Definition of artemisinin resistance
The working definition of partial artemisinin resistance was developed based on observations from routine therapeutic efficacy studies of ACTs, clinical trials of artesunate monotherapy, and pfK13 sequencing. Suspected partial artemisinin resistance is defined as: ≥ 5% of patients carrying pfK13 resistance-associated mutations; or ≥ 10% of patients with persistent parasitemia by microscopy on day 3 after treatment with ACT or artesunate monotherapy; or ≥ 10% of patients with a parasite clearance half-life of ≥ 5 h after treatment with ACT or artesunate monotherapy (WHO, 2014).
Confirmed partial artemisinin resistance is defined as: ≥ 5% of patients carrying pfK13 resistance-associated mutations, all of whom have been found, after treatment with ACT or artesunate monotherapy, to have either persistent parasitemia by microscopy on day 3, or a parasite clearance half-life of ≥ 5 h (www.who.int/malaria/publications/atoz/status-rep-artemisinin-resistance-sept2015.pdf, accessed on December 31, 2017).
In vivo ACT efficacy evaluation
The in vivo method is based on the administration of ACTs following the standard World Health Organization (WHO) Protocols for the Assessment of Therapeutic Efficacy of Antimalarial Drugs for Uncomplicated P. falciparum malaria (WHO, 2003). The 28-day follow-up protocol (Chanda et al., 2006; Denis et al., 2002; Marquino et al., 2003) or 42-day follow-up protocol (Leang et al., 2015; Mårtensson et al., 2005; Mayxay et al., 2004; Valecha et al., 2010; Yeka et al., 2008) were usually used. Few studies used 63-day follow-up protocols for efficacy monitoring of ACTs containing a long half-life partner drug (Sodiomon et al., 2016; Valecha et al., 2010). Patient follow-ups were scheduled for days 1, 2, 3, 7, 14, 21 and 28 plus any other day that the child was taken to the clinic on an unscheduled day. On each of these days clinical and parasitological assessments were performed.
In this review, articles that used the same follow-up protocol with the same investigational drug were represented by only one publication per group, for example all articles with 28 days or 42-day of follow-up comparing artemether-lumefantrine to artesunate-amodiaquine were represented by a single article. We included articles about artemether-lumefantrine (AL) alone (Chanda et al., 2006), on AL vs. artesunate-amodiaquine (AS-AQ) alone (Brasseur et al., 2009), AL vs. Artesunate-sulfamethoxypyrazine (AS-SMP) (Sagara et al., 2006), AL vs. dihydroartemisinin-piperaquine (DHA-PQP) (Kamya et al., 2007), AL vs. Artesunate-Mefloquine (AS-MQ) (Sagara et al., 2008), AL vs Chlorproguanil-Dapsone (CPD) (Kaye et al., 2008), AQ vs Argemone mexicana (AM) (Graz et al., 2010), DHA-PQP vs AS-MQ (Valecha et al., 2010), AQ-SP vs AS-SP (Yeka et al., 2005).
Endpoints and classification of treatment responses for the in vivo methods
Three categories of therapeutic response, namely Early Treatment Failure (ETF), Late Treatment Failure (LTF) and Adequate Clinical and Parasitological Response (ACPR) were used. This is based on the WHO classification system (WHO, 2003). Classification of the treatment outcome was based on an adequate clinical and parasitological response on day 28, day 42 or day 63 depending on the protocol used.
Delayed parasite clearance time as marker of artemisinin-resistant P. falciparum
Early studies showed that artemisinin based combination therapy resistance were characterized by slow parasite clearance in vivo. This in vivo phenotype was not coupled with any in vitro resistance phenotype as per the standard in vitro susceptibility testing (Ashley et al., 2014; Dondorp et al., 2012; Takala-Harrison et al., 2014; Thriemer et al., 2014; Tun et al., 2016).
In vitro/ex-vivo methods for ACTs efficacy evaluation
The principle of this method is based on measuring the maturation inhibition of laboratory adapted parasites in culture, in the presence of increasing doses of antimalarial drugs. This group includes optical microtest, standard isotopic methods, fluorometric methods, ELISA methods and the Ring Stage Survival Assay (RSA). Optical microtest was the first in vitro method widely used (Price et al., 2010; Tanariya et al., 2000). Standard in vitro and ex-vivo isotopic methods were developed initially for conventional antimalarial drugs but these methods were used to monitor ACTs partner drugs efficacy (Lim et al., 2010; Mwai et al., 2009; Pascual et al., 2012; Pradines et al., 1998, 2011). Because of biosafety concerns with radioactive reagents, and the high cost of equipment and reagents, fluorometric and ELISA methods were developed to monitor ACTs partner drugs. The main fluorophore SYBR Green I-based methods were used in several studies (Cheruiyot et al., 2016; Lim et al., 2013; Quashie et al., 2013). Two enzyme-linked immunosorbent assay (ELISA)-based methods that use mono-clonal antibodies to Plasmodium spp. or plasmodial lactate dehydrogenase (pLDH) and histidine-rich protein II (HRPII) have begun to be widely used in recent years due to their ease of use (Bacon et al., 2007; Kaddouri et al., 2008; Noedl et al., 2005; Noedl et al., 2004; Noedl et al., 2002). Because of the short half-life of artemisinins, the in vitro/ex-vivo methods described above, which were all based on the inhibition of schizont maturation were not appropriate for artemisinin derivatives monitoring. New methods called Ring Stage-survival Assays were developed to overcome the stage specific activity of artemisinin and were shown to be correlated with parasite clearance time in vivo (Witkowski et al., 2010, 2013).
