In 2017 and 2018, we respectively found fourteen and ten nesting pairs, of which six and three nesting pairs were with a one-egg clutch while eight and seven nesting pairs had two-egg clutches. The nesting pairs were classified as P1-17 to P14-17 and P1-18 to P10-18 with their respective nests N1-17 until N14-17 and N1-18 until N10-18. Nine nests were located along the forest edge and fifteen were inside the forests. In 2018, three pairs in 2017 (P1, P4 and P8) reused their same nests (P1, P4 and P5) and seven pairs constructed new nests (P2, P3, P6, P7, P8, P9, and P10-18). Newton (1979) stated that some large raptors do change their nests from year to year, but usually placed them near the previous years’ nests. In our study area, Madagascar Buzzards reused 30% of nesting pairs (N = 10) their previous years’ and were much lower than the same species (50%; N = 6) in Masoala (Berkelman, 1996). This difference is due to the distribution of good food sources and according to Gill (1990): when food sites are concentrated, a bird improves its success by staying in or near sites of high food density and by moving rapidly past sites of low food density. Newton (1979) stated “the habit of breeding in the same territory year after year is probably advantageous, so long as it is a good territory”. It would be better to collect more information about the fidelity to breeding areas because it shows relationship with other factors, notably good diet and quality of breeding areas. In more, for Yellow-billed Kites Milvus aegyptius at the Manambolomaty Lakes Complex PA, construction of a new nest not far from a previous nest might have a relationship with the size of the nesting territory they can defend (Andriamalala, 2005). Unfortunately, we did not observe this for Madagascar Buzzard.
In Bemanevika PA, at least 24 nesting pairs of Madagascar Buzzards were documented while Berkelman (1996) found 14 nesting pairs in Masoala Peninsula. The Madagascar Buzzard is among one of the most common and abundant raptor species in Madagascar (Thiollay and Meyburg, 1981) and this could be the factor for the high number of pairs found at these two sites. This abundancy could justify why the Madagascar Buzzard has a status as Least Concern (IUCN, 2020). During this study, nests were easily found in the rainforests of Bemanevika PA, while Berkelman (1993) mentioned that it was difficult to find nests in the lowland rainforests of Masoala Peninsula due to the steep topography and precipitation. In addition, our study documented Madagascar Buzzard nests were located at the forest edges and inside the forests of Bemanevika PA. However, in Masoala, the study area was primary lowland forest with some slash-and-burn clearings (Berkelman, 1995). These variations could be explained by several factors, including that the Madagascar Buzzard is an endemic species and prefers forests more than degraded habitat (Rene de Roland, 1994), and this species occupies a varied habitat such as savanna, forest edge, forest between edge and forest blocks in Bemanevika PA according to Razafindranaivo (2015). These findings are consistent with the characteristics of the species according to Langrand and Meyburg (1984): “the Madagascar Buzzard is reported to be common in wooded habitats throughout Madagascar”. This confirms that Buteo brachypterus frequently used the forest ecosystems and it is also a ubiquitous species. Like in Bemanevika PA, Madagascar Buzzards do not appear to be sensitive to localized habitat degradation on Masoala (Berkelman, 1996) and may be less vulnerable to the effects of forest fragmentation than species with more specialized requirements (Berkelman, 1995).
Pair formation
The observation time totaled 98.7 h during the pair formation period. In 2018, we followed pairs P1 and P2 and the pair formation period was recorded from the first week of August to the first week of September. We observed 18 copulations (n = 3 pairs), lasting from 6-10 s followed by vocalizations only emitted by females. Copulations were preceded by food provisioning and occurred at a distance from 10 to 300 m of nest trees.
Nest construction and reconstruction
Total observation time during this stage was 372 h. Nest building occurred from early August to early September. We monitored nest construction activity at N2-18 (new nest) and nest reconstruction activity at N1-18 (or N1-17) and N5-18 (or N8-17). In 2018, of 7 nests newly constructed, the nest construction period was only recorded at pair P2-18. Nest reconstruction lasted 23 and 15 days for the pairs P1-18 and P5-18. In 2018, for P2-18, it took 33 days for nest construction to be completed which was one and six days before egg laying occurred. Nesting material (sticks and twigs) was collected from nearby trees from 10-150 m of the nest tree, but only females arranged the material in the nest. Both sexes collected nesting materials. When the male returned to the nesting territory and brought a stick or twig, the female emitted a specific call. The male answered and delivered the material into the nest. Nest construction took place between 07:30 and 12:00 in the morning (n = 253 items) and in the afternoon between 12:00 and 14:30 (n = 101 items).
