ABSTRACT
Diversity of flowering plants, pollinators and microbial activity was assessed in five farms of Nzoia Sugar Company and one outgrower farm in Western Kenya. The overarching study objective was to determine differences in species diversity in the sugar farms using agrochemicals and those not using. To sample flowering plants, transects were laid along sugar farms with quadrats 20 m apart. Sweep nets captured fauna flying or attached to the flora. Microbial activities were assessed following application of 2,4 D and Hexazinone in soil from an outgrower farm (OGF) where these herbicides had not been applied before. Microbial activity expressed as formazan concentration following triphenyltetrazolium chloride (TTC) reduction in total Dehydrogenase activity (DHA) test. Results indicated that outgrower farm had higher flowering plant species richness than the sugar farms although diversity of both flowering plants and flower visitors in the sugar farms was higher. Results indicated that hexazinone significantly (p<0.05) increased microbial activity in soils to a high of 172.945 ug g-1 following 7 days incubation while 2,4-D suppressed microbial activity to less than 2 ug g-1 in first three days of incubation. From this study, it is recommended that bee pastures composed of plants frequently visited by bees should be left to grow around farms to increase pollinators and bee hotel structures for cavity nesting bees erected in agro-ecosystems. Use of pesticides on farms should be minimised and integrated weeds and pest management promoted to increase pollinators and beneficial fauna.
Key words: Pollinators, microorganisms, pesticides, out growers, sugarcane.
Agricultural intensification has led to a more homogenous landscape characterized by large crop fields and fewer non-cultivated habitats. In this context, many weed species within and around fields offer many important requisites for beneficial insects, such as pollen or nectar, as well as microhabitats that are not available in weed-free monocultures making natural assemblages of pollinators to suffer (Landis et al., 2005).
Cultivars of approximately two-thirds of the world’s crops require pollination by bees or other insects (Roubik, 2002). Between 78 and 94% of angiosperm species rely on the cross-pollination services provided by some 300 000 animal species (Altieri et al., 2015). There is now strong evidence that populations of both wild and managed insect pollinators are declining in both abundance and diversity (Potts et al., 2010). Among the many threats that agriculture poses to pollinators (changes in land use, loss and fragmentation of habitat, introduction of exotic organisms, modern agricultural practices, pesticide use, etc.), removal of weeds that provide forage for pollinators has been suggested as an important factor in the decline of native pollinators in agroecosystems (Richards, 2001; Steffan-Dewenter et al., 2005).
A lack of available nutrition in non-crop floral resources found in simplified agricultural landscapes leaves many pollinators susceptible to other pressures, particularly those with narrow diet breadths (Goulson et al., 2008). Providing floral resources within farmland ecosystems improves pollinator diversity or abundance or both (Carvalheiro et al., 2010). An increase in local availability of floral rewards is expected to result in greater fitness benefits such as longevity and fecundity of pollinators (Altieri et al., 2015).
Agricultural intensification has led to a more homogeneous landscape characterized by large weed-free fields and fewer uncultivated habitats. Habitat loss and degradation, for example, loss of complex landscape structures between farmland and adjacent ecosystems, as well as the increased use of agrochemicals, have been linked to the reduction in beneficial arthropod species richness in agricultural landscapes (Kevan, 1999). Sugarcane farms are usually expansive monocultures characterised by use of agrochemicals to increase soil fertility and for weed control.
Various physico-chemical factors and biological component of the soil influence transformation and decomposition of organic matter in the soil. It has been reported that bacteria and fungi make the greatest role in decomposition due to their high biomass and respiration in soil (Persson, 1980). Bacteria also constitute largest component of microbial biomass in soil. Pesticide application can also influence microbial diversity, biomass and their activity in the soil. Certain pesticides have been shown to affect microbial numbers and total dehydrogenase activity (Järvan et al., 2014). Thus, apart from investigating the effects of pesticides on weed and pollinators diversity, this study also was interested in the effects of 2,4-D and hexazinone two commonly used herbicides in Nzoia sugar farms on microbial degraders of organic matter in the soil. The purpose of this study was to determine whether there were any differences in diversity and abundance of pollinators between sugar farms and outgrower farms as a result of different farming practices.
Study site
The study was conducted in Nzoia Sugar Company farms in Bungoma County, Kenya. The sampling sites are shown in Figure 1. Nzoia River Basin lies between latitudes 1° 30′N and 0° 05′S and longitudes 34° and 35° 45′E. It runs approximately South-West and measures about 334 km with a catchment area of about 12,900 km2, with a mean annual discharge of 1777 × 106 m3/year. The area is characterised by two rainy seasons, the long rains from March to July, and short rains from August to October. The annual rainfall ranges from 400 to 1,800 mm. The annual temperature in the 5 and 32°C due to different levels of attitude. The soils are well drained, deep and vary from dark-red nitro sols to dark brown acrisols. For the study of microbial activities, soils were obtained from outgrower (OGF) farm which lies near Nzoia sugar Farms but had no history of application of 2,4-D and hexazinone.
