ABSTRACT
Population size, structure, activity time budget and feeding behavior of Guereza (Colobus guereza) were studied in Borena-Sayint National Park (BSNP), Ethiopia, from August 2008 to March 2009. Line transect survey technique was applied to investigate the population size and structure. Guerezas were observed only in the forest habitat. The mean group size, group density and group encounter rate in the forest habitat were 7.7 individuals, 14.8 groups per km2 and 1.43 groups per km, respectively. In addition, the majority of the groups contained one adult male. The total population was estimated to be 2170 individuals. The population was skewed towards females. The ratio of male to female, young to adult and infant to female were 1.0:1.45, 1.0:4.16 and 1.0:4.9, respectively. The age structure was 47.9% adult, 32.7% sub-adult, 11.5% young, and 7.9% infant. No significant variation was observed in group size between seasons. Guereza consumed 31 plant species which consisted of 15 trees, 12 shrubs and 4 herbs. Dombeya torrida and Olinia rochetiana were the most consumed plant species which accounted for 18.2 and 12.6% of the diet of guereza. Leaves comprised of the largest proportion of the food items consumed (71.6%). Their diurnal activity is dominated by resting periods. This study contributes greatly to add information on the status of guereza in Ethiopia, and for its conservation and management.
Key words: Activity, age structure, Borena-Sayint, diet, guereza.
The Ethiopian highlands are extremely rugged and varied, with some regions characterized by steep escarpments and deep valleys (Yalden, 1983). The Ethiopian biodiversity has high level of endemicity that needs much attention from government officials and other stakeholders for conservation because of the presence of a very diverse set of ecosystems (Bekele and Yalden, 2013). The country possesses high diversity of flora and fauna that occurs throughout the highland and lowland areas. Ethiopia consists of 315 species of mammals, out of these about 50 are endemic (Bekele and Yalden, 2013). So far, 11 species of primates are known to occur in Ethiopia (Yalden and Largen, 1992), and more are being discovered (Mekonnen et al., 2012).
The colobine monkeys are found in Africa and Asia (Fashing, 2006). The African colobines can be split into three genera and 15 species (Groves 2007), which include Colobus (five species of black-and-white monkeys). These colobines occur in Africa, and inhabit many of the forested regions of Equatorial and West Africa (Fashing, 2006). Most species of the genus Colobus are identified by differences in their pelage. C. guereza (guereza) is one of the five species of the genus Colobus (Groves, 2007). This species is widely distributed across eastern and western Africa (Groves, 2005; Kingdon et al., 2008).
Guerezas are medium-sized, black and white arboreal monkeys (Kingdon et al., 2008). They can be found in moist and deciduous forests and savanna woodlands, often extending into highland or montane forests (Oates et al., 1994; Kim, 2002). Guerezas can exist in riparian (close to rivers), colonizing and upland forests, with some preference for water edges (Kim, 2002). They favor the main canopy levels in the forest and partially disturbed habitats, especially secondary forests. The preference of such habitat is associated with high species diversity of food trees in some secondary growth forests (Thomas, 1991). They can also be found in areas under human use, such as eucalyptus plantation (Harris and Chapman, 2007).
Even though Guerezas display great flexibility in their ecology, they exhibit little variability in social organization throughout their wide ranges (Newton and Dunbar, 1994). Their social groups are generally small and cohesive, ranging between 3 to 15 individuals (Bocian, 1997; Fashing, 1999). In general, group sizes tend to be larger in continuous forest and smaller in riparian or interrupted forest (von Hippel, 1996). In many cases, guereza groups include one adult male, several adult females and immature individuals. However, more than one adult male can be present as group size increases, and in several populations multi-male groups are common (Oates, 1994; Fashing, 2001a).
A population density estimate of a given species is vital for determining future conservation and management of that species (Muoria et al., 2003). Knowledge on the diurnal activity patterns and time budget of the animal can serve as an important tool in developing the species’ conservation strategies (Kivai et al., 2007). Information on daily activity time budget is also useful in the overall analysis of primate behavior and habitat use, and has been used widely in primate research (Di Fiore and Rodman, 2001).
Out of the eight subspecies of Colobus guereza, two subspecies, namely C. g. guereza and C. g. gallarum, are found in Ethiopia (Groves, 2007; Kingdon et al., 2008). Little is known about the status and behavioral ecology of guereza in Ethiopia. Dunbar and Dunbar (1974) have attempted to study the ecology and population dynamics of C. guereza in Ethiopia specifically in Bole Valley. Hence, the present study aimed to investigate the population ecology of guereza in Borena-Sayint National Park, in northern Ethiopia. This study will provide base line information about the ecology and behavior of the animal for future in depth study. In addition, conservation initiatives may be launched for better protection of the animal and its habitat.