Molecular markers of artemisinin-resistant P. falciparum malaria
Early studies showed even before official ACTs implementation that SERCA-PfATPase6 could be the target for artemisinins and that the S769N PfATPase6 mutation was associated with artemisinin IC50 (Jambou et al., 2005; Mugittu et al., 2006). Several studies showed a statistically significant increase of pfmdr1 wild-type allele, N86 and pfcrt wild type allele K76 in recurrent parasite populations collected after treatment with ACTs (Duah et al., 2013; Lim et al., 2009; Lobo et al., 2014; Mwai et al., 2009; Sisowath et al., 2005, 2007, 2009; Witkowski et al., 2010). The best molecular marker for large-scale surveillance of artemisinin resistance in the Greater Mekong Sub-region was P. falciparum Kelch13 propeller gene polymorphisms (Ariey et al., 2014; Mbengue et al., 2015; Mita et al., 2016).
Data extraction
A systematic search for studies reporting on ACTs efficacy surveys was conducted using PubMed and WHO database. Abstracts from congresses were not included in this study. We searched PubMed with the terms “artemisinin”, “resistance” and “evaluation” and limited our search to in vivo efficacy, in vitro/ex-vivo assay and PK assay. We used no date or language restrictions.
This paper highlights efforts made in the past 20 years to monitor resistance to artemisinin and partner drugs and their metabolites, and to understand the mechanisms involved. The publication search identified 870 records. After removal of 803 records because of duplication, non-relevance, and non-availability of full text articles, sixty-seven articles were identified for inclusion (Figure 1). For each article, methods used and results were discussed. All methods used for P. falciparum susceptibility to ACTs were described in a chronological order of use.
28-day in vivo protocol to test ACTs efficacy
Studies realized in Zambia to assess therapeutic efficacy of AL showed that PCR corrected ACPR after 28 days of follow up was 100% (95% confidence interval 96.0 to 100%). There were no early treatment failures reported and all the analyzable LCF and LPF were due to re-infections. The gametocytes detected on Day 0 and Day 2 were reduced significantly by Day 7 with none being recorded on Days 21 and 28. No clinically detectable drug reactions were reported during the study period. MSP1 and MSP2 were genotyped in order to differentiate recrudescence from re-infections for parasites that appeared after Day 14 and the re-infection rate was similar between study sites (Chanda et al., 2006).
The Kaplan-Meier estimates of the crude cure efficacy rate was 94.6% (95% CI 93.0; 95.9) for all years combined from 2000 to 2005 after treatment with ASAQ in Senegal. There were no differences (p = 0.12) in cure rates between years by the log rank test for homogeneity over time (Brasseur et al., 2009). A non-randomized ASAQ efficacy study showed that on day 28, the PCR-uncorrected cure rate was 83%; this cure rate is lower than that reported in previous studies. After PCR adjustment, the overall treatment efficacy was 94.4%. The risk of treatment failure was not different between children aged ≤ 5 years and those aged >5 years, including adults (p = 0.6), and between patients living in urban and suburban areas (p = 0.4) (Ndounga et al., 2013).
DHA-PQP efficacy was assessed in Cambodia; there was a 96.9% cure rate at day 28. The cure rate among children < 14 years old was 98.6%, and the rate among adults was 92.3%. The data from this study suggested that DHA-PQP could prove to be suitable for use as combination antimalarial therapy but that pharmacokinetic studies and further efficacy evaluation with close monitoring of clinical outcomes should be carried out (Denis et al., 2002).
Day-28 protocol was used to compare efficacy of AL to ASAQ in Mozambique and showed that PCR- uncorrected cure rate was 89.6 (95% CI 86.0- 92.5) for AL, and 99.6 (95% CI 97.6-99.9) for ASAQ. There were 65.8% of new infections according to PCR, in AL group yielding a day-28 PCR-corrected cure rate of 96.0 (95% CI 93.4-97.8). In the ASAQ, only one recurring parasitemia was detected, which proved to be a recrudescence of the original infection according to PCR. Thus, day-28 PCR-corrected cure rate was identical to the uncorrected one (99.6 [95% CI 97.6-99.9]) (Nhama
et al., 2014).