Flora and fauna collection and identification
Collection of the flowering plants was done on a 100 m transect along each sampled sugar farm and within the outgrower farm. One by one metre quadrants were dropped along the transect every 20 m and all flowering plants in the quadrant were collected. After collection of the plants from the field they were pressed and dried at Egerton University and voucher specimens were mounted and preserved at the University’s herbarium. Identification was done by the assistance of botanist at the University. The pollinators and flower visitors were collected along the transects in the sugar farms and within the outgrower farm by use of sweep nets. These organisms were then transferred in killing jars having ethyl acetate. Flower visitors were identified by an expert at Egerton University whereas the pollinators were identified an expert at the National Museums of Kenya, Nairobi.
Simpson’s diversity index
Simpson’s index (D) was used to measure diversity of weed species in the farms. The index takes into account both species richness, and an evenness of abundance among the species present. Basically, it measures the probability that two individuals randomly selected from an area will belong to the same species. The formula for calculating D is:
Equation 1 Simpsons index of diversity
Where, ni = the total number of organisms of each individual species and N = the total number of organisms of all species.
The value of D ranges from 0 to 1. With this index, 0 represents infinite diversity while 1 represents no diversity. Thus the bigger the value, the lower the diversity (Simpsons, 1949).
Soil sampling, preparation and determination of microbial activity
The soil samples were collected from a depth of 0-10 cm using soil auger after removing the surface litters. Three sub-samples were collected. The samples were dried and sieved 2 mm sieve to remove stones and other debris.Total microbial activity in the soil can be determined using Dehydrogenase activity (DHA) measurements (Casida et al. 1964). In this test, soil is incorporated with 2,3,5-triphenyltetrazolium chloride (TTC). Microbial dehydrogenase activity during this incubation results in reduction of the water-soluble, colourless TTC to the water-insoluble, red 2,3,5-triphenyltetrazolium formazan which is extracted with a solvent and intensity measured spectrophotometrically and quantified using formazan standard curve.
Soils were incubated with field application dosage of either hexazinone or 2,4-D and incubated. Total DHA measurements were made using the procedure described in Lenhard (1956) and refined by Casida et al. (1964) in soil incubated for seven consecutive days. Details of the procedure are explained in Njue (2017). Positive results were based on reduction of 2,3,5-triphenyltetrazoliumchloride (TTC) from clear to formation of red coloured formazan (TPF) whose intensity was measured spectrophotometrically at 485 nm using Genesys 10-S 10uvspectrophotometer.
Microbial activities
The amount of the TPF expressed as µg TPFg-1 soil sample were established from a standard calibration curve of Formazan (Figure 2). The data was presented as means of triplicates samples ±Std.Dev.
Generally, higher microbial activities increased with days of incubation. Hexazinone herbicide seemed to possess a growth promoting activity and 2,4-D had a growth suppressing activity when compared to untreated soil. Thus, higher values were recorded for treatments with hexazinone compared to 2-4D. Highest microbial activities both for treated and non-treated soils occurred with activities reaching a maximum from day 5 to 7 with a yield mean of 172µg g-1 of TPF in the soil. The values seemed to improve within farm OGF from day 5 to day 7. Of notable concern is that Microbial activities significantly (P< 0.5) increased in nearly all days.
Results from this study shows that some pesticides such as 2,4-D may have a growth suppressive effects to soil microorganisms and the effects decrease with increased time in soil probably as the compound becomes degraded. From literature, reduction of microbial activities in soils treated with certain pesticides has been recorded (Järvan et al., 2014). In others microbial activity decrease initially and as the microbes become adapted to the herbicides, the normal activity is restored (sebiomo et al., 2010). In their preliminary study on effects of hexazinone application on soil biota in a forest plantation, Busse et al. (1986) did not find any inhibitory effects of hexazinone to soil microbial and arthropod communities. In the current study hexazinone application seemed to promote microbial activity but the mechanism it does this is not clear and probably it is used as a nutrient.
Flowering plants species found in the study area
A total of 114 plant species were identified in the study area after sampling. For those not yet identified a code was assigned to them. The five sugar farms had in aggregate more species than the outgrower farm. In comparison, the sugar farm area had 68 species whereas 37 species were identified in the outgrower farm. However, the outgrower farm had more species than each of the sugar farms. The outgrower farm had more than half of the species in five sugar farm area.
Flowering plants species richness
In terms of species richness, the outgrower farm had the highest number of flowering plants species (Table 1) with 37 species, while the next highest farm had 33. The least had only 26 species.
