A systematic survey of termite species in Northeastern Puducherry which is part of peninsular India, was carried out. As there is no pre-existing report on the richness or diversity of termifauna in this region, the present work aims to fill this major knowledge gap. The findings are discussed in the context of the quantitative studies on termifauna carried out across the world, as also in terms of the defining traits of the species identified in the survey vis a vis their possible use in biodegrading ligninous biowaste. The latter aspect is particularly relevant to the controlled use of termites by the process named ‘termigradation’, which denotes termite-based biodegradation of waste.
Among the nature’s scavengers and earth-movers, termites play the most dominant role alongside ants and earthworms. But while the other two are very efficient in assisting in decomposition of non-ligninous organic matter, termites are capable of processing lignin as well. Abbasi and coworkers (Abbasi et al., 2007; Ganesh, 2008; Kaur, 2014; Anantharaju, 2016) have developed processes with which termites could be used in a controlled fashion to treat ligninous and other hard-to-degrade solid bio-waste. The word ‘termigradation’ was coined by these authors to denote termite-assisted degradation of waste (Abbasi and Gajalakshmi, 2015). To ensure that use of termigradation does not lead to the introduction of invasives, it is necessary to identify the species already established in a given region and develop a repertoire of such species and the types of waste they prefer to feed on. Till now, little quantitative information on the richness and diversity of termifauna of India is available. There exists a lot of information, of which a good part has been compiled by the Zoological Survey of India (Kaur et al., 2013; Harit et al., 2013), on species available in different regions of India and on ways to control them but much less is available, if any, in the form of quantified measures of species richness, diversity, prevalence, etc.
Moreover, most of the termite species surveys reported in India so far have been based on sampling of the animals and where the surveyors spotted them. The usual practice has been to collect the animals, where they are seen present in good number, by sweeping them into a container by very soft alcohol-moistened brush, and identify the species (Pardeshi et al., 2010, Kumar and Thakur, 2010). There have also been studies wherein the entire termite colonies (mounds) have been excavated and the animals enumerated (Gupta, 1953). These studies are very useful in their context which was essentially termite control/eradication but have little use in the study of beneficial aspects of termite. As these surveys have not been based on properly randomized and representative methods of sampling and enumeration, the findings are not amenable to quantification of species richness, diversity, or evenness as truly representing any study area. This also precludes a proper comparison across regions because of the subjective nature of the surveys. Despite a general consensus among ecologists of the importance of termites, considerable knowledge gap exists on the functional roles of different termite taxa and the significance of termite diversity to soil function. Most of the published data on termite species richness and population density is not only location-specific but is difficult to generalize because different studies have used different sampling methods and experimental designs (Kaur et al., 2013, 2014). As a part of the efforts to cover the existing knowledge-gap, a systematic survey of termite species in Northeastern Puducherry which is the area where the authors are located, was carried out.
Study area
The study was conducted at Pondicherry University campus, located in the Northeastern Puducherry. An authentic map of the campus was obtained from the Engineering wing of the University. It is to a 1:3000 scale and represents an area of 780 acres harbouring rich tropical floral (537 species) and faunal diversity (197 species) (Parthasarathy et al., 2010; PriyaDavidar et al., 2010). The most diverse plant families in the campus include Euphorbiaceae (32 species), Poaceae (28 species), Rubiaceae (26 species), Mimosaceae (24 species), Papilionaceae (23 species), Acanthaceae (21 species), Araceae (18 species), and Agavaceae, Apocynaceae and Arecaceae (16 species each). Herbs are the most diverse: 94 species (36%), followed by trees - 73 species (28%), lianas - 26 species (10%), grasses and sedges - 26 species (10%), herbaceous climbers - 23 (9%) and shrubs - 16 species (6%) (Parthasarathy et al., 2010).