28-day in vivo study conducted in Mali showed that crude cure rates were 79.6 and 67.2% for participants receiving AS-MQ or AL, respectively (P < 0.003). After PCR correction, the 28-day cure rates were 96 and 96.9% for those receiving AS-MQ or AL, respectively. This difference between AS-MQ and AL was no longer significant (P = 0.6). AS-MQ showed an additional benefit of preventing new infections compared with AL; the 28-day re-infection rates were 15.4 and 29.9% (P < 0.001). Differences in elimination half-life may explain the advantage of MQ over lumefantrine (LM) (3 to 6 days) in preventing reinfection (Sagara et al., 2008).
Efficacy and safety study comparing artesunate-pyronaridine (AS-Pyr) versus AL in Mali showed that day-28 crude ACPR was 92·7% (95% CI 91·0-94·3) with AS-Pyr and 80·4% (77·8-83·0) with AL (treatment difference 12·3%, 95% CI 9·1–15·4; p<0·0001). ACPR was similar between AS-Pyr and AL treatments and greater than 95% at day 28. The difference in crude ACPR between two treatment groups resulted from a higher re-infection rate in the AL group than in the AS-Pyr group and might be explained by the longer elimination half-life of Pyr (14 days) than that of LM (Sagara et al., 2015).
Study comparing AS-AQ versus artesunate-sulfadoxine-pyrimethamine (AS-SP) in Sudan showed that the day 28-cure rate was 95.3% for AS+AQ and 98.2% for AS+SP. Both of the artemisinin-based combination therapies tested here were found to be highly efficacious in the treatment of uncomplicated P. falciparum malaria. AS+SP appears to be a better treatment option on the basis of non-PCR corrected responses. This potent action could be explained by the longer elimination half-life of SP than AQ (van den Broek et al., 2005).
Study comparing efficacy of DHA-PQP versus AL in Uganda showed that the risk of recurrent falciparum parasitemia unadjusted by genotyping was significantly lower for participants treated with DP than for those treated with AL after 28 day of follow-up (11% versus 29%; risk difference [RD] 1⁄4 18%, 95% confidence interval [CI] 11-26%) and after 42 day of follow up (43% versus 53%; RD 1⁄4 9.6%, 95% CI 0-19%). Similar trends were seen when results were adjusted by genotyping. The risk of recurrent parasitemia due to possible recrudescence was significantly lower for participants treated with DP than for those treated with AL after 28 days of follow-up (1.9% versus 8.9%; RD 1⁄4 7.0%, 95% CI 2.5-12%) (Kamya et al., 2007).
The day-28 PCR-uncorrected cure rates in the per-protocol analysis also showed no significant difference in efficacy (p = 0.077): 91% (79-97%), 86% (74-94%), 93% (84-98%) and 98% (91-100%) for the AL, AS-SMP, AS-AQ and SP-AQ groups, respectively. This comparative evaluation of four treatments shows satisfactory, comparable efficacy of AL, AS-SMP, AS-AQ and SP-AQ in Central African Republic (Djallé et al., 2014).
The results from these studies that were assessing efficacy of different ACTs in open-label studies or in randomized controlled or not studies showed that these combinations were highly efficacious after PCR correction and could still be used. Recurrence rates were higher in AL and AS-SMP group than all of the rest of ACTs.
42-day in vivo protocol to test ACTs efficacy
A study that was monitoring and comparing efficacy of AL versus AQ-MQ versus CQ-SP in Lao People's Democratic Republic showed that the cure rates, excluding patients who were lost to follow-up or experienced reinfections, were 100, 97 and 92% for subjects receiving AS-MQ, AL, and CQ-SP, respectively (Mayxay et al., 2004). By use of intention-to-treat analysis, the 42-day cure rates, adjusted for re-infections, were 100, 97 and 93% for the groups receiving AS-MQ, AL, and CQ-SP, respectively. This study demonstrates that oral AS-MQ and AL were highly effective for the treatment of uncomplicated falciparum malaria at the time (Mayxay et al., 2004).
Monitoring of therapeutic efficacy and tolerability of AL versus AS-MQ in Senegal showed that the cure rate was 98.5% in the AS-MQ group versus 98.2% in the AL group at day 42 (p = 1). However, dizziness was more frequent in the AS-MQ group than AL group (Tine et al., 2012).
Study conducted in Lao People’s Democratic Republic showed after 42 days, cure rates were 93.6% (95% CI 1⁄4 82.5-98.7%) for AL and 100% (95% CI 1⁄4 93.3-100.0%) for AS-MQ. The results show the excellent efficacy and tolerability of both AL and AS-MQ in Northern Laos (Stohrer et al., 2004). In bordering Thailand, similar cure rates were reported for the AS-MQ combination after 42 days (89 and 98%) (Price et al., 1997; Suputtamongkol et al., 2003), as well as for 28 days follow-up (cure-rates 98-100%) (van Vugt et al., 1998).
