Full Length Research Paper
ABSTRACT
Saba senegalensis fruits have gained more attention in recent years due to their antioxidant compounds such as carotenoids, phenolics, vitamin C. This study was designed to identify the main carotenoids from S. senegalensis fruits including phenolics and vitamin C quantifications. Carotenoid profiles from tissues of these fruits have been characterized by High Performance Thin Layer Chromatography- mass spectrometry (HPTLC-MS) for the first time. Phenolics and vitamin C contents were studied using spectrophotometric and high-performance liquid chromatography-diode-array detector (HPLC-DAD) methods, respectively. Using the Folin-Ciocalteu’s reagent, total phenolics content was estimated to be around 630 mg gallic acid equivalents/100 g fresh fruit of S. senegalensis. The HPLC analysis showed vitamin C content of about 1511 µg/100 g fresh weight. Three xanthophylls (antheraxanthin, lutein, and β-cryptoxanthin), and two hydrocarbon carotenes (β-carotene and phytoene) were identified in the saponified extract of fruits. For radical-scavenging activity, using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, S. senegalensis fruits extract was estimated to be 75 mg of Trolox equivalents/100 g fresh weight for whole fruit. Results obtained indicate that S. senegalensis fruits are a cheap source of carotenoids and other micronutrients. They are important for industrials and as an ingredient used in functional food formulation.
Key words: Carotene, β-carotene, pigment, antioxidants, fruit extract, functional food.
INTRODUCTION
Carotenoids form a distinguished group of natural pigments in the plant kingdom. They are responsible for yellow, orange, and red colors in flowers, fruits, and vegetables (Cano et al., 2017). These colors are caused by their chemical structure with a long polyenic carbon chain. 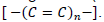 The remarkable coloring power in the flowers is essential in the attraction of pollinators (birds, bees, butterflies) that can convey the seeds and thus ensure species perennity (Tchuenguem et al., 2014; Rivera et al., 2013). In addition to their physiological functions such as photosynthesis, photoprotection, and nutrition, carotenoids play significant roles in promoting human health and reducing the risk of chronic diseases due to their vitamin A activity and antioxidant properties (Hartz et al., 2018; Abid et al., 2013; Papaioannou et al., 2011). So, carotenoids are used by the food industry, as well as in pharmaceutics and cosmetic products, and their demand is increasing (Vila et al., 2018). The negative perception of synthetic dyes by consumers has increased the demand for natural pigments like anthocyanins and carotenoids. Numerous scientific publications furthermore confirm the beneficial effect of natural pigments on human health.
The remarkable coloring power in the flowers is essential in the attraction of pollinators (birds, bees, butterflies) that can convey the seeds and thus ensure species perennity (Tchuenguem et al., 2014; Rivera et al., 2013). In addition to their physiological functions such as photosynthesis, photoprotection, and nutrition, carotenoids play significant roles in promoting human health and reducing the risk of chronic diseases due to their vitamin A activity and antioxidant properties (Hartz et al., 2018; Abid et al., 2013; Papaioannou et al., 2011). So, carotenoids are used by the food industry, as well as in pharmaceutics and cosmetic products, and their demand is increasing (Vila et al., 2018). The negative perception of synthetic dyes by consumers has increased the demand for natural pigments like anthocyanins and carotenoids. Numerous scientific publications furthermore confirm the beneficial effect of natural pigments on human health.
Native to Senegal, S. senegalensis is a widespread liana in West Africa. These ripe fruits possess nutritive and therapeutic properties (Kini et al., 2008). Due to these high nutritive values such as high contents of carotenoids, phenolic compounds, and vitamin C, S. senegalensis is a promising fruit for juice production. As mentioned earlier, some studies have been able to quantify carotenoids content, but their complete characterization in S. senegalensis fruit tissues was not reported. The aim of this study is to identify the main carotenoids present in S. senegalensis fruits by HPTLC-ESI-MS. Complementary information of other constituents of these fruits is also shown and the correlation of each chemical compound with the antioxidant activities (DPPH and FCR assays). This study may lead the necessary knowledge to the development of future functional foods and functional ingredients or nutraceuticals with potential health benefits from S. senegalensis ripe fruits.