Study area
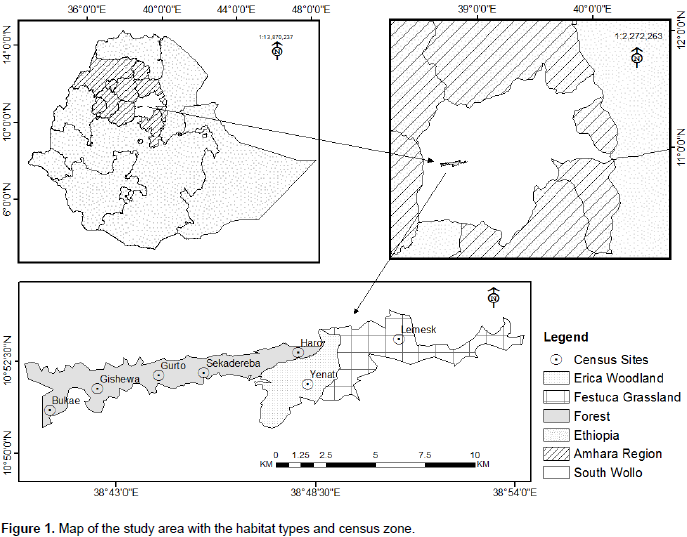
The present study was carried out in Borena-Sayint National Park (BSNP), which is located 600 km north of Addis Ababa, Ethiopia, situated between 10°50'45.4"-1053'58.3" latitude and 38°40'28.4" -38054'49" longitude (Figure 1). Currently, BSNP covers an area of 4,375 ha. It is part of the Eastern Afromontane Biodiversity Hotspot which is characterized by high species richness and endemism as well as severe human pressure (Mittermeier et al., 2005). The altitudinal range of BSNP in its current extent is 2,188 m to 3,732 m.. Due to the altitudinal range of the park, it comprises afromontane forest in this lower and sub-afroalpine in the middle and afroalpine vegetation types in the upper part of the park (EWNHS, 1996). The landscape of the park consists of rough topography, gorges, deeply incised valleys, steep escarpments, and strips of plateaus and cliffs (Ayalew et al., 2006). It harbors many mammalian species, including several primate species, including the eastern black and white colobus monkey (Colobus guereza guereza), hamadryas baboon (Papio hamadryas), grivet monkey (Chlorocebus aethiops) and gelada monkey (Theropithecus gelada). Farmland surrounds the park in all directions. The main agricultural products are cereal crops such as wheat (Triticum aestivium) and barely (Hordeum vulgare), and legumes such as lentils (Lens culinaris).
Preliminary survey
The study was conducted from August, 2008 to March, 2009 covering both wet and dry seasons. Before the period of actual data collection, a preliminary survey was conducted to get information regarding accessibility, climatic conditions, vegetation types, fauna, water sources, and the distribution of Guereza in the area. This period allowed the observer to become familiar with the forest environment within the home range of the studied groups and enabled to distinguish accurately the age/sex classes, recognize when the Guerezas performed different behavioral activities, and to know how close the observer could approach them. Three habitats were also identified: forest habitat, Erica woodland and Festuca grassland with Lobelia. Farmland habitat was ignored as the Guerezas were never seen in this habitat.
Population structure
Line transect survey technique was used to estimate the population size of guereza in BSNP (Peres, 1999; Plumptre, 2000). Line transects were used by stratified random sampling approach in which placement of transects was proportional to the area of different habitats where Guerezas were inhabited. Transects were measured and marked every 50 m (Chapman et al., 1988; Mekonnen et al., 2010). A total of 20 random transects (census zones), ranging from 1.5 to 2.5 km in length, were placed in the study area within the three habitat types. In the forest habitat, 16 transects were marked in five different specific census zones: 3 different transects in each of Bukae, Gishewa, Gurto and Haro areas, while 4 transects in Sekadereba area. In addition, two different transects were marked in each of Erica woodland and Festuca grassland with Lobelia habitat types. Each of these transects was surveyed with the help of experienced scouts during the wet and dry seasons in the morning (from 06:00 to 10:00 h) and afternoon (from 14:00 to 18:00 h) (Mekonnen et al., 2010) (Figure 1).
The observer recorded the time, group size (with age/sex), group spread, animal to observer distance, sighting angle, tree height and habitat types where Guerezas were detected (Fashing, 1999; Fashing and Cords, 2000). Animal to observer distance, and tree height were estimated by the observer. In addition, sighting angle was determined by the aid of compass. During the census, the observer walked on foot along a transect line with the help of Global Positioning System (GPS), and stopped frequently (every 5 min) to listen and scan the surrounding area. A walking pace of 2 km/hr was maintained (Peres, 1999).
In recording the number of individuals in a group, age and sex were determined primarily based on the work of Fashing (1999, 2001a) and Grimes (2000). Males have fused gray-colored ischial callosities encircled by an unbroken ring of white hair, but in females, the gray-colored ischial callosities are separate and the encircling ring of white hair is broken into two patches. The different age classes of guereza are identified on the basis of size, general appearance and behavior. Infants were small in size, clinging to the mother and dependent on her in all of the major group movements. At birth, infants are white in color with pink skin on face, ears, hands and feet, but gradually attain adult coloration within 3 to 4 months. They receive very intense attention from the other members of the group, especially from females. Young were small, but larger than infants, and roughly half the size of an adult female.
Activity patterns
Daily behavioral activities of Guereza were recorded by using instantaneous scan sampling method (Altmann, 1974) at 15 min intervals of 5 min duration starting from 06:00 to 18:00 h. Different activity types and dietary data were collected from two selected and partially habituated neighboring study groups of guereza for a total of 24 days per each season. The activities of individual Guereza were recorded by approaching the animals and observing them with naked eye or by the aid of binoculars to identify the specific types of activity they performed and food items consumed. The activity recorded for each individual was the first activity that sustained for 5 s once Guerezas came into view (Fashing, 1999, 2001a; Grimes, 2000).