The termite survey experiments was based on methods employing transects and quadrats (Jones and Eggleton, 2000). Each of these has been extensively used in faunal surveys and yields data that can be resolved into indices. In the present study, Shannon-Weiner Index and Simpson Index of diversity and Pielou’s eveness index were calculated as follows (Hill, 1973; Bibi and Ali, 2013):
s
H = -Σ (Pi * ln Pi)
i=1
where, H’is the Shannon diversity index; Pi is the fraction of the entire population madeup of species i (proportion of a species I relative to total number of species present, not encountered); S is the numbers of species encountered and

A protocol described by Jones and Eggleton (2000), adapted from a similar method developed by Eggleton et al. (1996), was used for the survey. The protocol has been used in many tropical forests around the world (Gathorne-Hardy et al., 2002; Davies et al., 2003). Transect of 100 m length and 2 m width, was marked and divided into 20 contiguous sections (each 5 × 2 m) and numbered sequentially. Sampling was done in each section for 30 min (a total of one hour of collecting per section). In each section, microhabitats were searched for termites: 12 samples of surface soil (each 12 × 12, to 10 cm depth); accumulations of litter and humus at the base of trees and between buttress roots; the inside of dead tree stumps, logs, branches and twigs; the soil within and beneath very rotten logs; all mounds and subterranean nests encountered (checking for inquiline species); arboreal nests, carton runways, and sheeting on vegetation up to a height of 2 m above ground level. Termites specimens (Figure 1) collected for identification were stored in 80% isopropyl alcohol. The collected animals were identified by the authors with the key developed by them from earlier compilations (Bose, 1984; Chottani, 1997; Abe et al., 2000). After identification, the species were assigned to feeding groups as per classification of Donovan et al. (2001).

A total of thirteen species were identified by the survey. They belong to six genera of family, Termitidae and one genus of the family Rhinotermitidae.
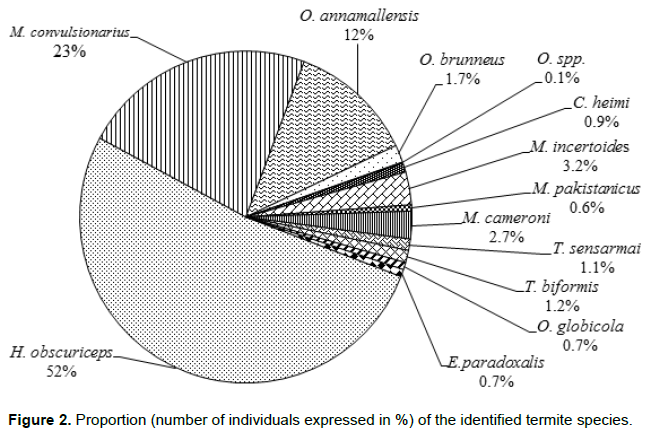
In turn, Termitidae is represented by three sub-families. Of these, Hypotermes obscuriceps Wasmann, Macrotermes convulsionarius Konig, Odontotermes anamallensis Holmgren and Holmgren, Odontotermes brunneus Hagen, Odontotermes globicola Wasmann, Odontotermes spp., Microtermes incertoides Holmgren and Eremotermes paradoxalis Holmgren belong to the sub-family Macrotermitinae. Microcerotermes cameroni Synder and Microcerotermes. pakistanicus Akhtar are of the sub-family Amitermitinae, while Nasutitermitinae is represented by Trinervitermes sensarmai Bose, Trinervitermes biformis Wasmann. The Rhinotermitidae is represented by Coptotermes heimi Wasmann, belonging to the sub-familiy Coptotermitinae (Table 1). The proportion of the identified species based on the number of individuals sampled is shown in Figure 2. H. obscuriceps was the most abundant (52 %) followed by M. convulsionarius (23 %).
Feeding groups of Donovan et al. (2001): I= dead wood and grass-feeders, II= Termites with a range of feeding habits including dead wood, grass, leaf litter, and micro-epiphytes, III= feeding in the organic rich upper layers of the soil, IV= true soil-feeders, ingesting apparently mineral soil).(Feeding group of DeSouza and Cancello (2010): I=Wood and grass feeders, II= Litter feeders, III= Soil feeders, IV= Soil feeders) (Lifeway classification of Eggleton and Tayasu (2001): Sing(I)ww= Group I [wood (wet and dry), grass, detritus], lifetype single; Int(II)=Group II (wood, fungus, grass, detritus, litter, microepiphytes), lifetype intermediate; Sep(II)=Group II (wood, fungus, grass, detritus, litter, microepiphytes), lifetype separate; Group III= soil–wood interface, soil feeder;Group IV=soil feeder.Group III and IV are not classified by life types.