Of 354 nesting materials recorded, 157 were sticks and 197 fresh cut twigs with leaves. Of 157 nesting material (sticks) delivered, males contributed 72% (n = 113 items) and females delivered 28% (n = 44 items). In 2017, the males and females delivered 71% (n = 27 items) and 29% (n = 11 items), respectively. In 2018, males delivered 72.3% (n = 86 items) and females delivered 27.7% (n = 33 items). Of 197 fresh cut twigs with leaves observed, females and males delivered 80.7% (n = 159 items) and 19.3% (n = 38 items), respectively. In 2017, males and females delivered 75% (n = 44 items) and 25% (n = 11 items), respectively. In 2018, males and females delivered 82.4% (n = 126 items) and 17.6% (n = 27 items), respectively.
The observations of sticks and fresh cut twigs with leaves making up the Madagascar Buzzard nests at Bemanevika PA is in agreement with findings of several earlier studies (Berkelman, 1993, 1995), suggesting nests are made of various kinds of plant matter, including twigs, grass, lichens, and leaves (Wimberger 1984; Collias and Collias, 1984). Moreover, these authors stated certain kinds of plants apparently help combat disease and ectoparasite infection, which can be a serious problem in fouled unsanitary nests and in reused nests such as cavities and artificial nest boxes. Gill (1990) reported green vegetation seems to be particularly useful in this regard. Similar patterns have been reported by Shutler and Campbell (2007) and imply that the green vegetation eliminate odors that may attract predators to the nest but some authors also mentioned that these green vegetation could have a repulsive action against ectoparasites, by insecticide and acaricide properties. In more, these statements could be possible according our observations which more sticks and fresh cut twigs with leaves were collected, respectively during nest building and nestling periods. Also, we think the use of sticks and fresh cut twigs with leaves are related to minimizing parasite infestation and to avoid their nestlings from falling out of the nest. Therefore, monthly monitoring would be necessary to accurately determine responses to choice of sticks and twigs in relation to specific flora taxa with its insecticide and acaricide properties in Madagascar Buzzard nests.
Egg laying
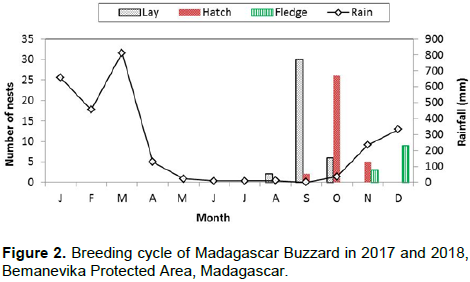
Eggs were laid during the dry season from August to October and the laying period peaked in September (n = 24 pairs) (Figure 2). The earliest laying date was 25 August 2017 while the latest was 04 October 2017. Eggs were laid within an interval of one (n = 4 nests) to three days (n = 11 nests). Eggs were mainly white; but sometimes contained red spots. Mean egg dimensions were 53.3 ± 14.6 mm in length and 42.1 ± 9.5 mm in width (n = 28 eggs). The average egg mass was 54.8 ± 12.1 g (n = 28 eggs).
Brown and Amadon (1989) stated that laying period for Madagascar Buzzard was in October, and on Masoala the laying period was from late September to early October (Berkelman, 1993). During this study, the egg laying period was from late August to early October. In Masoala, from June to August, the rainy season was still present and it may be the reason the breeding season was one month later than observed at Bemanevika PA (Rene de Roland, pers._comm.). According to Brown (1976), before and during the laying period, the female needs sufficient food to form eggs. Other than climate, the availability of prey could be a factor of the timing of the laying dates (Newton, 1979). We believe these factors could explain the difference that was observed for the initiation of the laying period between Bemanevika PA and Masoala.