The record of the behavior of 1 individual during the scan represents 1 observation (Wong and Sicotte, 2007). Intense attention was given to avoid scanning the same guereza more than once in a given scan (Di Fiore, 2004). During each scan, the age/sex class of the visible individual Guerezas, along with the category of behavior they were seen performing, was recorded. Infants were excluded from scan sampling, and sex of young Guerezas was not identified. The following five exclusive behavioral categories were recorded on the standardized data sheet: resting, feeding, moving, socializing (playing, aggression, grooming and sexual activity), and other activities (defecation, urination, drinking or others) (Bocian, 1997; Grimes, 2000; Fashing, 2001a; Fashing et al., 2007). Feeding was recorded when the Guerezas were seen manipulating, masticating or placing food in their mouth.
Diet
During scan sampling, dietary data along with other behavioral activities were collected every 15 min interval from the focal groups. During the feeding activity of Guereza, the type of food item: young leaves, matured leaves, flowers, fruits, roots, bark, shoots, and wood (from dead plants) and the type of species consumed were recorded. The type of plant species consumed were given local names and taken to National Herbarium, Addis Ababa University for taxonomic identification.
Data analysis
Data collected during the survey were analyzed by using statistical package for social science (SPSS) 15.0 Software for Windows Evaluation Version. Statistical tests were two-tailed with 95% confidence intervals and level of rejection was set at p=0.05. Analysis of sex ratio and age structure was carried out using one-way analysis of variance (ANOVA). Density, encounter rates and mean group size of guereza population from the line transect survey were analyzed using “DISTANCE 5.0” software program (Buckland et al., 1993). The mean group size used to calculate density was recorded only from guereza groups counted during transect walks (Plumptre, 2000). The total population of guereza in the BSNP was estimated by multiplying the average individual density with the total area of suitable habitat (Chiarello, 2000; Mekonnen et al., 2010). Sex structure and age category were compared using Mann-Whitney U-test. Group size and distribution were also compared using Mann-Whitney U-test for independent samples. The differences in the amount of time spent for different activities at different seasons were also analyzed using Mann-Whitney U-test. Descriptive analysis of feeding time, plant species and plant parts consumed by guereza were used to identify the feeding behavior of the species.
Population structure
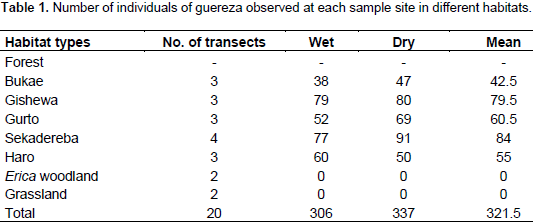
A total of 306 and 377 individuals were recorded from the
forest habitat during the wet and dry seasons, respectively. The total population estimate for wet and dry seasons were 2145 and 2195 individuals, respectively. In addition, individual density/km2 for wet and dry seasons were 112.9 and 115.5, respectively. Among the different sites sampled during the study period, the highest sample count was in the forest and no individuals were found in the Erica woodland and Festuca grassland habitats (Table 1). Analysis of the population size of guereza using Mann-Whitney U-test showed that there was no significant statistical difference between wet and dry seasons (P> 0.05).
Out of the average 321.5 individuals of guereza sighted during the observation period, 259 were adults and sub-adults, and the rest were young and infants (Figure 2). In addition, on the average, 47.9% of the individuals observed were adults, 32.66% sub-adults, 11.51% young and 7.93% infants. During the study period, more adults were counted than sub-adults, young and infants.
The ratio of adult male to adult female during wet and dry seasons was 1.0:2.75 and 1.0:2.68, respectively. The ratio of sub-adult female to sub-adult male was 1.0:1.71 and 1.0:1.67 during the wet and dry seasons, respectively. Analyses of the age structure and sex ratio for both adult and sub-adult males and females revealed that there was no significant difference (p> 0.05) in the age and sex distribution during the wet and dry seasons. In general, the ratios of male to female, young to adult and infant to female were 1.0:1.45, 1.0:4.16 and 1.0:4.9, respectively.
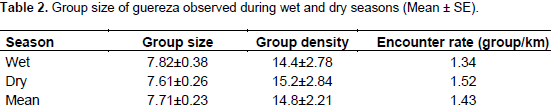
The average individual density was estimated to be 114.2 individuals per km2 (range 82.5-148.1). The average encounter rate (groups/km) for guereza groups was 1.43. The total area of the forest habitat in the BSNP was 19 km2. Hence, the total population of guereza in the BSNP was estimated to be 2170 individuals. The range of group size of guereza encountered was between 4 to 13 individuals. Group size never exceeded 13 individuals during the present observations (Table 2). Three multi-male groups, containing two males per group, were observed during the wet season, but during the dry season only one multi-male group was observed. All-male groups were never observed during both wet and dry seasons.