MATERIALS AND METHODS
Plant material
S. senegalensis fruits were collected from Ouid-tenga in the Central Region of Burkina Faso. The identification of the plant was made at University Joseph KI-ZERBO by Dr. Issouf ZERBO. A voucher specimen under the code N° 6908 was deposited in the Herbarium of the University of Joseph KI-ZERBO (Ouagadougou, Burkina Faso). The arils were removed from the cockle and kept in the freezer for further work.
Chemicals and standards
All the solvents used without acetonitrile (HPLC grade) were of analytical grade and were purchased from Sigma-Aldrich (Taufkirchen, Germany). Water was purified by a Millipore instrument (MOLSHEIM France). Gallic acid, 2,2-diphenyl-1-picrylhydrazyl, Folin-Ciocalteu phenol reagent, and L-ascorbic acid (HPLC grade) were obtained from Sigma Chemical Co. (St. Louis, MO). β-carotene (HPLC grade) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Buffer salts and all other chemicals were of analytical grade. Samples and standards solutions were filtered before chromatographic analysis using through Millipore membrane of 0.2 µm.
Extraction
Carotenoid z pigments were extracted by maceration using n-hexane-acetone (1:1, v/v) solvent system in the dark and at low temperature (4°C). So, one gram of fresh arils was extracted to color exhaustion with 5 ml of n-hexane-acetone (1:1, v/v) for 24 h. The extract was then filtered, and the filtrate was stored in the freezer until the spectrophotometer and HPTLC-MS analysis. Phenolic and hydrophilic antioxidant compounds of S. senegalensis fruits were extracted with methanol by maceration. 1 g of fresh arils was introduced into a 50-mL Erlenmeyer flask to which 15 ml of methanol was added, and the mixture was kept at room temperature for 72 h. After filtration, the filtrate was stored in the freezer until the spectrophotometer analysis.
Vitamin C was extracted by maceration with acidified methanol. Now 1 g of fresh arils was put into an Erlenmeyer flask to which 15 ml of acidified methanol (4% oxalic acid) was added, and the mixture was kept at room temperature for 24 h. The extract was then filtered, and the filtrate was stored in the freezer until HPLC analysis.
Lipophilic antioxidant compounds were obtained by maceration extraction with n-hexane. So 16 g of fresh arils was extracted with 25 ml of n-hexane for 24 h at low temperature (4°C) in the dark. The mixture was filtered with filter paper, and then the residue was taken up with 15 mL of n-hexane. After filtration, the extraction was repeated, and the different filtrates were collected, and dry concentrated on the rotatory evaporator at low temperature (<40°C). The dried extract was re-dissolved to 1 ml methanol-acetone (1:1, v/v) for lipophilic antioxidant activity evaluation by spectrophotometer analysis.
Saponification
Saponification was conducted by using potassium hydroxide in methanol. To 10 ml of n-hexane-acetone (1:1, v/v) extract, 15 ml 30% (w/v) of potassium hydroxide in methanol was added. The mixture was kept for 3 h at room temperature in the dark (Cano et al., 2017; Kini et al., 2008). The saponified extract was transferred into the separatory funnel containing 15 ml of n-hexane, shaken and left to settle. Then, the mixture was washed five times with distilled water, discarding each time the aqueous phase to obtain a neutral pH. The final extract was completely evaporated on the rotatory evaporator at low temperature (<40°C). The dried extract was re-dissolved to 2 ml with n-hexane, filtered through a filter paper and analyzed by HPTLC.
Carotenoids analysis by HPTLC-MS
Chromatography was performed on a 20 cm x 10 cm HPTLC plate silica gel 60 F254 (Merck, Germany). 2 µl of saponified and non-saponified extracts was applied as 5 mm bands with Automatic Thin layer chromatography (TLC) Sampler (CAMAG, Switzerland) Linomat 5. The identification of carotenoids in saponified fruits extract of S. senegalensis was carried out using a mass spectrometer Quadrupole Time of Flight (QTOF, Series 6520) with Electrospray ionization source. Mass spectra were obtained in positive ionization mode in a mass range of m/z 400-600. MS parameters were as follows: collision gas, helium; capillary temperature, 250°C.