Simpsons diversity indices
The species diversity was calculated for the six farms by use of Simpson’s Index (D) and Simpsons Index of Diversity (1-D). Species diversity by Simpsons Index is given by:
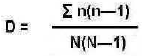
For instance, for Farm 139; ∑n(n-1) = 230 and N = 67 (Table 2), hence D = 230 / (67x66) = 0.052.
Simpsons Index of Diversity is given by 1-D, hence for Farm 139, 1-D = 0.95.
The higher the Simpsons Index of Diversity (1-D) the greater the diversity of the species. In this regard, the outgrower farm had the third highest diversity, Farm 212 and 242 had higher diversity.
Diversity of Fauna species
Diversity by orders
During the wet season the number of species was much higher than in the dry season. The total number of species across all order were 34 during the wet season and they were slightly less than half this number during the dry (15) (Table 3). The orders Hemiptera, Coleoptera, hymenoptera, and orthoptera had the greatest number of species and the highest population with at least 5 species each and at least a population of at least 10 during the wet season. In the dry season the orders Odonota, Chilopoda and Blattodea were not collected while the population was within the other orders much less as compared to the wet season. More species and in greater numbers were collected from the sugar farms as compared to the outgrower farm. Seven orders were identified in the collection from the sugar farms whereas only six orders were identified in the outgrower farm. The total population of all the species across the orders was 91 in the sugar farms, whereas it was 8 in the outgrower farm.
Pollinators
Bees were the most common pollinators in the study area whereby all the pollinators were collected in the sugar farms (Table 4). Three bee species were identified with the most common being Apis mellifera. Two species of butterflies were collected. No pollinator was collected in the outgrower farm. Some of pollinators captured in the study are shown below (Plates 1 and 2).
Fauna species diversity index
The fauna species diversity during the wet season was highest in two of the sugar farms (1-D=0.98) (Table 5). On the other hand, four sugar farms had a higher index that the outgrower farm. During the dry season the diversity index was calculated for only two farms, one sugar farm and the outgrower farm with the sugar farm having higher diversity. For the other farms, the population of each species in the dry season was too low to allow for the calculation of the index.
For the pollinators the diversity index was calculated for the wet season for two farms both of which were sugar farms (Table 6). It was not possible to calculate the index for the other farms during the wet season or for any farm during the dry season because of absence or very few numbers of the pollinators. Uses of agrochemicals have been associated with a variety of impacts to beneficial organisms. Some insecticides like neonicotinoids have been found to have sublethal effects on pollinators that may include negatively affect their productivity, reproduction or behaviour (Sgolastra et al., 2020). In many cases, some chemicals have been banned due to their environmental consequences like sublethal effects, synergestic interaction with other chemicals or toxicity to some species (Mancini et al., 2019). Whereas, Calatayud-Vernich et al. (2019) reported that insecticides may have lethal effects on bees upon exposure, Nicholsona (2017) and Main et al. (2020) observed that herbicides may enhance the adverse effects on insecticides either additively or synergistically.
Large scale colony loss has been reported in the USA by 50% in 28 years which has been ascribed to pesticide exposure among other factors. In addition, land use changes like fragmentation of habitats and improper use of herbicides has been cited stressing the pollinators (Fulton et al., 2019). Eeraerts et al. (2019) also notes that habitat loss and intensification of agricultural activities leads to decline of pollinators and pollination services. On the other hand, Eeraerts argues that maintaining seminatural habitats in agricultural areas can works to reverse or minimise the negative effect of loss of habitat on pollinator diversity. This is supported by Nicholsona et al. (2017) who observed that amount of natural areas in an area supports abundant and stable pollinators communities. This is similar to the study area where uncultivated strips are left around the sugar farms. However, intensive use of herbicides may reduce the habitat value of these strips to pollinators by availability of flowering plants.
Weeds and plants growing close to monocultural farms act as refuge for pollinators when crops are not flowering. In sugarcane farms bee pastures composed of plants frequently visited by bees should be left to grow around farms to increase pollinators and bee hotel structures for cavity nesting bees erected in agro-ecosystems. Similarly, weed strips can be left around farms and clearing weed in these strips, the activity should be staggered so that a sizeable area is at all times under these flowering plants so as to maintain a healthy population of pollinators. Efforts should be made to promote growing of hedgerows or strips of indigenous weeds. Integrated weeds and pest management should be promoted to increase pollinators and natural enemies for pest control.
The authors have not declared any conflict of interests.