A randomized non-inferiority study conducted in Democratic Republic of Congo comparing AL to AS-AQ at 42-day follow-up showed that PCR corrected cure rates were 98.3% (95%CI, 94.1-99.8) in the AS-AQ group and 99.1% (95%CI, 94.9-99.9) in the AL group (difference -0.7%, one sided 95% CI -3.1) (Espié et al., 2012). Kaplan-Meier PCR-adjusted cure rates were similar. Both treatment regimens were generally well tolerated. High PCR adjusted cure rates of 98.3 to 98.4% (depending on the analysis population) were seen in patients assigned to AS-AQ, compared with rates of 99.1 to 99.2% in patients assigned to AL. These results are comparable to those from previous studies in other sub-Saharan countries (Zongo et al., 2007).
A Randomized Trial using 42-day follow-up to Guide Policy in Uganda showed the risk of treatment failure unadjusted by genotyping was significantly lower for participants treated with DHA-PQP than for those treated with AL after 28 (3.8% vs. 17.3%; Risk Difference (RD) = 13.6%, 95% CI 7.7-19.4%) and 42 days of follow up (12.2% vs. 33.2%; RD = 20.9%, 95% CI 13.0–28.8%). Recurrent parasitemia were seen 28 days or more after therapy in the AL group and 35 days or more after treatment with the DP. After PCR correction, there was no statistically significant difference in the risk of treatment failure after 28 days of follow up (0.9% vs. 3.2%; RD = 2.2%, 95% CI 20.6-5.1%); and although the risk of failure tended to be lower in those treated with DHA-PQP than for AL after 42 days of follow up, the difference was not statistically significant (Yeka et al., 2008).
Efficacy of AS-AQ versus AS-SP combinations at 42-day follow-up in Sudan showed PCR corrected cure rate was 95.3% for AS-AQ and 98.2% of AS-SP. There was no difference between the age, temperature, parasitemia, hemoglobin on admission of the two group (van den Broek et al., 2005).
63-day in vivo protocol to test ACTs efficacy
Monitoring of therapeutic efficacy and tolerability of AL versus AS-MQ in Thailand showed that the PCR corrected cure rate at day 63 was 98.2% in AS-MQ group compared to 97.7% in AL group (p = 0.32). The two treatments were well tolerated with a similar safety profile. The risk of dizziness in patients treated with AS-MQ was dose-related. Indeed, an increase risk was observed after each treatment course (Odd ratio [OR] at day 1 = 2.3, OR at day 2 = 12, OR at day 3 = 10) (van Vugt et al., 1998).
A multi-centric randomized trial using 63-day follow-up showed the PCR-corrected ACPR rate was 90.9% in the AS-MQ group and 89.7% in the AL group (treatment difference 1·23%, 95% CI -2·84% to 5·29%). In both the per-protocol and intention-to-treat (ITT) analyses, the mean time to parasite clearance did not differ between treatment groups (Sodiomon et al., 2016).
Randomized open label trial conducted in Asia showed the PCR-corrected cure rate at day 63 confirmed that DHA-PQP was non-inferior to AS-MQ. For the ITT population, PCR-corrected cure rates were 87.9% for DHA-PQP and 86.6% for AS-MQ. As expected, better absolute but comparatively similar results were obtained for the per protocol population with PCR-corrected cure rates of 98.7% for DHA-PQP and 97.0% for AS-MQ (Valecha et al., 2010). Comparison of the data of this study to data from other country found no differences between the primary outcome measures, further confirming that DHA-PQP was similarly active against the P. falciparum found in India, Laos and Thailand, relative to AS-MQ. The PCR-corrected cure rates observed for DHA-PQP in this study were in line with the day-63 PCR-corrected cure rates noted in a previous study conducted in Thailand (Ashley et al., 2004).
Studies assessing delayed parasite clearance time as marker of artemisinin-resistant P. falciparum and ring stage survival assay
To characterize treatment responses to artemisinin based combination therapies and provide evidence that P. falciparum are being resistant to artemisinin and derivatives, WHO established a multipartite task force. The first trials reported here were conducted as part of this initiative to understand more in resistance phenotype (Dondorp et al., 2009). The first study conducted in Thailand in two locations (Pailin and Wang Pha). Patients were treated with AS monotherapy or AS-MQ. The standard 48-hour 3H-hypoxanthine uptake inhibition method was used to monitor in vitro sensitivity of freshly collected parasite. Copy numbers of the P. falciparum multidrug-resistance gene pfmdr1 were assessed. Gene encoding sarco-endoplasmic reticulum calcium ATPase6 (PfSERCA) was amplified by PCR. The main results showed overall median parasite clearance times were 84 h in Pailin and 48 h in Wang Pha (P<0.001). Witkowski and collaborators used artemisinin resistant P. falciparum strain that can survive a drug dose up to 7,000-fold the initial IC50 (Witkowski et al., 2010). The main results of this study indicated that drug tolerance is mediated by cell cycle arrest at the ring stage, as they were the only viable forms observed during the 48-h ART pressure period (Witkowski et al., 2010).