Feeding and nesting habits
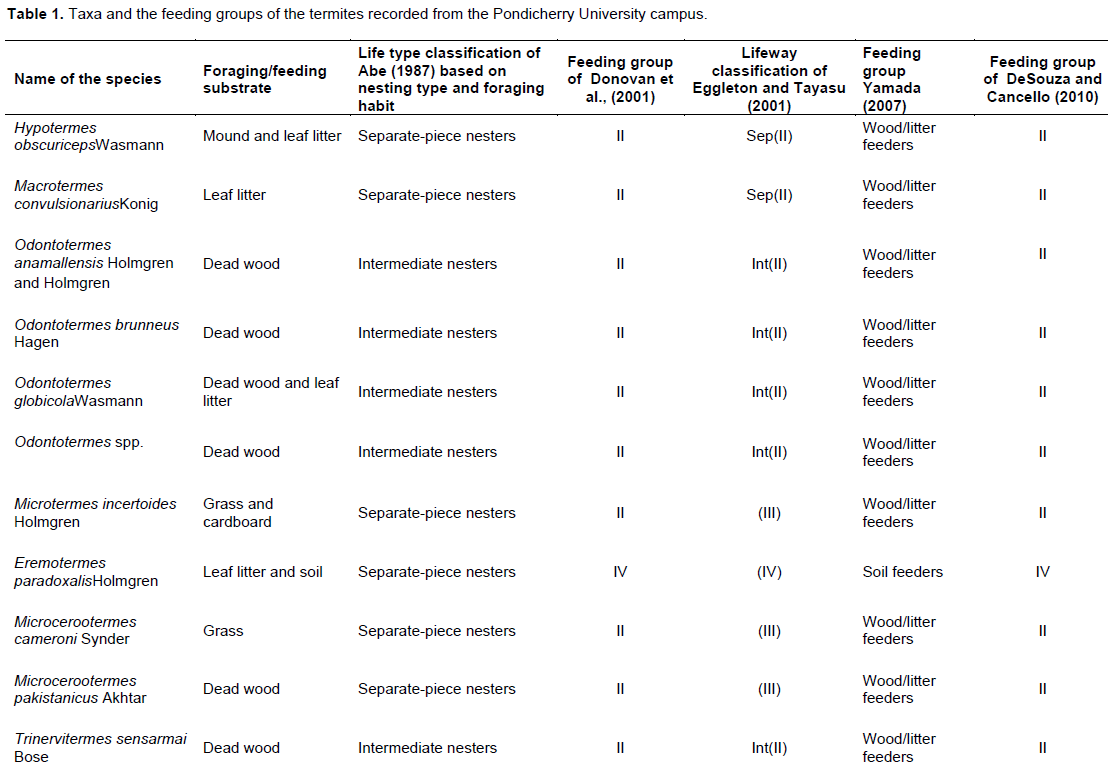
Table 1 presents the species identified in the present survey and the six types of classifications to which they belong. Of these, the classification of Abe (1987) is based on nest type and foraging habit. It distinguishes between single-piece, intermediate and separate-piece nesters. Single-piece nesters feed and nest in the same discrete substrate; wood-feeding termites are in this category. Intermediate nesters nest in their feeding substrate but also forage out from the colony centre to find other patches of feeding substrate nearby. Again, these are all wood-feeding termites. Separate-piece nesters do not nest in their feeding substrate and actively forage for their feeding substrate away from the nest, which does not act as a primary feeding substrate.
The other classifications on which these authors’ assessment has been done (Table 1) include the scheme of Donovan et al. (2001a), based on gut content analysis correlated with the morphology and anatomy of worker termites. This classification has been followed widely (Jones and Prasetyo, 2002; Davies et al. 2003; Bignell, 2011). The classification of Eggleton and Tayasu (2001) which is also called lifeway classification, combines the features of Abe’s lifetypes and Donovan’s feeding groups. It comprises eight groups– six categories of non-single piece nesters, and one each of dry wood and wet wood nesters. The eight groups are distributed across the gradients of humification and the degree to which the feeding and nesting substrates overlap. In the scheme of Yamada et al. (2007), termites are slotted into two major feeding groups - wood/litter feeders (including fungus-growers) and soil feeders. Lastly DeSouza and Cancello (2010) classified termites into four feeding groups or functional taxonomic groups, according to the proportion of the humification gradient they feed on. The substrates from where the termites were collected indicate their feeding preference. Based on this, the species were matched with the six classifications summarized (Table 1). C. heimi is a single piece nester, feeding on dead wood. E. paradoxalis is the only true soil feeder with separate piece nest type. The other eleven species are wood/litter feeders having a wide range of feeding habits including dead wood, grass, leaf litter, micro-epiphytes, fungus-comb, and conidia. They are either separate piece nesters or intermediate nesters. Hence, it can be deduced that except E. paradoxalis, all the other species found in the present survey are suitable for use in termigradation as they all have orgainc material as part of their diet.