Activity patterns
A total of 9600 individual behavioral records were made. Guerezas used their time resting (61.7%), feeding (22.6%), socializing (8.2%), moving (5.4%), and other activities (2.1%) such as urination and defecation. Guerezas on average spent more time resting (64.2±2.3%) during the wet season than the dry season (59.2±3.6%). Mann-Whitney U-test showed that there was a significant seasonal difference in resting between seasons (P< 0.0 5). Levels of feeding activity were closely related to resting. Guerezas on average spent more time feeding (23.8%±1.0%) during the dry season than the wet season (21.4±1.9%). However, there was no statistically significant difference between seasons in feeding (P> 0.05). Guerezas engaged in social activities (9.9±1.3%) more during the wet season than the dry season (6.5±2.4%). Mann-Whitney U-test showed that there was a significant seasonal difference in social activities between seasons (P< 0.05). Moving records were low followed by high resting records. Adult females usually initiate the moving activity of the group and members usually followed the same arboreal pathway. The amount of daily time spent in moving is 5.4±3.7%. Guerezas on average spent 2.5±0.3% of their moving time during the wet season and 8.3±2.8% during the dry season. There was a significant seasonal difference in moving (P< 0.05). They also spent more time engaged in other activities during the dry season 2.2 ±0.5% than the wet season 2.0±0.3%. However, there was no statistically significant difference between seasons in other activities (P> 0.05).
Diet
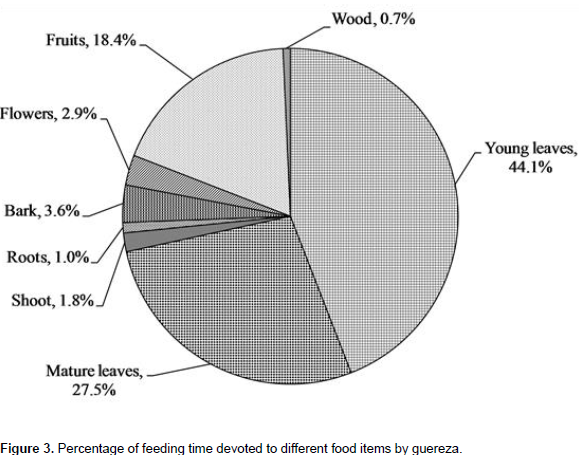
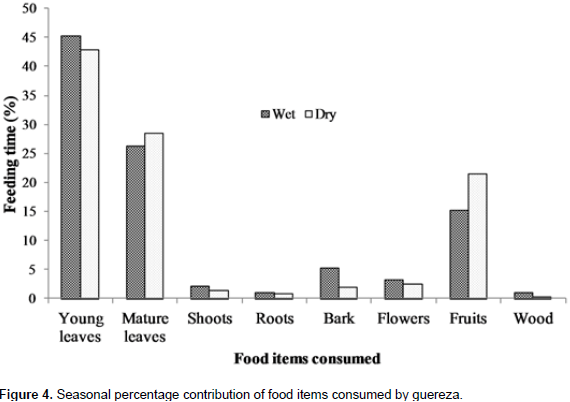
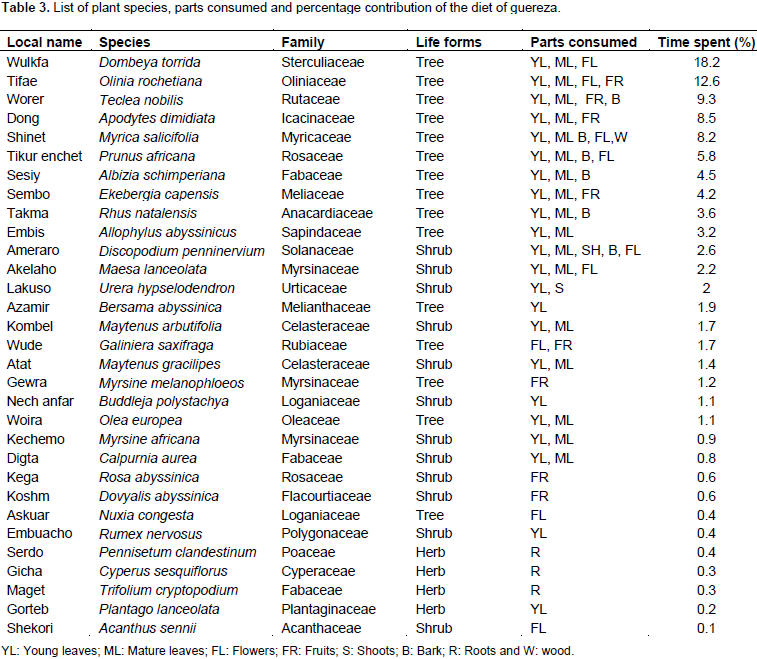
A total of 2153 feeding observations were recorded from scan sampling of guereza. The overall diet of guereza during the study period is shown in Figure 3. Young leaves contributed to 44.1±1.9% of the overall diet. Mature leaves and fruits were the second and third favored food items. The fourth and fifth contributors of guereza diet were bark and flowers. Shoots, roots and wood were consumed infrequently. However, they were never observed feeding on animal material. The Mann-Whitney U test showed that there was a statistically significant difference in time spent for feedingon young leaves, mature leaves, bark, fruits and wood (P< 0.05) between wet and dry seasons. However, there was no significant difference (P> 0.05) between seasons in time spent feeding on shoots, roots and flowers (Figure 4). Guerezas consumed a total of 31 plant species that are grouped in 24 plant families. The percentage contribution and food items consumed are given in Table 3 and the top ten consumed species are given in Figure 5. Out of the 31 plant species contributed for the overall diet of guereza, 15 species were trees, 12 were shrubs and 4 were herbs.