Total phenolics analyses
Phenolics were analyzed spectrophotometrically with the Folin-Ciocalteu reagent according to the procedure described in the literature (Ouédraogo et al., 2017; Dudonné et al., 2009; Miliauskas et al., 2004). After a suitable dilution, 60 µl of methanolic extract of S. senegalensis fruits and gallic acid (standard) were mixed with 60 µl of Folin-Ciocalteu reagent previously diluted ten times with distilled water. After vortexing, the mixture was incubated for 8 min at room temperature, and 120 µl of 7.5% saturated sodium carbonate solution was added to destroy the residual reagent. Samples were stirred and stored for 30 min in the dark. The spectrophotometric determination was performed in a 96-microwell plate by placing 240 µl of sample and a reagent blank in corresponding microwells. The reading was done at 760 nm spectrophotometrically. The phenolics content of S. senegalensis fruits was determined using the equation of the calibration curve (y = 7.3194x + 0.1309,R² = 0.998), and the results are expressed in mg of gallic acid equivalents (GAE) per gram of fresh fruits weight. All measurements were performed in three replications.
Spectrophotometric analysis of vitamin C
Vitamin C was analyzed by High-Performance Liquid Chromatography (SHIMADZU LC 20A) with the Shodex Asahipark NH2-NP column (5 µm, 250 × 4.6 nm from Showa Denko K.K. USA) at 40°C. Then 8 g of fresh almond of S. senegalensis’s fruits was extracted with 12 mL of 5% metaphosphoric acid solution for 15 min at room temperature. After filtration, the residue was mixed with 8 ml of 5% metaphosphoric acid solution for two successive extractions. The three filtrates were combined and centrifuged for 10 min at 4,000 g and 5°C. The supernatant was collected and
made up to 40 ml and then filtered with a 0.2 mm Advantec filter for HPLC analysis. The mobile phase was acidified with 0.1%phosphoric acid in distilled water (solvent A) and acetonitrile (solvent B), performed at the ratio 25:75. The flow rate was 1 ml/min, and the injection volume was 20 µl. HPLC-Diode Array Detector (HPLC-DAD) was used, and L-ascorbic acid was detected at 245 nm.
Hydrophilic and lipophilic antioxidant activities determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
Hydrophilic and lipophilic antioxidant activities were measured using the DPPH decoloration method (Lizárraga-Velázquez et al., 2018; Ouédraogo et al., 2017; Dudonné et al., 2009). The method is based on the capacity of S. senegalensis fruit extracts to scavenge compared to the DPPH radical (DPPH•) and the Trolox (a standard antioxidant) in a dose-response curve. DPPH radical absorbs in the visible at 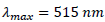 and disappears with reduction by an antioxidant compound (Dudonné et al., 2009). Briefly 200 µl of DPPH• the solution (0.04 mg/ml in methanol) was mixed with 50 µl of fruit extract. After 8 min of incubation at 37°C under dim light, then the decrease in absorbance at 515 nm was measured spectrophotometrically (MP96, SAFAS Monaco). A linear regression curve (y = -11.777x + 0.5516; R² = 0.998) was established in order to calculate antioxidant content in fruits of S. senegalensis. The antioxidant activity was expressed as Trolox equivalents per 100 g of fresh weigh of S. senegalensis fruits (mg TE/100 g).
and disappears with reduction by an antioxidant compound (Dudonné et al., 2009). Briefly 200 µl of DPPH• the solution (0.04 mg/ml in methanol) was mixed with 50 µl of fruit extract. After 8 min of incubation at 37°C under dim light, then the decrease in absorbance at 515 nm was measured spectrophotometrically (MP96, SAFAS Monaco). A linear regression curve (y = -11.777x + 0.5516; R² = 0.998) was established in order to calculate antioxidant content in fruits of S. senegalensis. The antioxidant activity was expressed as Trolox equivalents per 100 g of fresh weigh of S. senegalensis fruits (mg TE/100 g).
