ABSTRACT
An extensive research on deciphering lichen diversity in the high Nepalese Himalaya was undertaken in two subsequent years in Manaslu Conservation Area (MCA) and Sagarmatha National Park (SNP). Altogether, 621 specimens were collected from these two regions, viz., 173 from MCA and 448 from SNP, which resulted in the occurrence of a total of 13 species of lichens from MCA (belonging to 4 families) and 69 species belonging to 15 different families from SNP. Among the lichen families reported from these two study sites, family Parmeliaceae (7 and 29 species from MCA and SNP, respectively) was the dominant one followed by Physciaceae (12 species from SNP) and Cladoniaceae (4 and 8 from MCA and SNP, respectively). Thus, this research work, to some extent, reveals the lichens enrichment in the study region, furnishing much new insights that can be used as a composite signal of environmental quality and future bio-monitoring studies.
Key words: Bio-indicators, endemism, geographical gradients, speciation.
Lichens, frequently called ‘Jhyau’ or ‘Tare’ in Nepal are classified as a cluster of lower life-form of fungi (dual organisms assumed as a single one) and are formed by an intricate and mutualistic combination of both fungal (mycobiont) and algal (phycobiont/cyanobiont) partners (Sharma, 1995; Shah, 2014). Approximately, about 771 species belonging to 167 genera are known to Nepal as of now, of which more than 50 species are endemic (Baniya et al., 2010). However, current estimation of over 2,000 lichen species belonging to Nepal has now been made (Bhuju et al., 2007), but, lack of adequate knowledge continues to keep them undocumented. Though, the Nepalese Himalaya possess enormous pristine glories enriched with higher diversity and a very wide range of eco-climatic zones (Dobremez, 1976), most of its regions remained still unexplored. As it is well known that scientific discourse on ecological and biogeographical patterns and theories of species richness may prove an excellent system in bio-monitoring (Körner, 2002), this contemporary study was designed to abridge the existing gap in understanding and elucidating the diversity of lichens in Nepal.
Topography of the sampling areas
The Manaslu Conservation Area (MCA) lying on the lap of Manaslu Himalaya (Figure 1A), is spread in an area of 1,663 km2 and has an elevation gradient ranging from 1,400-8,163 m asl. It is bordered by Tibet autonomous region of China to the north and east, while Manang district to the west and Gorkha district to the south. The Sagarmatha National Park (Figure 1B) stretched in an area of 1,148 km2 lies in the Solukhumbu district and has an elevation gradient ranging from 2,845-8,848 m asl. The dominant tree vegetation in both the study area comprises of species of Rhododendron, Betula, Quercus and Juniperus.
Lichen sampling
The field trips were executed during 2010 (September-October) in Manaslu Conservation Area (MCA; coordinates Table 1), and in 2012 (April-May) in Sagarmatha National Park (SNP; Mount Everest Region; coordinates Table 2). Lichens growing on different substrata were sampled at an elevation above 2,350 m asl. With the special focus made on higher elevations, the sampling of lichens below 2,300 m asl was not incorporated in the present study. All species of lichens found in the sampling areas were harvested and species that tightly adhered to bark or soil were uprooted with the help of iron scalpels. Immediately after collection, samples were cleansed to eliminate the bark residues and other extraneous materials for their proper identification, followed by storage in air tight zip-lock plastic bags. All specimens were shade dried and the herbaria prepared according to method devised by Nash et al. (1993), was lodged in Nepal Academy of Science and Technology (NAST). All specimens collected were also coded with the British Lichen Society recording code numbers (Coppins, 2002).
The lichen cohort richness patterns in the studied areas were performed at different altitudinal ranges. Random-samplings were performed according to the availability of the lichens and the lichens found were harvested and the geographical co-ordinates and substratum were recorded. The specimens were identified through examination of their morphology, anatomy and habitation as per published floras (Awasthi, 2007).