Regarding clutch size, this study confirmed that Madagascar Buzzards laid one to two-egg clutches, similar to that the Mountain Buzzard B. oreophilus (Rudebeck, 1957). Our findings were also similar to those found by Berkelman (1993) on Masoala. The size of Mountain Buzzards averaged 45 cm (Birdlife International, 2016) and the Madagascar Buzzards averaged 49.5 cm (Langrand, 1995). The minimum and maximum clutch size for raptor species related to the size of each species and the environment they inhabit. In more, clutch size can be an indirect measure habitat quality, and also an indirect indicator of the physiological condition of reproductively active females (Jacobs and Jacobs, 2002). According to our observation, both proximate factors determined eggs’ number produced: Madagascar Buzzard breed in a good habitat and the female is able to produce eggs during the months when food is plentiful.
King (1973), Ricklefs (1974) and Gill (1989) confirmed the lack of food could reduce or freeze eggs productions and affect clutch size. Additionally, the maximum clutch size of two eggs for the Madagascar Buzzard is more or less small compared with other species in the same genus such as B. platyperus (Burns, 1911), B. polyosoma (Marchant, 1960) and B. buteo (Moore, 1957; Vanegue, 2015). The aforementioned Buteos laid three eggs and, having respectively a body size of 44, 48 and 49 cm, nearly similar to B. brachypterus (49.5 cm). The environment they live in is a major factor for the difference. It means Buteos in temperate climates lay larger clutches than Buteos living in the tropical environment (R. Thorstrom, pers. comm.). Also we believe that this difference is due to the body size between the species but it is a secondary factor to explain the variation. It would be better to conduct a specific study focused on relation between clutch size and foods. It could be used to elucidate the difference of clutch size for same or different Buteos species (especially B. brachypterus), from one to another region.
Incubation
We totaled 114 h of nest observations during the incubation period. We only monitored incubation activity of adults at N1-18 and N5-18. The females incubated while the males provided some incubation and all the food during nest observations. At N1-18 and N5-18, the observation times were 62 and 52 h, respectively. At N1-18, the female incubated 83.8% of observation time (n = 52 h) while the male incubated for 3.2% (n = 2 h) and the nest was unattended during 13% (n = 8 h). At N5-18, the female incubated for 75% (n = 39 h), the male 2% (n = 1 h) and the pair was absent from the nest for 23% (n = 12 h) of the observation time. The incubation period was 36.2 ± 1.1 days (range 35-38 days, n = 16 nests). At the N1-18 and N5-18, the following behavior marked the hatching period: when the first egg hatched, the females incited the males to deliver the prey item into the nest. All eggs hatched at an interval of one (n = 2 nests) to three days (n = 9 nests). Hatching occurred between the end of the dry season and the start of the rainy season, with peak hatching in October (Figure 2).
In this study, the incubation period for the Madagascar Buzzard was from 35 to 38 days and nearly the same for Buzzards on Masoala with 34-37 days (Berkelman, 1993). These results agree with Newton (1979) who stated that in raptors, the incubation period ranges from four to eight weeks. On the other hand, the variation of incubation period of the species could be due to the difference in sizes of each species. Based on this, our result and other records confirm it. In fact, our result is more or less similar compared with other species in the same genus such as Broad-winged Hawk B. platypterus (28 to 31 days) (Goodrich et al., 2014.) and Common Buzzard B. buteo with duration of 33 to 35 days (Mebs, 1964). These two species have a body size of 44 and 49 cm that are more or less similar to B. brachypterus (49.5 cm). Also, the Buteos above inhabit a temperate climate whereas the Madagascar Buzzard is found in a tropical climate (Brown and Amadon, 1968).
Nestling period
The observation time during the nestling period totaled 665 hours. During the first two weeks after hatching, the female spent 45.8% (n = 305 h) of the time brooding and feeding young while the male provisioned the female and nestlings. Males called from a tree near the nest upon arrival with food and the female took and carried the prey item to the nest. Starting at 15 days of age, adult females started spending less time brooding and feeding their nestlings. At 20 days of age, females fed and assisted young for 37.4% (n = 249 h) or perched near the nest waiting for prey deliveries for 8.8% (n = 58 h) of total observation time. Female Madagascar Buzzards brooded and fed the young while males never brooded or fed the young (Figure 3). When two nestlings were present in the nest, the older first-hatched young was more aggressive and always received the most food. The young were able to feed themselves at 35 days of age. At this period, females spent 8% (n = 53 h) of the observation time hunting. Of 347 prey items delivered to the nest (n = 11 nests), males delivered 206 and females 141 prey items for the young in the nest. The quantity of food delivered by both sexes did not differ significantly during the feeding of young (chi-square test: χ2cal = 11.34, df = 12, p = 0.5). Trail cameras recorded adult females brood the young during the night. Adult females came into the nest at 17:45 (when it started becoming dark) and went out at 5:05. This occurred from the first day of age to 25-30 days of age (n = 5 nests). After this period, the adult female rarely came into the nest or stayed near the nest during the night.