Novel phenotypic assays for the detection of artemisinin-resistant P. falciparum malaria in Cambodia were conducted. To perform this study, samples from patients with long and short parasite clearance half-lives from a study done in Pursat, Cambodia were tested. Novel in-vitro survival assays were used to explore the stage-dependent susceptibility of slow-clearing and fast-clearing parasites to DHA. Ex-vivo survival rates from 0 to 3 h ring-stage parasites significantly correlated with in vivo parasite clearance half-lives. The in vitro RSA of 0 to 3 h ring-stage (RSA0-3h) parasites provided a good platform for the molecular characterization of artemisinin resistance (Witkowski et al., 2013).
The reduced Artemisinin susceptibility of P. falciparum Ring Stages in Western Cambodia was assessed in two study locations (Pailin and Ratanakiri) (Witkowski et al., 2013). The RSA identified marked differences between ring stages from Pailin and Ratanakiri after exposure to a 6-h pulse of 700 nM DHA. The median percentage of viable parasites at 72 h was significantly higher (P < 0.0002) for parasites from Pailin than for parasites from Ratanakiri or laboratory reference clones (Witkowski et al., 2013).
The 42-day in vivo and in vitro efficacy study of was conducted in Quang Nam Province in Central Vietnam to evaluate delayed parasite clearance after treatment with DHA-PQP in P. falciparum Malaria patients (Thriemer et al., 2014). The median PCT was 61.7 h and the median parasite clearance rate had a slope half-life of 6.2 h. The IC50 of isolates with delayed PCT (>72 h) was significantly higher than those with normal PCT for DHA and PQP. Delayed parasite clearance (PCT, >72 h) was significantly higher among day 0 samples carrying the mutant allele at codon 543 of Kelch 13 propeller domain (47.8%) than those carrying the wild-type allele (1.8%; P < 0.048) (Thriemer et al., 2014).
An open-label, randomized trial was conducted at 15 sites in 10 countries from the Democratic Republic of Congo to Thailand-Cambodia border. The median parasite clearance half-lives ranged from 1.9 h in the Democratic Republic of Congo to 7.0 h at the Thailand-Cambodia border. Slowly clearing infections (parasite clearance half-life >5 h) strongly associated with single point mutations in the “propeller” region of the P. falciparum kelch protein gene (Ashley et al., 2014). The study realized in Myanmar indicated median parasite half-life was prolonged and clearance half-life was also greater than 5 h in 21% of enrolled patients. There were a significant association between delayed parasite clearance and polymorphisms in propeller kelch 13 region of P. falciparum (Tun et al., 2016).
Studies assessing in vitro and ex-vivo efficacy methods of ACTs and partner component
In vitro activity of dihydroartemisinin against clinical isolates of P. falciparum in Cameroun was assessed. Classical isotopic (48-h) method was used for in vitro DHA monitoring (Desjardins et al., 1979). DHA was the most potent drug (geometric mean IC50 = 1.11 nM, 95% confidence interval = 0.96-1.28 nM, range = 0.25-4.56 nM, n = 65) among the panel of drug compounds tested (Ringwald et al., 1999).
In vitro activities of Piperaquine, Lumefantrine, and Dihydroartemisinin in Kenyan P. falciparum isolates. The sensitivities of the Kenyan isolates to drugs were assessed in vitro by the use of a classical isotopic (48-h) test (Desjardins et al., 1979). The median IC50s of CQ, PQP, LM, and DHA were assessed. As expected, DHA was the most active drug, followed by PQ, CQ, and LM. Interestingly, LM IC50s were > 100 nM for about 20% of isolates (Mwai et al., 2009).
A standard in vitro isotopic method was used to monitor imported P. falciparum isolate from Asia to France. The result of this finding showed decreased in vitro susceptibility to DHA associated with decreased susceptibility or resistance to quinine (Q), MQ, LM, Pyr and PQP in a patient returning from South-East Asia after trekking along the Mekong from the south of Laos to the north of Thailand. Surprising, these isolates were susceptible in vitro to chloroquine and monodesethyl amodiaquine. It was also susceptible to doxycycline (Pradines et al., 2011).