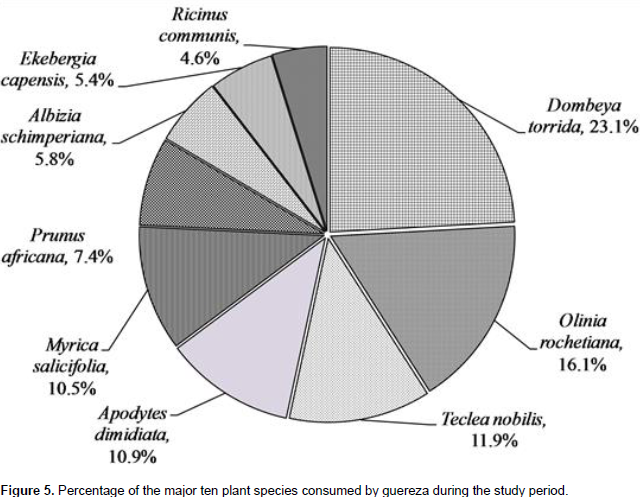
The highest contribution of the diet is from the family Sterculiaceae (18.2%), Oliniaceae (12.6%), Rutaceae (9.3%), Icacinaceae (8.5%) and Myricaceae (8.2%). The ten most consumed plants accounted 78.1% of the overall diet of guereza as shown in Figure 5. Based on the overall percentage contribution, Dombeya torrida was the most consumed plant species which accounted for 18.2%, Olinia rochetiana and Teclea nobilis ranked second and third (12.6 and 9.3%), respectively. The fourth and fifth ranked plant species were Apodytes dimidiata and Myrica salicifolia which accounted for 8.5% and 8.2% of the overall diet of guereza, respectively. Based on the overall percentage contribution of plant parts to the diet of guereza from each species, young leaves of D. torrida were highly consumed accounting for 11.3%, while fruits of O. rochetiana accounted for 7.4%. Mature leaves of D. torrida and Prunus africana contributed for 10.1% of the diet of guereza. M. salicifolia wood exclusively contributed for 0.7% consumption of guereza. Plant species that were used by this monkey as sleeping and sheltering were D. torrida, Ekebergia capensis, A. dimidiata, Hagenia abyssinica and P. africana.
In the present study area, Guerezas inhabited only the forest habitat of BSNP. They were never observed in the Erica woodland and Festuca grassland habitats. Guerezas avoided these habitats may be due to the unsuitable leaves and grasses of Erica arborea and Festuca spp., respectively. Sample count of Guereza individuals in BSNP revealed that there were 306 and 337 individuals during the wet and dry seasons, respectively. The increase in the number of individuals during the dry season might be due to an increase in visibility, presence of additional five groups throughout the census sites, and groups were observed feeding more in lower strata of the forest during the dry season. New born white infants were observed both during wet (3) and dry (5) seasons.
Infants were born throughout the year (Fashing, 1999; Harris and Monfort, 2006). Considering the census sites during the transect survey, differences in the number of individuals were observed between seasons in Gurto and Sekadereba areas. This is probably due to the availability of fruits and mature leaves of O. rochetiana, the young leaves of Apodytes dimidiata and Ekebergia capensis, and the mature leaves and flowers of Dombeya torrida during the dry season. The census result of the present study showed that the individual and group densities, and mean group size of guereza were 114.2 individuals per km2, 14.8 group per km2 and 7.71 individuals, respectively. The density of guereza in BSNP is high compared to some other areas of Africa (Bocian, 1997; Grimes, 2000). This may be due to the availability and distribution of food trees in the present study area (Chapman and Chapman, 2002). In a number of colobine species, when population density increases home range size becomes compressed (Dunbar, 1987; Newton and Dunbar 1994). For instance, Fashing (2001b) found a significant negative correlation between population density and mean home range size.
Guereza groups in BSNP ranged from 4 to 13 individuals. However, the mean group size was 7.7 individuals. In most cases, one adult male, three adult females, two sub-adults, two young and/or infant are typical to the area (Dunbar, 1987; Bocian, 1997). A total of four multi-male groups (containing two males in a group) were observed during the study period. Three of them were during the wet and one was during the dry seasons. The three multi-male groups that were seen during the wet season might change to one male unit through group fission during the dry season (Bocian, 1997).
The number of adult males in guereza groups is related to habitat type (von Hippel, 1996). Larger multi-male groups usually live in continuous forests but smaller one male group likely resides in patchy forests. The group size of African colobines can be influenced by logging history, predation risk and feeding competition (Fashing, 2006). Guerezas are capable of conserving energy by traveling short distances each day, spending most of the day time resting, and feeding on relatively ubiquitous food items (Oates, 1977). Similarly, Guerezas in BSNP spend larger proportion of their time resting and feeding than engaging in socializing and moving.Considering seasonal distribution of different behavioral activities, there were significant differences in time spent on resting, socializing and moving during wet and dry seasons. These seasonal variations might be corresponding with changes in the consumption of major dietary items or food availability and environmental factors (Oates, 1994; Bocian, 1997).
Guerezas spent more time resting and less time moving during the wet season than the dry season. This probably is due to the availability of young leaves during the wet season, as a result, they spent more time digesting and fermenting after consuming young leaves (Chivers, 1994). On the other hand, during the dry season, they had to travel relatively longer distances to obtain fruits of O. rochetiana and Galiniera saxifraga, young leaves of A. dimidiata and E.capensis, and mature leaves of P. africana and Albizia schimperiana. However, there was no significant difference between seasons in activity time allocation for feeding and other activities. This is due to the consumption on easily obtainable plant materials around their home ranges (Fashing, 2001b). In general, Guerezas spend more than half of their time resting leading inactive lifestyles throughout the year (Oates, 1977; Bocian, 1997; Fashing, 2001a).