Statistical analysis
All analyses were conducted in three replications, and data processed in Microsoft Excel 2016. An ANOVA with Bonferroni post hoc test was performed to determine significant differences. The level of significance was defined as p≤0.5.
RESULTS AND DISCUSSION
Carotenoids identification
Carotenoids in S. senegalensis fruits were characterized and identified according to their chemical, chromatographic and spectroscopic properties (UV-vis and mass spectroscopic analysis). Figure 1 shows the High-Performance Thin-Layer Chromatography profiles of the saponified (A) and unsaponified carotenoids (B) extracts obtained from S. senegalensis mature fruits. After saponification, only one spot (Rf= 0.73) was observed on the HPTLC chromatogram (Figure 1). The UV-Vis spectrum obtained from saponified extract presented three maxima around 450 nm, which is characteristic of carotenoid compounds (Vila et al., 2018). In addition, the standard (β-carotene) and the saponified extract UV/Vis spectra were almost identical. The ultraviolet spectrum of the saponified extract is moved 2-5 nm towards the low wavelengths in comparison with the β-carotene spectrum (Figure 2), due to the interference from other carotenoids. The mass spectrum of this spot shows several peaks (Figure 3) corresponding to the molecular mass of free carotenoids. Out of the many detected carotenoids, five carotenoids were tentatively identified (Table 1). The main carotenoid of S. senegalensis fruits was identified as β-carotene by comparison of the chromatographic, UV/Vis, and spectrometric characteristics with standard and with literature data (Etzbach et al., 2018; van Breemen et al., 2012; de Rosso and Mercadante, 2007). The non-protonated molecule [M]+• of β-carotene was detected at m/z 536.1, and a typical fragment was found at m/z 430.4 formed by the loss of xylene [M-106]+• following the mechanism in Figure 4. Concerning the protonated molecule [M+H]+ of β-carotene detected at m/z 537.4, the typical fragment was formed at m/z 445 [M+H-92]+ corresponding to the elimination of a neutral molecule of toluene (Figure 4). The protonated and non-protonated molecules of β-carotene were both detected in ESI-MS in positive ionization mode by Vallverdú-Queralt et al. (2012) and Rivera et al. (2013). Also, the mass spectrum of a saponified extract shows that the non-protonated and the protonated molecules of β-carotene were both more abundant peaks. Based on data reported by Kini et al. (2008), β-carotene was the main carotenoid in S. senegalensis fruits. Lutein was identified in the saponified extract of fruits, as it presented the protonated molecule [M+H]+ at m/z 569 corresponding to the formula C40 H56 O2. It also produced a most abundant fragment ion at [M+H-18]+ m/z 551, confirming the loss of a neutral molecule of water (presence of hydroxyl group). Besides, zeaxanthin and lutein are isomers, which differ in the position of the double bond in one of the end rings. Only lutein contains an allylic hydroxyl group, therefore, easier to eliminate than the other hydroxyl group on the secondary carbon atom with adjacent saturated C-C bonds. The fragment ion [M+H-H2 O]+ at m/z 551 is stabilized by mesomeric effects (Figure 4) and therefore has a more abundant peak than the protonated molecule [M+H]+ at m/z 569. Zeinoxanthin, α-cryptoxanthin, and β-cryptoxanthin, possessing one hydroxyl group, have the same chemical formula C40 H56 O. They share identical protonated molecules [M+H]+ at m/z 553 and a fragment ion at m/z 535 [M+H-H2 O]+. Unlike β-cryptoxanthin and zeinoxanthin, α-cryptoxanthin possesses an allylic hydroxyl group. This structural difference can be used to distinguish between them, owing to the ease of the elimination of hydroxyl group allylic to the double bond (Etzbach et al., 2018; de Rosso and Mercadante, 2007). The mass spectrum of the saponified extract of S. senegalensis fruits revealed a most intense signal of a protonated molecule [M+H]+ at m/z 553 than the fragment ion signal at [M+H-H2 O]+ m/z 535 .The increase of the stability in the protonated molecule prevents the loss of water molecule, which could explain the presence of β-cryptoxanthin and zeinoxanthin in the fruits of S. senegalensis. The non-protonated [M]+ was also detected at m/z 552. It is most abundant than the fragment ion at m/z 534 [M-H2 O]+ confirming the stability of the molecule ion.