The sensitivities of the Cambodian isolates to drugs were assessed in vitro by the use of a classical isotopic (48-h) test. The overall geometric means of IC50s (GMIC50s) of AS and MQ tested were significantly higher in the western than in the eastern provinces (P < 0.001) (Lim et al., 2010). This difference between western and eastern province could be explain by the higher resistance artemisinin rate in west compare to east of Cambodia.
Ex-vivo isotopic study of the ACT new components Pyr and PQP in comparison with conventional ACT drugs against isolates of P. falciparum was conducted in France. P. falciparum isolates were collected from hospitalized patients with imported malaria from a malaria-endemic country. A significant positive correlation was shown between responses to PQP and Pyr responses and between PQP and MDAQ (Pascual et al., 2012). Ex-vivo isotopic study of P. falciparum clinical isolates to artemisinin derivatives and partner drugs in Niger (Issaka et al., 2013) showed that GMIC50s were below the resistance threshold.
The SYBR Green-I in vitro and ex-vivo assays were run without adding tritiated hypoxanthine to the culture suspension by using the classical isotopic method. The geometric mean IC50 values from SYBR Green 1-based ex-vivo study of susceptibility of Ghanaian P. falciparum clinical isolates to a panel of anti-malarial drugs were below the resistance threshold for atovaquone (ATV), AS, DHA, artemether (ATM), LM, AQ, MQ, PQP and CQ, respectively (Quashie et al., 2013). SYBR Green 1-based ex-vivo study of susceptibility of laboratory culture adapted parasite line D6 and clinical isolates from the Worldwide Antimalarial Resistance Network with various initial parasitemia levels were all sensitive to ATV, ART, MQ and CQ (Cheruiyot et al., 2016).
The ex-vivo ELISA/pLDH based assay was used to monitor susceptibility of P. falciparum of field isolates from Mali and Senegal. These studies were conducted in 2007 and 2011 respectively in Mali and Senegal (Fall et al., 2011; Kaddouri et al., 2008). The following artemisinin derivatives and partner drugs were used: MDAQ, LM and DHA. The pLDH ELISA assays were run without adding tritiated hypoxanthine to the culture suspension. The GMIC50s of LM and DHA were higher in Senegal compared to Mali. This difference could be explained by the period of study, Malian study was conducted in the same year of ACTs implementation. The difference between MDAQ geometric means could be explained because of fact that during the Malian study, AQ was still using in monotherapy that could select resistant parasite compared to Senegalese study that was conducted in period AQ was used in combination.
HRPII was performed to compare generic antigen capture HRP2 ELISA and commercial kit HRP2 ELISA. There was no difference in IC50 means between two techniques (Noedl et al., 2005). In vitro and ex-vivo ELISA based HRPII versus SYBR Green I were used to monitor efficacy of reference strains 3D7, D6, W2 and fresh clinical isolates of P. falciparum collected from imported cases of malaria in Lyon. The IC50 obtained by both methods were similar for reference strains and clinical isolates (Bacon et al., 2007).
Studies assessing genetic and molecular markers for ACTs resistant P. falciparum
Role of pfmdr1, pfmdr2 and pfcrt, ferredoxin, apicoplast ribosomal protein S10 and plasmepsin 2 in artemisinin-based combination therapies susceptibility iIn early 2005, studies in Tanzania showed a statistically significant increase of pfmdr1 wild-type allele N86 in recurrent parasite population after treatment with artemether-lumefantrine (Sisowath et al., 2005). In the same Tanzanian region, a yearly increase of pfmdr1 N86, 184F, D1246 and pfcrt K76 between 2006 and 2011, from 14 to 61% was reported. The temporal selection of molecular markers associated with artemether-lumefantrine tolerance/resistance may represent early warning sign of impaired future drug efficacy (Malmberg et al., 2013).
Further investigations in Zanzibar showed a strong correlation between two allele of pfmdr1 (184F, 86N) and reinfection parasites, suggesting that pfmdr1 can be used to monitor artemisinin tolerant parasites (Sisowath et al., 2007).
AL and AS-AQ exerted opposing selective effects on single-nucleotide polymorphisms in pfcrt and pfmdr1. Monitoring selection and responding to emerging signs of drug resistance are critical tools for preserving efficacy of artemisinin combination therapies; determination of the prevalence of at least pfcrt K76T and pfmdr1 N86Y should now be routine (Venkatesan et al., 2014). Studies conducted in Mali showed that AS + AQ selected pfcrt and pfmdr1 point mutations associated with CQ and AQ resistance (P < 0.001) (Djimdé et al., 2008).