From one to two weeks after hatching (7- 14 days of age), young had black beaks, yellowish ceres and tarsus, and grey nails. During this period, young were directly fed by the female. By 12-16 days after hatching, the young actively moved around in the nest, preened and looked out of the nest. They emitted calls similar to the female, but much weaker. At 25-28 days of age, primary and secondary wing and tail feathers were emerging, and feathers around chest and abdomen were predominately complete. From 33 days of age, down feathers disappeared slowly and head, chest, wing and tail feathers were emerging. At 35 days of age, the young began feeding themselves and emitted specific repetitive calls during prey deliveries. At 38-40 days of age, the young remained on a branch within 1-2 m of the nest. Prey was delivered by both adults into the nest and the young fed themselves. The young were silent except when prey was delivered to the nest. In nests with two youngs (n = 3 nests), the first hatched young left the nest before the second hatched young.
At 45 days of age, the young were completely covered with feathers and their plumage, ceres, nails and tarsus colors were nearly close to those of adults. Three to seven days before fledging, the young exercised their wing when the wind blew. Madagascar Buzzard young fledged at an average of 48 ± 3.9 days of age (range 43-56 days, n = 17 young, N = 22 young). During the nestling period, the nesting activities differed between males and females. Females spent almost half of their time in the nest protecting and feeding the nestlings while the male provisioned food to the female and nestlings. This is typical of nearly all raptors (Newton, 1979), for example the Common Buzzard (Moore, 1957) Jackal Buzzard Buteo rufofuscus (Steyn, 1983) and Yellow-billed Kite Milvus aegyptius (Andriamalala, 2005). However, we also suspect that the activities of this hawk considerably depend on the period and provisioned food. In fact, from the third week of the nestling period, Berkelman (1993) and this study documented that the adult females spent less time at their nests and started hunting for provisioning their nestlings. According to Simmons (1983), for raptors, the time when adult females start to provision food for the nestlings depends on prey delivered by adult males. In spite of this, in our study area, the nestling period ranged from 43-56 days and was similar to those in Masoala (39-51 days) (Berkelman 1996).
During this study, the nestling period ended between mid-November and mid-January while Berkelman (1993) found the nestling period ended between early December and mid-January. The breeding season for Madagascar Buzzards coincides with the driest period of the year (Donque, 1972), and young fledge from nests at the start of the summer rains. Most species in eastern Madagascar breed between September and January (Langrand, 1990). We think the length of the nestling period may be determined by the variation of climate at each site, food availability and the number of young at the nest.
Fledging period and dispersal
From 49 -56 days of age, the young always perched in a tree from 10-30 m of the nest tree and waited for food delivered by adults. Young were fed in or near the nest until 60 days of age (n = 7 young, n = 5 nests). The young stayed within 150 m of the natal territory with their parents until 67 to 76 days old during fledging period. Prey transfers took place on the perch of female and female incited young to move between trees, at 57-65 days of age. For example, at 60 days of age, young began to fly farther from their nest sites, from 50 to 300 m.
The observation time during the post-fledging period was 145 h. During the post-fledging period, young became independent of food provided by their parents at 68 days of age. Young started catching prey from 69.7 ± 2.5 days (n = 4 young), and consequently the amount of prey delivered by adults decreased (one prey per day compared to one to three prey items per day during the first and second weeks after hatching period). Young were observed catching insects like caterpillars from leaves and branches in perching trees within their natal territory. We observed young from pairs P1-17 and P5-18 capturing insects, chameleons and a small bird. At 70 days of age, the young flew far, and adults rarely visited their nests. The restriction of prey may have stimulated the dispersal of young from natal area. For pairs that successfully fledged two young (P3-17, P5-18 and P8-18), the second hatched young became independent later than the first young. For instance, for P3-17, first young was independent at 68 days of age and second was at 77 days. Young became completely independent at an average age of 73.3 ± 3.8 days (range 68-78 days, n = 14 young).