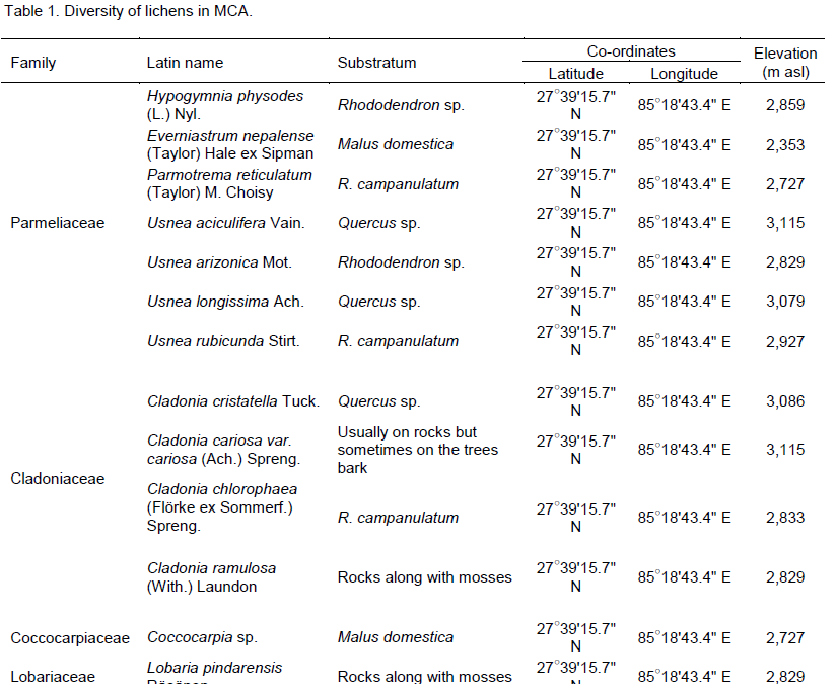
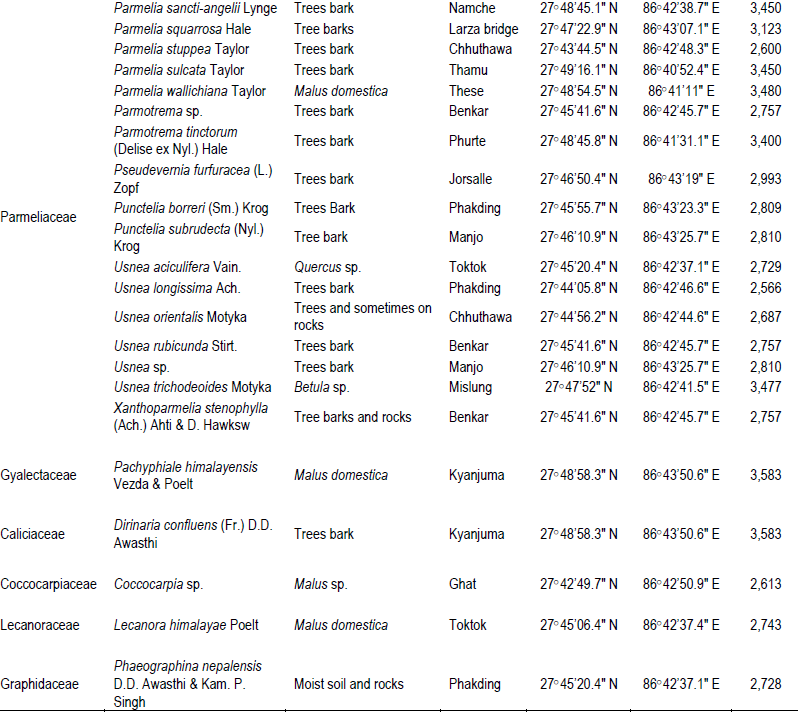
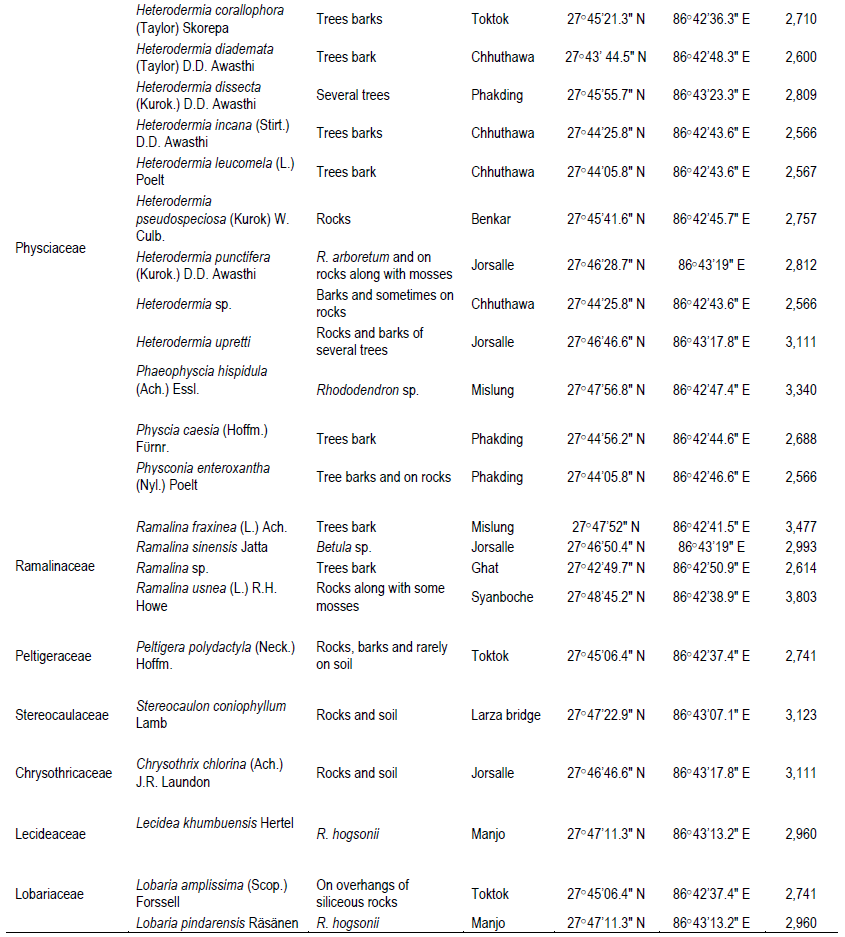
The two studied areas (Figure 1) possess an ample amount of lichens diversity, which are summarized in Tables 1 and 2. Through this discourse, we traced a total of 621 lichens specimen from the studied areas, viz., 173 from MCA and 448 from SNP. Altogether, 13 species of lichens from MCA (lying on 4 families) and 69 different species lying on 15 different families from SNP were identified, among which family Parmeliaceae (7 and 29 species from MCA and SNP, respectively) was largest followed by Physciaceae (12 species from SNP) and Cladoniaceae (4 and 8 from MCA and SNP, respectively) (Figure 2). From the study, the high altitudinal lichens located on the studied areas were Leptogium indicum (3,900 m asl) and Cladonia coniocraea (3,803 m asl). Lichens enrichment was found to be maximum in the range of 2,800 m asl in MCA, while the species richness were found to be maximum at an elevation of 2,700 m asl in SNP.
.png)
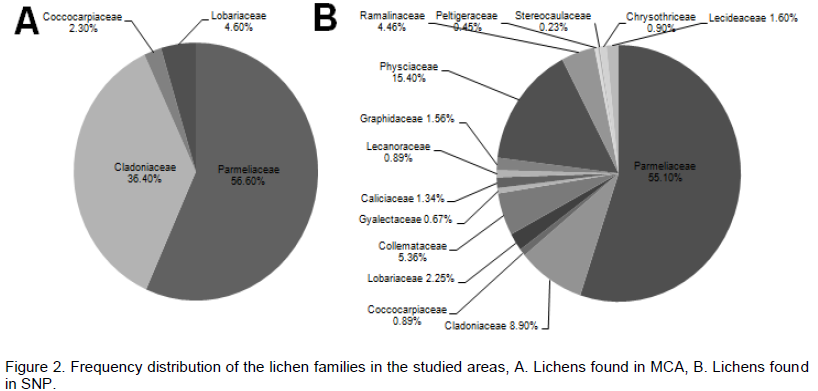
Most of the lichen species traced in the present study have previously been reported from the adjacent regions, such as India, Pakistan and Bhutan (Kumar et al., 2011) and also other parts of Nepal (Baniya et al., 2010). Thirty-three percentage of the total lichens reported in the present study were found above the tree line (> 4,300 m asl) of which 53% were endemic. This number seems to be enormous which suggests that the environmental conditions are quite favorable for the luxuriant growth of lichens and the topography and varied altitude somehow contribute towards rich lichen diversity and endemism in the study sites. But, it would be too early to predict that the results of the present study are somewhat similar to that of Baniya et al. (2010) who traced altogether 525 species of Nepalese lichen distributed between elevations of 200-7,400 m asl and documented 55 species endemic to Nepal, because, in the present study, lichens sampling was done up to the elevation of 4,200 m asl. The members of the Parmelioid clade belonging to lichen family Parmeliaceae were dominant in the study sites and are followed by the Usneoid clade of the same family. This study corresponds to the findings of Thell et al. (2012) who mentions that the Parmeliaceae is the largest family across the globe and till date includes 79 genera and ca. 2,726 spp. belonging to 5-main clades, viz., Parmelioid, Cetrarioid, Usneoid, Alectorioid and Hypogymnioid.