Present work in the context of past surveys
Most surveys of termifauna done so far have largely been of an ad-hoc nature and have not been based on any structured methodology amenable to statistical analysis such as line transect, belt transect, quadrat or other systematized survey method (Abbasi et al., 2015). Pardeshi et al. (2010) and Kumar and Pardeshi (2011), in separate surveys conducted in Vadodra, recorded fifteen termite species in agricultural fields. As the focus of these studies was to assess the damage to the agricultural crops, the samples of termites were taken only from the individual plants. In similar studies, Kumar and Thakur (2010, 2013) recorded fifteen species and twenty seven species, respectively, in the states of Haryana and Punjab.
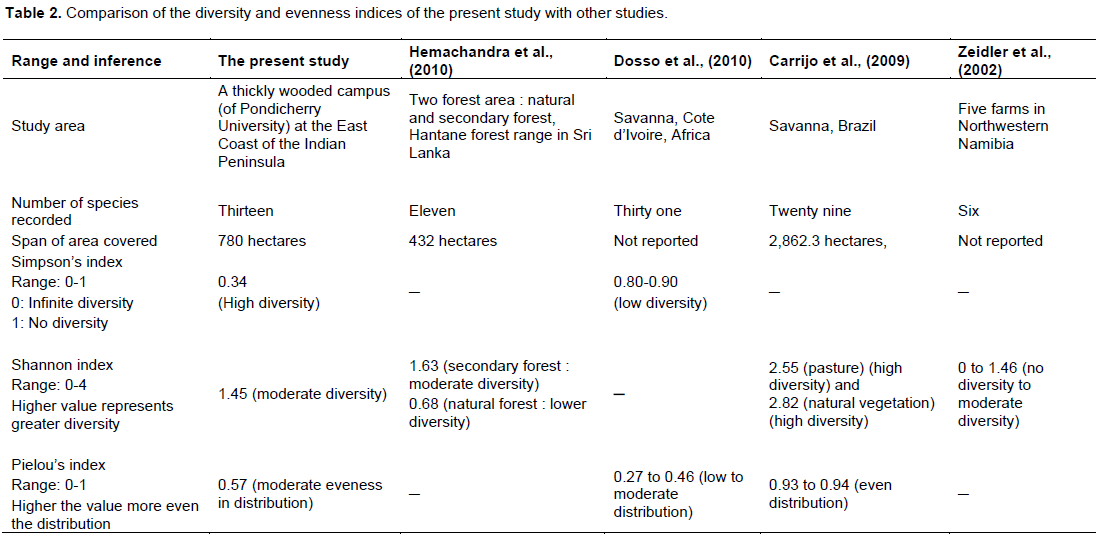
Attempt was made to compare the species richness and diversity of termites sampled in the present study with that of others who have also followed similar methods of sampling and indices development. Hemachandra et al. (2010) examined termite assemblages in patches of undisturbed natural forest and secondary forest spanning 432 ha. In addition, random collections of termites were carried out in both the forests for species determination. They recorded eleven species overall: nine species in the secondary forest (four species by transect sampling, three by random sampling and two by both methods), and two species in the natural forest of which neither was recorded from secondary forest. As a consequence, the Shannon diversity index as computed by them was higher for the secondary forest (1.63) as compared to the natural forest (0.68).