During the post-fledging period, there was a decrease in prey deliveries by the adults, possibly forcing the young to disperse from their natal areas (Moreno, 1984; Edwards, 1985). Young Madagascar Buzzards dispersed at 73 days of age, almost similar to Jackal Buzzards (70 days of age) (Steyn, 1983).
We believe that age and morphology related to faculty of young to hunt their own prey could determine the variation of dispersion period, from one to another site. Moreover, at this age, young are able to disperse from their natal areas because they were able to capture their own prey. The ability of fledged young to catch their own prey is an indication that they are close to dispersing from their natal area as also reported for M. aegyptius (Andriamalala, 2005). The dispersion of young might have a relationship with most raptors do not feed their young once they have left the breeding area (Newton, 1979). This author also mentioned, food is not only the factor influencing dispersion but the nesting places are also involved. It means where suitable places are widespread, many species nest solitarily in contiguous or overlapping home ranges, as described; but where suitable nesting places are concentrated, pairs of the same species may have no choice but to nest close together, and range over surrounding land to feed. Newton (1979) mentioned that the young continue to be fed by their parents until they become self-sufficient. It was concluded that Madagascar Buzzard young were able to disperse in Bemanevika and with a complete development at 73 days of age.
Rand (1936) thought that the breeding cycle extended from at least July to November for this species. In the Bemanevika PA, the breeding cycle of the Madagascar Buzzard was from the last week of July to mid-January whereas in Masoala, the breeding cycle was from August to mid-January (Berkelman, 1993). The availability of prey is the most important factor for determining the breeding cycle of raptors (Newton, 1979). Furthermore, the variation of the beginning and the end of breeding cycles are determined by the locality and season (Ferguson-Lees and Christie, 2001). For another endemic Malagasy raptor, the Madagascar Harrier-hawk Polyboroides radiatus, breeding activities started August and ended January (Thorstrom and La Marca, 2000). Yet, nestling hatched when conditions were at the driest (November) and most passerines were breeding and fledging occurred when the rainy season had begun (January). Consequently, for the Madagascar Buzzard, we suspect as in all such areas, increased food availability, facilitating improved body condition and egg production are the most likely proximate factors controlling the timing of breeding (Newton, 1979).
Reproductive success
In 2017 and 2018, 39 eggs were laid in 24 nests composed of 15 nests with two-egg clutches and nine nests with a one egg clutch (Table 1). In the 24 nests, 87.2% hatched (n = 34) and 64.7% (n = 22) of the nestlings fledged. At N3-17, N5-18 and N8-18, both hatchlings fledged and all were completely independent. Overall productivity was 0.91 young fledged per breeding attempt. Overall nest success for 24 fully-documented was 79.2% (19 successful nesting attempts) in the two study years.
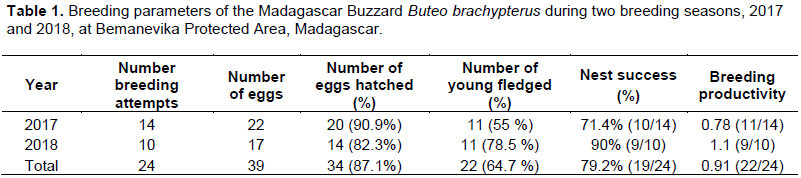
During this study, the average productivity for the Madagascar Buzzard was 0.91 young per pair (range: 0.78-1.1) for the two years combined. This result was different to that recorded from Masoala of 0.7 young per pair (Berkelman, 1996). Furthermore, we found three pairs which had two-egg clutches, hatched and fledged both young: one pairs in 2017 and two pairs in 2018. However, Berkelman (1996) noted only one young survived to fledge in each successful nest although two eggs were laid in at least four nests during his study. This could explain the difference of productivity between two sites. In fact, this study showed that the reproductive success of Madagascar Buzzard could hatch and fledged two young from the same nest. In any case, the average productivity of this species varied between 0.7 and 0.91 young per pair. This study highlighted that the nest success of Madagascar Buzzard is normal in a species that the causes of breeding failures do not affect its population productivity. Consequently, the population is suspected to be stable in the absence of any declines or substantial threats (Birdlife International, 2016).