A large multicenter genome-wide association study showed that non-synonymous polymorphisms in fd (ferredoxin), arps10 (apicoplast ribosomal protein S10), pfmdr2 (P. falciparum multidrug resistance protein 2) and pfcrt (P. falciparum chloroquine resistance transporter) also showed strong associations with artemisinin resistance. Polymorphisms of the fd, arps10, pfmdr2 and pfcrt could be used as markers of a genetic background on which kelch13 mutations are particularly likely to arise and that they correlate with the contemporary geographical boundaries and population frequencies of artemisinin resistance (Miotto et al., 2015). A genotype-phenotype association study showed that multi copy of plasmepsin 2 could constitute a surrogate molecular marker to track piperaquine resistance (Witkowski et al., 2016).
In vitro studies were conducted in Kenya to monitor the selection of P. falciparum pfcrt and pfmdr1 variants by artemisinin. Parasite strains D6 and W2 were exposed to artemisinin and D6 was exposed to lumefantrine only to generate resistance parasites in vitro. The change in parasite susceptibility was associated with pfmdr-185K mutation, a mutation never reported before. The pfcrt-CVMNK genotype (pfcrt codons 72-76) was retained and notably, the study did not detect any polymorphisms reported to reduce P. falciparum susceptibility in vivo in the coding sequences of the Pfk13 gene (Njokah et al., 2016).
Role of P. falciparum Kelch13 propeller gene polymorphism
Whole-genome sequencing of an artemisinin-resistant parasite line from Africa and clinical parasite isolates from Cambodia (Ariey et al., 2014) showed an association between mutations in the PF3D7_1343700 kelch propeller domain ('PfK13-propeller') with P. falciparum resistance to artemisinin in vitro and in vivo. The prevalence of mutant allele was higher and correlated with in vitro parasite survival rates and in vitro parasite clearance rates. PfK13-propeller polymorphism constitutes a useful molecular marker for large-scale surveillance efforts to contain artemisinin resistance in South- East Asia region and prevent its global spread (Ariey et al., 2014). In the study realized in Asia, samples collected from Bangladesh, Cambodia, Laos, Myanmar, and Vietnam were genotyped by Whole genome Sequence Technology and generated 33,716 genome-wide single-nucleotide polymorphisms (SNPs). The presence of non-reference PfK13 alleles was associated with prolonged parasite clearance half-life (P = 1.97 × 10-12). Parasites with a mutation in any of the PfK13 propeller domains displayed longer parasite clearance half-lives than parasites with wild-type alleles (Takala-Harrison et al., 2015).
A study realized in Mali showed that PFK13-propeller mutations previously described and associated with delayed parasite clearance in Cambodia were not present, some new PfK13-propeller mutations were identified in both recent samples and samples from 7 years before ACTs implementation in Mali (Ouattara et al., 2015). Parasite clearance time was comparable between infections with non-synonymous PfK13-propeller mutations and infections with the reference allele.
A study assessed PfK13-Propeller polymorphisms in P. falciparum parasites from Sub-Saharan Africa and found none of the PfK13-propeller mutations previously reported in Southeast Asia in a multicentric dataset, but 22 unique mutations were detected, of which 7 were non-synonymous. Allele frequencies ranged between 1 and 3%. Three mutations were observed in >1 country and the A578S was present in parasites from 5 countries (Kamau et al., 2015).
Studies assessing pharmacokinetics (PK) and clinical efficacy of ACTs
A pharmacokinetics study conducted during AL efficacy monitoring was performed by Novartis Pharma group, the variability in bioavailability of artemether and dihydroartemisinin was large both between doses and between patients, but was less pronounced for lumefantrine. Compared with the first dose, lumefantrine bioavailability was estimated to increase three-fold by the third and fourth doses (Ezzeyt et al., 1998). Higher artemether or dihydroartemisinin area under the plasma concentration time curve (AUC) was found to decrease parasite clearance time. Higher lumefantrine AUC was found to significantly increase the chance of cure and could be used as a marker (Ezzet et al., 1998).
A study investigated the relation between population pharmacokinetics and clinical response for artemether-lumefantrine in pregnant and non-pregnant women with uncomplicated malaria in Tanzania. The finding of this study showed that pregnant status and diarrhea were significantly associated with lumefantrine PK; the bioavailability of lumefantrine was lower and higher for its metabolites. The high day 7 concentration was significantly associated with adequate clinical and parasitological response (ACPR). The high level of treatment failure in pregnant women group may be explained by the lower bioavailability (Mosha et al., 2014).
Efficacy and correlation with day 7 plasma Piperaquine concentrations in African children treated with DHA-PQP in Bobo-Dioulasso showed that children with successful treatment outcomes had significantly higher median plasma concentrations of PQP compared to those with recurrent malaria within 42 days after therapy, considering either capillary samples (68 ng/ml [50–85] compared to 48 ng/ml [36-55], p < 0.001) or venous samples (42 ng/ml [29-59] compared to 25 ng/ml [19-44], p < 0.001). This study showed that recurrent malaria was mainly due to new infections after treatment and was correlated with low day 7 PQP concentration in the youngest patients (Zongo et al., 2014).