The mass spectrum of fruits of S. senegalensis showed a protonated molecule [M+H]+ at m/z 545 and was tentatively identified as the phytoene with the chemical formula C40 H64. Phytoene is a colorless carotenoid expected to be found in fruits of S. senegalensis. The presence of phytoene in a variety of carotenoid-rich fruits, including mango, papaya, orange, and tomato, has been reported (Al-Yafeai et al., 2018). Antheraxanthin (C40 H56 O3) was detected in a saponified S. senegalensis fruits extract by MS (ESI+) spectrum with a peak ion for non-protonated molecule [M]+ at m/z 584.2. Also, a significant fragment was observed at m/z 566.5 [M-H2 O]+, related to the loss of a water molecule due to the presence of a hydroxyl group located in β-ring. Some authors reported that the cis-antheraxanthin did not give two fragments possibly due to the low stability of this ion 548.5 [M-2H2 O]+ under the specific ionization conditions used (Cano et al., 2017; Meléndez-Martínez et al., 2005).
Micronutrient contents in fruits of S. senegalensis
In this study, the complete analysis of total phenolic compounds and vitamin C was conducted to know the antioxidant composition in fruits of S. senegalensis. Previous studies attributed the antioxidant activities of S. senegalensis fruits to its bioactive compounds like carotenoids, phenolic compounds, vitamin C (Kini et al., 2008). Total phenolic compounds were determined by the Folin-Ciocalteu assay. Folin-Ciocalteu reagent detects electron transfer by measuring the reductive capacity of the sample. For this reason, total phenolics may also be considered as an antioxidant activity assay. Table 2 showed the total phenolics content of 630 mg gallic acid equivalents/100 g fresh fruit of S. senegalensis. This value obtained was higher than that reported by Lamien-Meda et al. (2008). This difference could be attributed probably to environmental conditions, the use of different solvents, and extraction methods (Lizárraga-Velázquez et al., 2018).
Vitamin C is an essential nutrient in organic systems because it has antioxidant properties. In S. senegalensis fruits, vitamin C is one of the main vitamins found with concentrations that are related to fruit maturity. Vitamin C content of the studied S. senegalensis fruits was 1511 µg/100 g fresh weight for whole fruit (Table 2 and Figures 5 and 6). The antioxidant activity of fruits of S. senegalensis was evaluated using the established assay that uses the DPPH radical (DPPH•) as a chromogen. The hydrophilic and lipophilic antioxidant activities of S. senegalensis fruit extracts were obtained with this assay. Table 2 shows the hydrophilic and lipophilic antioxidant activities in the fruits of S. senegalensis. Lipophilic antioxidant activity exhibited the highest scavenging ability (340 ± 6 mg TE/100 g), which was about 5-fold higher than hydrophilic antioxidant activity (75 ± 0.4 mg TE/100 g). The presence of carotenoids in the fruits of S. senegalensis might explain the high lipophilic antioxidant activity. Carotenoids are bioactive compounds that react quickly with free radicals to produce radical adducts with a resonance stabilized carbon-centered radical due to their conjugated double bonds and the presence of ring structures at the end of the polyenes (Lizárraga-Velázquez et al., 2018). Therefore, we think that enhanced scavenging ability of lipophilic antioxidant activity is likely due to the presence of a mix carotenoids, to their high reactivity with free radicals, and the possible synergy among carotenoids.
Besides, S. senegalensis fruits also exhibited significantly phenolic and vitamin C contents (Table 2), which are known as the two major natural hydrophilic antioxidants. Hydrophilic antioxidant activity of S. senegalensis fruits presented relatively DPPH radical scavenging ability. This effect is attributed to the mix of phenolic compounds and vitamin C identified in this study. Total phenols are considered to be efficient hydrogen donors that are capable of neutralizing free radicals by forming resonance stabilized phenoxy radicals (Siangu et al., 2019, Dudonné et al., 2009).