The present study showed that different proportion of the hours of daylight was used by guereza for different activities. At BSNP, Guerezas mostly begin by exposing their body to the sun in resting condition and then followed by periods of feeding and resting (Bocian, 1997). This activity pattern may be related to the guereza’s high fiber content of their diet (Oates, 1977) forcing them to spend more time on fermenting such food items in their specially designed multi-chambered stomach (Chivers, 1994). During the present study period, only one case of intragroup feeding competition was observed in one of the studied groups when individuals of the group competed to feed on flowers of Acanthus sennii during the dry season. This showed that intragroup aggression within guereza groups in BSNP was a rare phenomenon (Oates, 1977; Fashing, 2001a). Such phenomenon might occurs due to the relatively even distribution of most food items in their largely folivore diets (Fashing, 2006). In addition, smaller groups face less within group competition than larger groups (Bonaventura et al., 2008).
Classifying primate diets is usually accomplished by categorizing them from observations made over the total duration of the study, in terms of the proportion of feeding time spent consuming different plant parts (Chapman, 1987). However, categorization of primate species into folivores and frugivores is usually imprecise, given that a given species often depends on several kinds of food sources and that its diet varies locally and seasonally (Hill, 1997). Based on this categorization, Guerezas in BSNP are folivores since leaves accounted 71.6% of their overall diet. In addition, Kay and Davies (1994) noted that the physiology of the colobine gastrointestinal tract allows them to harvest abundant plant food sources such as mature leaves indicating folivory.
Members of Guereza groups in BSNP devoted more time consuming their favorite food, young leaves, rather than mature leaves. Wasserman and Chapman (2003) indicated that young leaves have more protein, low fiber content and are more easily digestible than mature leaves. They showed seasonal variation by consuming excess young leaves during the wet season as young leaves were abundant. Mature leaves were the second largest contributors of guereza diet in BSNP. These were consumed in excess during the dry season. This may be due to a relatively high consumption of mature leaves of Dombeya torrida and Prunus africana. In addition, young leaves were relatively scarce during the dry season and Guerezas fairly switch to the consumption of mature leaves.
Guerezas also spent portion of their time feeding on fruits after young and mature leaves. They spent more time feeding on fruits during the dry season due to the high availability of fruits and relatively low abundance of young leaves (Fashing, 2001b). Olinia rochetiana was the largest fruit contributor of guereza diet followed by Teclea nobilis. The fruits of Olinia rochetiana were abundant during the early dry season. Fruits make up substantial proportion of Guerezas diet (Fashing, 2001b). Bark was the next food item and consumed in higher proportion during the wet season. This may be due to the relatively higher moisture content of bark during the wet season (Bocian, 1997). In addition, wood was consumed by Guereza more during the wet season. However, flowers shoots and roots were consumed more or less in equal amount during both wet and dry seasons. But, Guerezas were never observed feeding on animal material; their diet consisted exclusively plant materials (Oates, 1977; Fashing, 2001b).
Guerezas have the most variable diets compared to other African colobines (Fashing, 2006). They heavily feed on leaves at some sites such as, the present study area; Kibale, Uganda (Harris, 2005); and Ituri, D.R. Congo (Bocian, 1997). In addition, a more varied diet of leaves and fruits or seeds at other sites was observed in Kakamega, Kenya (Fashing, 2001b). In general, the habitat in which the species lives has a profound influence on shaping its dietary niche (Xiang et al., 2007). A total of 31 plant species were consumed by Guerezas during the study period. However, 10 plant species accounted for 78.1% of the overall diet of guereza. Fashing (2001b) suggested that Guerezas are adapted to feed on relatively few food species to maintain low dietary species diversity even in species- rich rain forest environments. Considering the seasonal variation of these plant species, Teclea nobilis and Rhus natalensis were consumed more during the wet season. However, Olinia rochetiana, Prunus africana and A. schimperiana were consumed more during the dry season. This indicates that Guerezas in BSNP manage to live on easily available plant materials during both dry and wet seasons (Bocian, 1997; Fashing, 2001b).
The collected data during the present study period will provide important information on the population structure and behavioral ecology of Guerezas in Ethiopia. The total population of guereza in the study area was estimated to be 2170 individuals. Due to the presence of a variety of dietary materials during both wet and dry seasons, the population status of guereza groups in Denkoro forest is high. The vast majority of the groups were small containing only one adult male per group. Guerezas live only in the forest habitat of the present study area. Erica woodland and grassland habitats are found at a higher elevation and do not possess suitable dietary materials for Guereza. Resting and feeding are the dominant behavioral activities as the favorite dietary materials of Guereza are abundant. Hence, there are prolonged rest periods after the consumption of these food items. During the entire study period, Guerezas consumed a variety of food items from a total of 31 plant species. Young leaves, mature leaves, fruits, bark and flowers are accessed from a wide variety of plant species during the wet and dry seasons. As a result, they subsist on easily available dietary materials around their home ranges. Since Guerezas never engaged in crop raiding there was no conflict with the local people. However, the human settlements and agricultural lands are in close proximity with the park that may pose great threat to Guerezas and other animals by habitat destruction.
The authors have not declared any conflict of interests.