World Wide Antimalarial Drug Resistance Network has reviewed over 30 studies on pharmacokinetics of lumefantrine. The result of this review showed that recrudescence was associated with low day 7 lumefantrine concentrations. For all other populations studied except in very young children under 3 years, day 7 concentrations ≥200 ng/ml were associated with > 98% cure rates (if parasitemia <135,000/µL) in areas of emerging artemisinin resistance and very low transmission intensity (World Wide Antimalarial Resistance Network (WWARN) Lumefantrine PK/PD Study Group1, 2015). The day 7-lumefantrine concentrations could be use as PK marker in addition to molecular marker to predict diminution sensitivity of P. falciparum to artemether-lumefantrine combination.
The weakness of in vivo 28, 42 and 63-day follow-up protocol are explained by the fact that in vivo protocol takes at least 28 days to be able to conclude to the effectiveness of ACTs. Genotyping recurrent parasite has a potential limitation (Table 1). The responses of in vivo studies are always adjusted by PCR by distinguishing recrudescence to new infection. The usual PCR techniques used for this purpose are insufficiently sensitive to pick up minority populations of parasites present at Day 0. It is therefore possible that “new” parasites identified during follow-up actually represent recrudescence of parasites from a resistant minority population that was present from the start rather than true reinfections.

Alleles with limited polymorphism may lead to misclassification of reinfections as recrudescence. Merozoite Surface Protein 2 (MSP2) alone was often used by other authors to make molecular corrections (yeka et al., 2005). Some authors used only polymorphism markers such as MSP1 and MSP2 to distingue recrudescence to new infection (Moses et al., 2007, Van den Broeak et al., 2005, Chanda et al., 2006); MSP1, MSP2 and GLURP were used in previous study to perform molecular correction (Nhema et al., 2014). Sometimes, microsatellites such as Ca1, Ta87 or Ta99 have been combined with polymorphism markers (Sagara et al., 2008). Most of the authors did not use the same polymorphism markers to discriminate recrudescence from re-infection. This poses a problem of standardization. MSP2 was the most widely used marker to perform molecular correction. It is often used alone or in association with the MSP1. For better molecular correction it may be preferable to use microsatellites (Ca1, Ta87, Ta99, etc.) and antigen polymorphism markers (MSP2, MSP1, GLURP, etc.) as the antigen markers are under immune selection pressure while microsatellites are non-coding neutral markers.
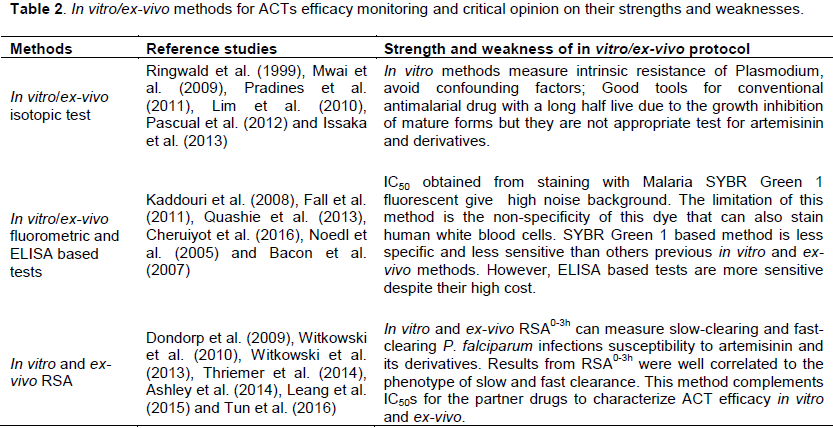
In vitro/ex-vivo optical microtest, in vitro/ex-vivo isotopic test, in vitro/ex-vivo fluorometric and ELISA based tests are good tools for conventional antimalarial drug witch have a long half live due to the growth inhibition of mature forms but they are not appropriate test for artemisinin and derivatives (Table 2). IC50 obtained from staining with Malaria SYBR Green 1 fluorophore give high noise background. The limitation of this method is the non-specificity of this dye that can also stain human white blood cells. SYBR Green 1 based method is less specific and less sensitive than others previous in vitro and ex-vivo methods. In vitro and ex-vivo RSA0-3h can measure slow-clearing and fast-clearing P. falciparum infections susceptibility to artemisinin and its derivatives. Results from RSA0-3h were well correlated to the phenotype of slow and fast clearance. This method gives more details on ACT efficacy monitoring in vitro and ex-vivo.
PfK13 propeller gene is highly polymorphic and sometimes associated with others genetic background such as fd, arps10, pfmdr1, pfmdr2 and pfcrt polymorphisms. Therefore, a molecular toolkit combining plasmepsin 2 with pfK13, pfmdr1 and pfcrt monitoring should provide more accurate information for ACT and resistance containment efforts.