Lipophilic and hydrophilic antioxidants contribute to the total antioxidant activity of S. senegalensis fruits. It was reported that phenolic compounds, vitamin C, and carotenoids contribute to antioxidant activity by inhibiting the oxidation of free radicals through an electron or hydrogen atom transfer mechanism (Vila et al., 2018; Cano et al., 2017; Mario et al., 2004). Several epidemiological studies showed a positive relationship between antioxidant consumption and good health (Hartz et al., 2018; Al-Yafeai et al., 2018; Thaipong et al., 2005). The high level of the plasma vitamin C reduces the risk of cataracts (Thaipong et al., 2005). Diets with a high level of phenolic compounds like flavonoids have been associated with lower rates of mortality from coronary heart disease (Trappey et al., 2005). Carotenoids (biofunctional compounds) help to promote human health, for example, decreasing the risk of cancer, cardiovascular diseases, Parkinson, or Alzheimer (Portarena et al., 2019; García et al., 2018; Hartz et al., 2018). Thus, regular consumption of senegalensis fruits could be beneficial to human health, such as reducing the risk of cardiovascular affections and cancers.
CONCLUSION
S. senegalensis fruits contain an interesting carotenoid profile in its fruits with the presence of xanthophylls and hydrocarbon carotenoids. β-carotene is the most abundant carotenoid, followed by antheraxanthin. In total, three xanthophylls (antheraxanthin, lutein, and β-cryptoxanthin) and two carotenes (β-carotene and phytoene) were identified. The fruits also contain a reasonable concentration of vitamin C and phenolic compounds that provide health benefits. S. senegalensis fruits extract contains a higher content of phytochemicals like carotenoids, vitamin C, and phenolic compounds. They are, therefore, of high value for the development of nutraceutical and functional foods.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors appreciate Prof. Pierre DUEZ of “Université de Mons” for technical assistance on mass spectrometry.
REFERENCES
|
Abid AL, Shaiq AG, Rayees AAr, Hilal AB, Tauseef AB, Imtiyaz AW (2013). Free radicals and antioxidants: Myths, Facts, and mysteries. African Journal of Pure and Applied Chemistry 7(3):91-113. |
|
|
Al-Yafeai A, Malarski A, Böhm V (2018). Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chemistry 242:435-442. |
|
|
Cano MP, Gómez-Maqueo A, García-Cayuela T, Welti-Chanes J (2017). Characterization of carotenoid profile of Spanish Sanguinos and Verdal prickly pear (Opuntia ficus-indica, spp.) tissues. Food Chemistry 237:612-622. |
|
|
de Rosso VV, Mercadante AZ (2007). Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. Journal of Agricultural and Food Chemistry 55:5062-5072. |
|
|
Dudonné S, Vitrac X, Courtiere P, Woillez M, Mérillon JM (2009). Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry 57(5):1768-1774. |
|
|
Etzbach Lara, Pfeiffer A, Weber F, Schieber A (2018). Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by 〖HPLC-DADAPCI-MS〗^n. Food Chemistry 245:508-517. |
|
|
García MJ, Giuffrida D, Dugo P, Mondello Li, Osorio C (2018). Development and characterization of carotenoid-rich microencapsulate from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technology, 339:702-709. |
|
|
Hartz P, Milhim M, Trenkamp S, Bernhardt R, Hannemann F, (2018). Characterization and engineering of a carotenoid biosynthesis operon from Bacillus megaterium. Metabolic Engineering 49:47-58. |
|
|
Kini F, Saba A, Ouédraogo S, Tingueri B, Sanou G, Guissou IP (2008). Nutritional and therapeutic potential of some "wild" fruit species in Burkina Faso. Pharmacopée et Médecine Traditionnelle Africaines, 15:32-35. |