The authors thank the Department of Zoological Sciences, Addis Ababa University for logistic and financial support. They are also grateful to the local administrators and people who helped them during the investigation period.
REFERENCES
|
Altman J (1974). Observational study of sampling methods. Behaviour 49:227-267.
Crossref
|
|
|
|
Ayalew A, Bekele T, Demissew S (2006). The undifferentiated Afro-montane forest of Denkoro in the central highland of Ethiopia: a floristic and structural analysis. SINET: Ethiop. J. Sci. 29:45-46.
|
|
|
|
|
Bekele A, Yalden DW (2013). Mammals of Ethiopia and Eritrea. AAU Printing Press, Addis Ababa.
|
|
|
|
|
Bocian CM (1997). Niche Separation of Black and White Colobus Monkeys (Colobus angolensis and Colobus guereza) in the Ituri Forest. Ph.D. dissertation. City University of New York, New York.
|
|
|
|
|
Bonaventura M, Visioli AB, Schino G (2008). Costs and benefits of group living in primates: group size effects on behaviour and demography. Anim. Behav. 76:1235-1247.
Crossref
|
|
|
|
|
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993). Distance Sampling. Estimating Abundance of Biological Populations. London: Chapman and Hall.
|
|
|
|
|
Chapman CA (1987). Flexibility in diets of three species of Costa Rican primates, Folia Primatol. 49:90-105.
Crossref
|
|
|
|
|
Chapman CA, Chapman LJ (2002). Foraging challenges of red colobus monkeys: Influence of nutrients and secondary compounds. Comp. Biochem. Physiol. 133:861-875.
Crossref
|
|
|
|
|
Chapman CA, Fedigan LM, Fedigan L (1988). A comparison of transect methods of estimating population densities of Costa Rican primates. Brenesia 30:67-80.
|
|
|
|
|
Chiarello AG (2000). Density and population size of mammals in remnants of Brazilian Atlantic Forrest. Conserv. Biol. 14:1649-1657.
Crossref
|
|
|
|
|
Chivers DJ (1994). Functional anatomy of the gastrointestinal tract. In: Davies AG, Oates JF (Eds). Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge University Press. Cambridge, pp. 205-227.
|
|
|
|
|
Di Fiore A (2004). Diet and feeding ecology of woolly monkeys in a western Amazonian Rain Forest. Int. J. Primatol. 25:767-801.
Crossref
|
|
|
|
|
Di Fiore A, Rodman PS (2001). Time allocation patterns of lowland woolly Monkeys (Lagothrix lagotricha poeppigii) in a Neotropical terra firma forest. Int. J. Primatol. 22:449-480.
Crossref
|
|
|
|
|
Dunbar RIM (1987). Habitat quality, population dynamics, and group composition in colobus monkeys (Colobus guereza). Int. J. Primatol. 8:299-329.
Crossref
|
|
|
|
|
Dunbar RIM, Dunbar EP (1974). Ecology and population dynamics of Colobus guereza in Ethiopia. Folia. Primatol. 21:188-208.
Crossref
|
|
|
|
|
Ethiopian Wildlife and Natural History Society (EWNHS), 1996. Important Bird Areas of Ethiopia: A First Inventory. Addis Ababa: Ethiopian Wildlife and Natural History Society.
|
|
|
|
|
Fashing P (1999). The Behavioral Ecology of an African Colobine Monkey: Diet, Range Use and Patterns of Intergroup Aggression in Eastern Black and White Colobus Monkeys (Colobus guereza). Ph.D. dissertation, Columbia University, New York.
|
|
|
|
|
Fashing PJ (2001a). Activity and ranging patterns of Guerezas in the Kakamega Forest: intergroup variation and implications for intragroup feeding competition. Int. J. Primatol. 22:549-577.
Crossref
|
|
|
|
|
Fashing PJ (2001b). Feeding ecology of the Guerezas in the Kakamega forest, Kenya: the importance of Moraceae fruit in their diet. Int. J. Primatol. 22:579-609.
Crossref
|
|
|
|
|
Fashing PJ (2006). African colobine monkeys: Patterns between group-interaction. Prima. Perspect. 17:201-224.
|
|
|
|
|
Fashing PJ, Cords M (2000). Diurnal primate densities and biomass in the Kakamega Forest: an evaluation of census methods and a comparison with other forests. Am. J. Primatol. 50:139-152.
Crossref
|
|
|
|
|
Fashing PJ, Mulindahabi F, Gakima J, Masozera M, Mununura I, Plumptre AJ, Nguyen N (2007). Activity and ranging patterns of Colobus angolensis ruwenzorii in Nyungwe Forest, Rwanda: possible costs of large group size. Int. J. Primatol. 28:529-550.
Crossref
|
|
|
|
|
Grimes KH (2000). Guereza Dietary and Behavioural Patterns at the Entebbe Botanical Gardens. Ph.D. dissertation, The University of Calgary, Calgary.
|
|
|
|
|
Groves CP (2005). Order Primates. In: Wilson DE, Reeder DM (Eds). Mammal Species of the World: A Taxonomic and Geographic Reference. Johns Hopkins University Press. Baltimore, pp. 111-184.
|
|
|
|
|
Groves CP (2007). The taxonomy of the Colobinae of Africa. J. Anthropol. Sci. 85:7-34.
|
|
|
|
|
Harris TR (2005). Roaring, Intergroup Aggression and Feeding Competition in Black and White Colobus Monkeys (Colobus guereza) at Kanyawara, Kibale National Park, Uganda. Ph.D. dissertation, Yale University, New Haven.
|
|
|
|
|
Harris TR, Chapman CA (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates 48:208-221.
