganisms, increased content of indicator microorganisms (hydrocarbon-oxidizing and methylotrophic in this case obligate) which gave room to talk about the entry of methane into the upper layers of soil.
One more feature justifying the leakage of methane in this gas bearing territories is the presence of iron-oxidizing microorganisms which utilize bacteriomorphic sub-nano dispersed magnetic oxides formed during the incomplete circle of methane oxidation as substrate for energy. Fermentative analysis also identified a distinction in the researched indicators between soil in the UGS and the control soil. A reduced dehydrogenase and catalase activity in soil of UGS within the boundaries of the internal and external contours of gas storages points to an inhibition of soil enzymes by pollutants. The increased activities of oxido-reductases within the boundaries of the mineral block allocation of the UGS in combination with the identified obligate methylotrophic bacteria in this zone justifies the fact that there was a stimulation of growth and metabolic activity of specialized microorganisms. So, an increased number of hydrocarbon-oxidizing and methylotrophic microorganisms in soil of the territory of UGS in comparison with the control samples, obtained outside boundaries of the UGS points to the presence of hydrocarbons. This is why the use of indicators such as the number of methylotrophic and hydrocarbon-oxidizing bacteria serve as perspectives for ecological soil monitoring in regions of gas storages and also for determining and locating areas of gas leakages.
The authors have not declared any conflict of interests.
This research was conducted as part of the Russian Scientific Foundation grant (research task No. 17-77-10040).
REFERENCES
|
Achuba FI, Okoh PN (2014). Effect of petroleum products on soil catalase and dehydrogenase activities. Open J. Soil Sci. 4:399-406.
Crossref
|
|
|
|
Cao Y, Staszewska E (2011). Methane emission mitigation from landfill by microbial oxidation in landfill cover. Proceedings, International Conference on Environmental and Agriculture Engineering IPCBEE, IACSIT Press, Singapore 15:57-64.
|
|
|
|
|
Dearing JA, Hay KL, Baban SMJ, Huddleston AS, Wellington EMH, Loveland PJ (1996). Magnetic susceptibility of soil: An evaluation of conflicting theories using a national data set. Geophys J. Int. 127:728-734.
|
|
|
|
|
Foght J, Aislabie J (2005). Enumeration of soil microorganisms. In: Margesin R, Schinner F, (eds): Manual for Soil Analysis – Monitoring and Assessing Soil Bioremediation. Springer-Verlag, Berlin, Heidelberg, Germany. pp. 261-280.
|
|
|
|
|
Gennadiev AN, Pikovskii YuI, Tsibart AS, Smirnova MA (2015).
|
|
|
|
|
Hydrocarbons in soils: origin, composition, and behavior (Review). Eurasian Soil Sci. 48(10):1076-1089.
Crossref
|
|
|
|
|
Granina LZ, Parfenova VV, Zemskaya TI, Zakharova YuR, Golobokova LP (2003). On iron and manganese oxidizing microorganisms in sedimentary redox cycling in lake Baikal. Berliner Palaobiologische Abhandlungen 4:121-128.
|
|
|
|
|
Guo H, Yao J, Cai M, Qian Y, Guo Y, Richnow HH, Blake RE, Doni S, Ceccanti B (2012). Effects of petroleum contamination on soil microbial numbers, metabolic activity and urease activity. Chemosphere 87:1273-1280.
Crossref
|
|
|
|
|
Hirsch P, Conti SP (1964). Biology of budding bacteria. Growth and nutrition of Hyphomicrobium spp. Arch. Microbiol. 48:358-367.
|
|
|
|
|
Ho A, Kerckhof FM, Luke C, Reim A, Krause S, Boon N, Bodelier PLE (2013). Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ. Microbiol. Rep. 5:335-345.
Crossref
|
|
|
|
|
Johnson JI, Temple KL (1964). Some variables affecting measurement of catalase activity in soil. Soil Sci. Soc. Am. J. 28: 207-209.
|
|
|
|
|
Jordanova D, Veneva L, Hoffmann V (2003). Magnetic susceptibility screening of anthropogenic impact on the Danube river sediments in northwestern Bulgaria preliminary results. Stud. Geophys. Geod. 47:403-418.
|
|
|
|
|
Kizilova A, Yurkov A, Kravchenko I (2013). Aerobic methanotrophs in natural and agricultural soils of European Russia. Diversity 5:541-556.
Crossref
|
|
|
|
|
Kumar S, Chaudhuri S, Maiti SK (2013). Soil dehydrogenase enzyme activity in natural and mine soil - a review. Middle-East J. Sci. Res. 13(7):898-906.
|
|
|
|
|
Magiera Т, Strzyszcz Z (2000). Using of field magnetometry in estimation of urban soil degradation. Proceedings of First International Conference on soils of Urban, Industrial, Traffic and Mining area 1:105-110.
|
|
|
|
|
Maila MP, Cloete TE (2005). The use of biological activities to monitor the removal of fuel contaminants – perspective for monitoring hydrocarbon contamination: a review. Intern. Biodeterior. Biodegr. 55:1-8.
Crossref
|
|
|
|
|
Makoi JHJR, Ndakidemi PA (2008). Selected soil enzymes: Examples of their potential roles in the ecosystem. African. J. Biotech. 7:181-191.
|
|
|
|
|
Mills A, Breuil LuC, Colwell RR (1978). Enumeration of petroleum degrading marine and estuarine microorganisms by the most probable number method. Can. J. Microbiol. 24:552-557.
|
|
|
|
|
Mozharova NV, Kulachkova SA (2008). Specificity of soil functioning and formation on gas-bearing areas. J. Soils Sediments 8(6):424-432.
Crossref
|
|
|
|
|
Mozharova NV (2010). Soil cover of gas-bearing areas. Eurasian Soil Sci. 43(8):935-944.
Crossref
|
|
|
|
|
Öhlinger R (1996). Dehydrogenase activity with the substrate TTC. In: Schinner F, Ohlinger R, Kandler E, Margesin R (eds): Methods in Soil Biology. Berlin, Springer Verlag. pp. 241-243.
|
|
|
|
|
Pascual JA, Garcia C, Hernandez T, Moreno JL, Ros M (2000). Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol. Biochem. 32:1877-1883.
|
|
|
|
|
Peterson EW, Martin LI, Malone DH (2015). Identification of potential vertical gas migration pathways above gas storage reservoirs. World J. Environ. Eng. 3(2):23-31.
Crossref
|
|
|
|
|
Sabrina H, Michael S, Johnson B (2011). The iron-oxidizing proteobacteria. Microbiology 157:1551-1564.
|
|
|
|
|
Sharafutdinov RF, Valiullin RA, Ramazanov AS, Sadretdinov AA, Sharipov AM, Vakhitova GR (2015). Environmental monitoring of underground gas storages. Paper presented at 77th EAGE Conference and Exhibition. Madrid, Spain.
Crossref
|
|
|
|
|
Shi ZJ, Lu Y, Xu ZG, Fu SL (2008). Enzyme activities of urban soils under different land use in the Shenzhen city, China. Plant Soil Environ. 54(8):341-346.
|
|
|
|
|
Sumampouw OJ, Risjani Y (2014). Bacteria as indicators of environmental pollution: Review. Int. J. Ecosyst. 4(6):251-258.
|
|
|
|
|
Utobo EB, Tewari L (2015). Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Env. Res. 13(1): 147-169.
Crossref
|
|