Cause of breeding failures
Of the 15 nests with two-egg clutches, in four nests (N10-17, N2-18, N4-18 and N6-18) the second egg did not hatch (possibly addle eggs). One of nine nests with a one-egg clutch failed because of predation (N6-17). Unfortunately, the predator was not identified but the corpse of female devoured was observed on the ground near the nest tree. At N13-17, both nestlings were found dead in the nest because adult female was killed by local residents for food when the nestlings were 12 days of age. At N10-17 and N7-18 respectively, both the first and second hatched nestlings were found death probably due to the insufficiency of food causing starvation during the first week after hatching.
Siblicide was also recorded as cause of death to several second-hatched nestlings. During this study, the trail camera recorded 31,165 photos in 2017 and 96,303 photos and 707 video sequences in 2018. The photos and videos were analyzed for the presence of siblicide. Siblicide was documented at six nests (N1-17, N2-17, N4-17, N8-17, N14-17 and N1-18) with two-egg clutches. The second sibling died between 7-15 days of age, from 21 October to 23 November, and at these six nests all first hatched young survived and fledged successfully. The interval of hatching of two-egg clutches was one day for nests without siblicide (NNS, n = 6 nests) and three days for nests with siblicide (NS, n = 5 nests). Prey delivery was lower at NS than at NNS, respectively 19 and 36 prey items. The siblicide recorded for this species was facultative may or may not occur, based on environmental conditions. A case of both siblicide and cannibalism was recorded by the trail cameras at N1-18 between 24 and 25 October 2018. The adult female also increased the second nestling’s death because of her pecking attacks on it. The aggression attacks were intensified on 25 October 2018 until the young died and then the adult female fed the dead young to her first-hatched nestling (Figure 4).

We recorded siblicide as a cause of breeding failures for Madagascar Buzzard in our study area. Moreover, photos and videos recorded during this study confirmed the presence of facultative siblicide in the Madagascar Buzzard. Despite the second-hatched nestling not surviving for three nests with two-egg clutches, Berkelman (1993) didn’t suspected that siblicide occurred in the Madagascar Buzzard in Masoala National Park. However, Rand (1936); Milon et al. (1973); Langrand and Meyburg (1984), Brown and Amadon (1989); stated that Madagascar Buzzards exhibits caïnism and R. Thorstrom suspected it for Berkelman’s study at Masoala. This observation of siblicide as cause of breeding failures for this species concurs with the result of Watson et al (1999), in the Madagascar Fish Eagle (MFE) (an Accipitridae in the same family as the Madagascar Buzzard). Additionally, siblicide is common in eagles as it occurs in at least 27 out of 59 (45.7%) eagle species worldwide (Meyburg, 1978; Brown and Amadon, 1989). Ingram (1959) also stated the existence of siblicide in five species of the genus Buteo such as Common Buzzard Buteo buteo, Rough-legged Buzzard B. lagopus, Swainson’s Hawk B. swainsoni, Red-tailed Hawk B. jamaicensis and Red-shouldered Hawk B. lineatus.
Our study suggests that siblicide doesn’t only occur in Madagascar Fish Eagles and Madagascar Harrier Hawks (Polyboroides radiatus) (Thorstrom and La Marca, 2000) in Madagascar raptors. Henceforth, our result and the statement of Ingram (1959) confirm that siblicide is found in six out of 25 (24%) species of Buteos in the world. In fact, the observation of siblicide by trail cameras suggests research has an important relationship with technology, especially research on difficult to study species like raptors. For the Madagascar Buzzard, it’s interesting to check if siblicide is normal or happens occasionally throughout Madagascar, like what we recorded with trail cameras in Bemanevika PA.
Siblicide is either obligatory (a nestling is always killed by its older sibling) or facultative (apparent mortality or not of the second hatched young) (Edwards and Collopy, 1983; Mock, 1984). Our observations were of facultative siblicide with a maximum of two-egg clutches for the Madagascar Buzzard at Bemanevika PA. Watson et al. (1999) reported the Madagascar Fish Eagle was an obligatory fratricide relative to the maximum clutch size of two eggs. Therefore, we believe the maximum of clutch doesn’t determine the categorization of siblicide. In addition, raptors with facultative siblicide generally have more than two eggs per clutch (Simmons, 1988). However, the Madagascar Harrier Circus macrosceles has a maximum clutch size of three eggs, but siblicide is obligate (the third-hatched nestling always dies on 10th day of age) (Rene de Roland et al., 2004).