In the present study, thirteen species were found; one soil feeder and the rest wood/litter feeders. The Shannon index of the study area is much higher (H’=1.45) as compared to the natural forest surveyed by Hemachandra et al. (2010). They recorded only soil feeders from natural forest, and attributed the absence of wood feeders there to the natural forest’s altitude and climate. Moreover, they reported only five dominant species of trees and the litter comprised of small twigs of pencil size and sparse leaf litter in the natural forest. The present study area has much more diverse tree species and the litter generated is of different types ranging from small to large leaves, small twigs to large barks, shallow patches of litter to thick mulch covering large spans. Hence, there is more number of litter/wood feeding termites in the study area than in the Hantane forest reported by Hemachandra et al. (2010).
Carrijo et al. (2009), who followed the same methodology as in the present study except that their transects were twice as long, surveyed two areas: pasture and natural vegetation of State Park, Goias, Brazil. They recorded a total of twenty nine species (seventeen in pasture and twenty one in natural vegetation). The Shannon diversity indices were 2.55 and 2.82 for pasture and natural vegetation, respectively. Brazilian savanna is the richest tropical savanna in the world (DESilva and Bates, 2002) and part of the world’s 25 biodiversity hotspots. Hence, as expected, the Shannon diversity index in both vegetations (2.55 and 2.82 at pasture and natural vegetation, respectively) are higher than that of the present study area (1.45).
Zeidler et al. (2002) surveyed for termites in five farms in the Southern Kuene region, Namibia. In each farm, they studied a site each of high and low land use intensity. In each area, 400 m2 was surveyed which is twice the area normally used for representative sampling (Jones and Eggleton, 2000). They reported a total of ten species and concluded that termite species assemblages differed between the various forms, as well as across the land-use intensity gradients. The Shannon indices obtained by them ranged from 0–1.46, indicating zero diversity to moderate diversity. Dosso et al. (2010) while studying four different habitats differing in their vegetation and fire history: annually burned savanna, savanna woodland, forest island and gallery forest, in Cote d’Ivoire, West Africa, recorded a total of thirty species. The Simpson index for the areas ranged from 0.80 to 0.90 which indicates generally a low diversity as compared to the present study in which the Simpson index value of 0.34 represents high diversity (Table 2).
Among the four habitats studied by them, the forest island was the richest, followed by the gallery forest and savanna woodland. The forest island and gallery forest has more number of species as they act as refuge to species that are sensitive to regular fire that occurs in annually burned savannah. Between savanna woodland and annually burned savanna, savanna woodland had more number of species as the woodland consisted of savanna patches randomly unburned for five years, whereas annually burned savannah being fuel rich is burned deliberately every year.
The Pileou’s indices reported by Dosso et al. (2010) ranged between 0.27–0.46 representing low to moderate evenness in distribution of species in four different study sites, whereas in the present study, the Pileous index of 0.57 indicate moderate evenness in that respect. Pielou’s evenness values reported by Carrijo et al. (2009) are 0.94, 0.93 for pasture and for natural vegetation, respectively. The higher value indicates less variation among the species distributed in the natural vegetation as compared to the study area (0.57).
In another study conducted by the authors (Anantharaju et al., 2014) in Pondicherry Engineering College spanning about 210 acres, adjacent to the present study area, Pondicherry University, Puducherry, ten species were identified. In Pondicherry Engineering College, three species (Microtermes obesi, Microcerotermes fletcheri and Neotermes assumuthi) were identified which were not sampled from the present study area. Six species (Microtermes incertoides, Eremotermes paradoxalis, Microcerotermes cameroni, Microcerotermes pakistanicus, Odontotermes spp., and Trinervitermes sensarmai) were only found in the Pondicherry University campus and were not sampled from the Pondicherry Engineering College. The Simpson index of the Pondicherry Engineering College is 0.20 and the Shannon index is 1.83. The Pileous index of 0.75 shows less even distribution of the species in Pondicherry Engineering College as compared to the present study (0.57).
Hence it can be concluded that termite species in the present study area exhibit moderate evenness in distribution. The Simpson’s index of 0.34 indicates more number of rare species (M. pakistanicus, O. globicola, E. paradoxalis) than abundant species. On the other hand, the high (1.45) Shannon diversity index indicates that there are a few abundant species as well (H. obscuriceps, M. convulsionarius). The authors have also conducted survey of termites by bait method in the study area to check if any species is missed in the survey reported in this study (Kaur et al., 2013). The baits attracted six species which were otherwise also sampled using transect and quadrats.