At high elevation, lichens of either in foliose or fructicose forms are found and they exhibit maximum species diversity value (Sipman, 1989), while crustose forms of lichens prevail greatly in lower altitude. The ubiquitous lichen species tend to establish and colonize early on every microhabitat occupying a larger chunk of area (Rose and Wolseley, 1984). The diversity of the epiphytic lichens may vary with tree species, age of the tree, bark pH and tree health status (Herk, 2001; Hauck and Runge, 2002; Nascimbene et al., 2013). However, actual mapping of lichens render difficulties at times. More often, the seemingly chaotic mingling of lichen species provides falsification to their actual identification and classification. Moreover, the implication of the molecular tools for their actual identification at the genomic level is needed, which decreases the falsified misinterpretations regarding the taxonomic classification. Thus, on such prevailing scenario, scare ongoing effort to document the lichen population in Nepal is below the threshold, and hence species identification via molecular studies may prove to be an effective milestone to know more about this charismatic group of organism in Himalayan ecozone.
The Nepalese Himalayas still harbors clusters of lichens that are not well-identified, so this study furnishes a preliminary finding about the diversity of lichens in some new localities of Nepal which may somehow be helpful in developing a general framework on the exploration of lichens in other regions of Nepal, and also to conduct future bio-monitoring studies by utilizing this baseline data.
The author declares there is no conflict of interest whatsoever.
The author thanks Mr. Niranjan Bhakta Ranjitkar and Mr. Basant Chhetri for providing their valuable inputs in the manuscript and to anonymous reviewers for critically evaluating the manuscript.
REFERENCES
|
Awasthi DD (2007). A compendium of the macrolichens from India, Nepal and Sri Lanka. Bishen Singh Mahendra Pal Singh, Dehra Dun, India.
|
|
|
|
Baniya CB, Solhoy T, Gauslaa Y, Palmer MW (2010). The elevation gradient of lichen species richness in Nepal. Lichenologist. 42: 83-96.
Crossref
|
|
|
|
|
Bhuju UK, Shakya PR, Basnet TB, Shrestha S (2007). Nepal biodiversity resource book: Protected areas, ramsar sites, and world heritage sites. ICIMOD, MOST and GoN.
|
|
|
|
|
Coppins BJ (2002). Checklist of Lichens of Great Britain and Ireland, British Lichen Society, London, p. 95.
|
|
|
|
|
Dobremez JF (1976). Le Nepal, ecologie et biogeographie. Centre National de la Recherche Scientifique, Paris.
|
|
|
|
|
Hauck M, Runge M (2002). Stemflow chemistry and epiphytic lichen diversity in dieback-affected spruce forest of the Harz Mountains, Germany. Flora. 4:250-261. (http://dx.doi.org/10.1078/0367-2530-00039)
Crossref
|
|
|
|
|
Herk CM (2001). Bark pH and susceptibility to toxic air pollutants as induces causes of changes in epiphytic lichen composition in space and time. Lichenologist. 33: 419-441.
Crossref
|
|
|
|
|
Nascimbene J, Thor G, Nimis PL (2013). Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe- A review. Forest Ecol. Manage. 298: 27-38.
Crossref
|
|
|
|
|
Körner C (2002). Mountain biodiversity, its causes and function: an overview. Mountain biodiversity: a global assessment (Eds. Körner C, Spehn EM), Parthenon, Boca Raton, FL. pp 3-20.
|
|
|
|
|
Kumar SR, Thajuddin N, Upreti DK (2011). Diversity of lichens in Kollihills of Tamil Nadu, India. Int. J. Biodivers Conserv. 3(2):36-39.
|
|
|
|
|
Nash TH, Wetmore CM, Anderson W, Brat C, Denison C, Eversman S, Murray B, Clari L (1993). Floristics, lichens as bio-indicators of air quality. U.S. Department of Agriculture, Fort Collins, Colorado, General, Technical Report, RM-224:6-15.
|
|
|
|
|
Rose F, Wolseley P (1984). Nettlecombe Park - its history and its epiphytic lichens: an attempt at correlation. Field Stud. 6:117-148.
|
|
|
|
|
Shah NC (2014). Lichens of commercial importance in India. Scitech J. 1:32-36.
|
|
|
|
|
Sharma LR (1995). Enumeration of the lichens of Nepal. (Ed. Verheught WJM) Ministry of Forest and Soil Conservation and Department of National Parks and Wildlife Conservation research report. Publ. No. 3-111.
|
|
|
|
|
Sipman HJM (1989). Lichen zonation in the parque Los Nevados Transect. Stud. Trop. Andean Ecosyst. 3:461-483.
|
|
|
|
|
Thell A, Crespo A, Divakar PK, Kärnefelt I, Leavitt SD, Lumbsch HT, Seaward MRD (2012). A review of the lichen family Parmeliaceae- history, phylogeny and current taxonomy. Nord. J. Bot. 30:641-664.
Crossref
|
|