|
|
Lamien-Meda A, Lamien CE, Compaoré MM, Meda RN, Kiendrebeogo M, Zeba B, Millogo JF, Nacoulma OG (2008). Polyphenol Content and Antioxidant Activity of Fourteen Wild Edible Fruits from Burkina Faso. Molecules 13(3):581-594. |
|
|
Lizárraga-Velázquez CE, Hernández C, González- Aguilar GA, Heredia JB (2018). Effect of hydrophilic and lipophilic antioxidants from mango peel (Mangifera indica L. cv. Ataulfo) on lipid peroxidation in fish oil. CyTA-Journal of Food 16(1):1095-1101, |
|
|
Mario CF, Carmelo D, Corrada G (2004). Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. Journal of Organic Chemistry 69(7):2309-2314. |
|
|
Meléndez-Martínez AJ, Britton G, Vicario IM, Heredia FJ (2005). Identification of isolutein (lutein epoxide) as cis-antheraxanthin in orange juice. Journal of Agricultural and Food Chemistry 53(24):9369-9373. |
|
|
Miliauskas G, Venskutonis PR, Van Beek TA (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry 85(2):231-237. |
|
|
Ouédraogo JCW, Koala M, Ouédraogo N, Kini FB, Pascal G ,Yvonne LB (2017). Total Phenolics and Total Flavonoid Contents, Antioxidant Activity and Flavonoids Identification by High-Performance Liquid Chromatography-Tandem Mass Spectrometry of Odontonema strictum (Acanthaceae) Leaves. Asian Journal of Plant Science and Research 7(5):54-63. |
|
|
Papaioannou EH, Stoforos NG, Liakopoulou-Kyriakides M (2011). Substrate contribution on free radical scavenging capacity of carotenoid extracts produced from Blakeslea trispora cultures. World Journal of Microbiology and Biotechnology 27(4):851-858. |
|
|
Portarena S, Anselmi C, Zadra C, Farinelli D, Famiani F, Baldacchini C, Brugnoli E (2019). Cultivar discrimination, fatty acid profile and carotenoid characterization of monovarietal olive oils by Raman spectroscopy at a single glance. Food Control 96:137-145. |
|
|
Rivera SM, Christou P, Canela-Garayoa R (2013). Identification of carotenoids using mass spectrometry. Mass Spectrometry Reviews 33(5):353-372. |
|
|
Siangu BN, Sauda S, John MK, Njue WM (2019). Antioxidant activity, total phenolic and flavonoid content of selected Kenyan medicinal plants, sea algae and medicinal wild mushrooms. African Journal of Pure and Applied Chemistry 13(3):43-48. |
|
|
Tchuenguem FFN, Kingha TBM, Brückner D (2014). Flowering insect diversity and its impact on fruit and seed yields of Arachis hypogaea L. (Fabaceae) in Dang (Ngaoundéré-Cameroun). International Journal of Biological and Chemical Sciences 8(3):983-997. |
|
|
Thaipong K, Boonprakob U, Cisneros-Zevallos L, Byrne HD (2005). Hydrophilic and lipophilic antioxidant activities of guava fruits. The Southeast Asian Journal of Tropical Medicine and Public Health 36:254-257. |
|
|
Trappey II Alfred, Bawadi AH, Bansode RR, Losso NJ (2005). Anthocyanin profile of mayhaw (Cretaegus opaca). Food Chemistry 91(4):665-671. |
|
|
Vallverdú-Queralt A, Martínez-Huélamo M, Arranz-Martinez S, Miralles E, Lamuela-Raventós RM (2012). Differences in the carotenoid content of ketchups and gazpachos through HPLC/ESI(Li+)-MS/MS correlated with their antioxidant capacity (2012). Journal of the Science of Food and Agriculture 92(10):2043-2049. |
|
|
van Breemen RB, Dong L, Pajkovic ND (2012). Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. International Journal of Mass Spectrometry 312:163-172. |
|
|
Vila E, Hornero-Méndez D, Azziz G, Lareo C, Saravia V (2018). Carotenoids from heterotrophic bacteria isolated from Fildes Peninsula, King George Island. Antarctica. Biotechnology Reports 20: e00306. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0