REFERENCES
|
Altieri MA, Nicholls CI, Gillespie M, Waterhouse B, Wratten S, Gbèhounou G, Gemmill-HB (2015). Weeds and pollinators: Understanding ecological interaction for better management. FAO, Italy.
|
|
|
|
Busse M, Rapport N, Powers R (1986). Hexazinone effects on soil biota and processes: Preliminary findings. 22nd Vegetation Management conference, USDA Forest Service, PSW Research Station, 2400 Washington Avenue, Redding CA 96001.
|
|
|
|
|
Calatayud-Vernich P, Calatayud F, Simo E, Aguilar JAP, Pico Y (2019). A two-year monitoring of pesticide hazard in-hive: High honey bee mortality rates during insecticide poisoning episodes in apiaries located near agricultural settings. Chemosphere 232:471e480.
Crossref
|
|
|
|
|
Carvalheiro LG, Seymour CL, Veldtman R, Nicolson SW (2010). Pollination services decline with distance from natural habitat even in biodiversity-rich areas. Journal of Applied Ecology 47:810-820.
Crossref
|
|
|
|
|
Casida LE, Klein DA, Santoro T (1964). Soil dehydrogenase activity. Soil Science 98:371-376.
Crossref
|
|
|
|
|
Eeraerts M, Smagghe G, Meeus I (2019). Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agriculture, Ecosystems and Environment 284:106586.
Crossref
|
|
|
|
|
Fulton CA, Hartz KEH, Fell RD, Brewster CC, Reeve JD, Lydy MJ (2019). An assessment of pesticide exposures and land use of honey bees in Virginia. Chemosphere 222:489e493.
Crossref
|
|
|
|
|
Goulson D, Lye GC, Darvill B (2008). Decline and conservation of bumble bees. Annual Review of Entomology 53:191-208.
Crossref
|
|
|
|
|
Järvan M, Edesi LA, Adamson, Võsa TV (2014). Soil microbial communities and dehydrogenase activity depending on farming systems., Plant Soil Environment 60(10):459-463.
Crossref
|
|
|
|
|
Kariuki CW (2016). Isolation and characterization of soil bacteria capable of degrading metribuzin in sugarcane farms of Nzoia River Drainage Basin, Western Kenya. MSc. Thesis, Egerton University, Kenya.
|
|
|
|
|
Kevan PG (1999). Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture, Ecosystems and Environment 74(1-3):373-393.
Crossref
|
|
|
|
|
Landis D, Menalled FD, Costamagna AC, Wilkinson TK (2005). Manipulating plant resources to enhance beneficial arthropods in agricultural landscapes. Weed Science 53(6):902-908.
Crossref
|
|
|
|
|
Lenhard G (1956). The dehydrogenase activity in soil as a measure of the activity of soil microorganisms. Z. Pflanzenernaehr. Dueng. Bodenkd 73:1-11.
|
|
|
|
|
Main AR, Hladik ML, Webb EB, Goyne KW, Mengel D (2020). Beyond neonicotinoids -Wild pollinators are exposed to a range of pesticides while foraging in agroecosystems. Science of the Total Environment 742 (2020):140436.
Crossref
|
|
|
|
|
Mancini F, Woodcock BA, Isaac NJB (2019). Agrochemicals in the wild: Identifying links between pesticide use and declines of nontarget organisms. Current Opinion in Environmental Science and Health 11:53-58.
Crossref
|
|
|
|
|
Nicholsona CC, Koha I, Richardsona LL, Beaucheminb A, Rickettsa TH (2017). Farm and landscape factors interact to affect the supply of pollination services. Agriculture, Ecosystems and Environment 250:113-122.
Crossref
|
|
|
|
|
Njue R (2017). Assessment and characterization of bacterial degraders of hexazinone and 2, 4-D herbicides from sugarcane cultivated soils in Nzoia River Drainage Basin in Western Kenya. MSc. Thesis, Egerton University, Kenya.
|
|
|
|
|
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010). Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution 25:345-353.
Crossref
|
|
|
|
|
Richards AJ (2001). Does low biodiversity resulting from modern agriculture practice affect crop pollination and yield? Annals of Botany 88:165-172.
Crossref
|
|
|
|
|
Roubik D (2002). Tropical Agriculture: The value of bees to the coffee harvest. Nature 417:708-708.
Crossref
|
|
|
|
|
Sebiomo A, Ogundero VW, Bankole SA (2010). Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. African Journal of Biotechnology 10(31):770-778.
|
|
|
|
|
Sgolastra F, Medrzycki P, Bortolotti L, Maini S, Porrini C, Simon-Delso N, Bosch J (2020). Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biological Conservation 241:1-5.
Crossref
|
|
|
|
|
Simpson HE (1949). Measurement of Diversity. Nature 163(688).
Crossref
|
|
|
|
|
Steffan-Dewenter I, Potts SG, Packer L (2005). Pollinator diversity and crop pollination services are at risk. Trends in Ecology and Evolution 20:651-652.
Crossref
|
|