Crossref
|
|
|
|
|
Harris TR, Monfort SL (2006). Mating behavior and endocrine profiles of wild black and white colobus monkeys (Colobus guereza): Toward an understanding of their life history and mating system. Am. J. Primatol. 68:383-396.
Crossref
|
|
|
|
|
Hill DA (1997). Seasonal variation in the feeding behavior and diet of Japanese macaques (Macaca fuscata yahui) in lowland forest of Yakushima. Am. J. Primatol. 43:305-322.
Crossref
|
|
|
|
|
Kay RNB, Davies AG (1994). Digestive physiology. In: Davies AG, Oates JF (Eds). Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge University Press. Cambridge, pp. 229-249.
|
|
|
|
|
Kim K (2002). Colobus guereza (On-line). Animal Diversity Web.
|
|
|
|
|
Kingdon J, Struhsaker T, Oates JF, Hart JA, Groves CP (2008). Colobus guereza. In: IUCN 2008. 2008 IUCN Red List of Threatened Species.
<View>. Accessed on 28 November, 2008.
|
|
|
|
|
Kivai SM, Muoria PK, Bekele A, Ogue N (2007). Activity budget and group dynamics in Grevy's zebra on Samburu rangelands, Kenya. Discov. Innov. 19:162-169.
|
|
|
|
|
Mekonnen A, Bekele A, Fashing PJ, Lernould JM, Atickem A, Stenseth NC (2012). Newly discovered Bale monkey populations in forest fragments in southern Ethiopia: evidence of crop raiding, hybridization with grivets and other conservation threats. Am. J. Primatatol. 74:423-432.
Crossref
|
|
|
|
|
Mekonnen A, Bekele A, Hemson G, Teshome E, Atickem A (2010). Population size and habitat preference of the vulnerable Bale monkey Chlorocebus djamdjamensis in dobullu Forest and its distribution across the Bale Mountains, Ethiopia. Oryx, 44:558-563.
Crossref
|
|
|
|
|
Mittermeier RA, Robles Gil P, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, Da Fonseca GA (2005). Hotspots revisited: Earth's biologically richest and most threatened terrestrial ecoregions. Conservation International, Washington, DC.
|
|
|
|
|
Muoria PK, Karere GM, Moinde NN, Suleman MA (2003). Primate census and habitat evaluation in the Tana delta region, Kenya. Afr. J. Ecol. 41:157-163.
Crossref
|
|
|
|
|
Newton PN, Dunbar RIM (1994). Colobine monkeys. In: Davies AG, Oates JF (Eds). Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge University Press. Cambridge, pp. 311-346.
|
|
|
|
|
Oates JF (1977). The guereza and its food. In: Clutton-Brock TH (Ed). Primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys and Apes. Academic Press. New York, pp. 275-321.
Crossref
|
|
|
|
|
Oates JF (1994). The natural history of African colobines. In: Davies AG, Oates JF (Eds). Colobine Monkeys: Their Ecology, Behaviour and Evolution, Cambridge University Press. Cambridge, pp. 75-128.
|
|
|
|
|
Oates JF, Davies AG, Delson E (1994). The diversity of living colobines. In: Davies AG, Oates JF (Eds.). Colobine Monkeys: Their Ecology, Behavior and Evolution. Cambridge University Press. Cambridge, pp. 45-73.
|
|
|
|
|
Peres CA (1999). General guidelines for standardizing line-transect surveys of tropical forest primates. Neotrop. Prim. 7:11-16.
|
|
|
|
|
Plumptre AJ (2000). Monitoring mammal populations with line transect techniques in African forests. J. Applied Ecol. 37:356-368.
Crossref
|
|
|
|
|
Thomas SC (1991). Population densities and patterns of habitat use among anthropoid primates of the Ituri Forest, Zaïre. Biotropica 12:68-83.
Crossref
|
|
|
|
|
von Hippel FA (1996). Interactions between overlapping multimale groups of black and white colobus monkeys (Colobus guereza) in the Kakamega Forest, Kenya. Am. J. Primatol. 38:193-209.
Crossref
|
|
|
|
|
Wasserman MD, Chapman CA (2003). Determinants of colobine monkey abundance: the importance of food energy, protein and fibre content. J. Anim. Ecol. 72:650-659.
Crossref
|
|
|
|
|
Wong NPS, Sicotte P (2007). Activity budget and ranging patterns of Colobus vellerosus in Forest Fragments in Central Ghana. Folia. Primatol. 78:245-254.
Crossref
|
|
|
|
|
Xiang Z, Huo S, Xio W, Quan R, Grueter C (2007). Diet and feeding behavior of Rhinopithecus bieti at Xiaochangdu, Tibet: Adaptation to a marginal environment. Am. J. Primatol. 69:1141-1158.
Crossref
|
|
|
|
|
Yalden DW (1983). The extent of high ground in Ethiopia compared to the rest of Africa. SINET: Ethiop. J. Sci. 6:35-39.
|
|
|
|
|
Yalden DW, Largen MJ (1992). The endemic mammals of Ethiopia. Mammal Rev. 22:115-150.
Crossref
|
|