In Madagascar, obligatory siblicide is well documented in the Madagascar Fish Eagle (Watson et al., 1996). The second-hatched nestling always dies several days after hatching (Razafindramanana, 1995). In facultative siblicide species, the second hatched young sometimes dies (Edwards and Collopy, 1983). For the Madagascar Buzzard, we found siblicide in six of 11 nests with two-egg clutches and with both hatching. Edwards and Collopy (1983) mentioned that siblicide is related to asynchronous hatching and a high-percentage of volume difference within two-egg clutches, for two types of siblicide (obligate and facultative). In facultative siblicide species, siblicide usually occurred in relation to food restriction and egg volume difference is < 10%. Our study reported two reasons of siblicide occurrence: (1) asynchronous hatching of siblings, and (2) reduction food resources. Asynchronous hatching can be regarded as an adaptation to an unpredictable food supply, enabling all young to survive in times of plenty, but ensuring rapid reduction of the brood to an appropriate level in times of scarcity (Newton, 1979). Among medium-size raptor species of Buteo and Accipiter, attacks by older nestlings on smaller siblings occur only at times of great hunger (Balfour, 1957 and Newton, 1976). We believe that facultative siblicide in the Madagascar Buzzard does not affect its population at the moment. This species has a large distribution range and the bird prefers various habitat types, degraded to primary forests (Birdlife International, 2016).
Circumstantial evidence indicates that fratricide, in all probability invariably followed by cannibalism, occurs, far more frequently among birds of prey than is commonly reported and, indeed, in a few species is perhaps a normal, rather than an exception (Ingram, 1959). During this study, we discovered the presence of both siblicide and cannibalism, like in the Common Buzzard (Salter, 1904; Gilbert and Brook, 1924). A study on nesting biology and behavior of Madagascar Harrier Hawk Polyboroides radiatus reported the same cannibalism for this species (Thorstrom and La Marca, 2000). Hence, siblicide followed by cannibalism was not found in Madagascar Fish Eagle but only siblicide (Milon et al., 1973). Since, the Madagascar Buzzard and the Madagascar Harrier hawk are a dietary generalist while the Madagascar Fish Eagle is a dietary specialist suggesting that siblicide exists as an evolutionary breeding strategy that developed in Madagascar Buzzard. Therefore, this study reported the first case of siblicide followed by cannibalism in the community of raptors in Bemanevika PA. Based on the community of raptors, the occurrence of siblicide in the Madagascar Buzzard adds to the number of species with this behaviour in Madagascar. At present, siblicide occurs in Madagascar Fish Eagles, Madagascar Harriers, Madagascar Harrier Hawks and Madagascar Buzzards.
Food habits and hunting behavior
During this study, we recorded 541 prey items of which 312 (57.7%) were documented by trail cameras and 229 (42.3%) by direct observation for a total of 515 identified and 26 not identified. Of the 541 prey items, 28 and 513 were recorded during the incubation and nestling periods, respectively. Based on the 515 identified prey items, the diet of the Madagascar Buzzard was composed of 37.3% (192) reptiles, 35% (180) birds, 19% (98) mammals (Figure 5), 8.3% (43) invertebrates and 0.4% (2) amphibians. Reptiles were composed of 98% (188) chameleons and 2% (4) other lizards. Of the 98 mammals identified, rats made up 98% (n = 96) and lemurs 2% (n = 2) (Figure 6). The quantity of each prey type differed significantly between the two study seasons (chi-square test: χ2 = 241.7, df = 8, p = 0.0001). Madagascar Buzzards hunted alone and from a raised perch in the forest or on the ground in the savannas. In the forest, when prey was observed they flew directly at the prey. In the savanna, after a long stationary flight and when prey was spotted, they descended slowly to the ground and then dropping onto the prey grasping it with their beak or feet. Males delivered at least two preys items per day to the nest from the hatching to fledging periods. At N8-18, the female was observed delivering two small birds at the same time: one carried in a foot and the second one in the beak. The consumption of prey items varied from two seconds to five minutes (n = 904 beakfuls).
During the two breeding seasons, we recorded a variety of prey items in the Madagascar Buzzard’s diet such as reptiles, birds, amphibians, invertebrates and micro-mammals. In Masoala, micro-mammals were not reported as a prey item by this species (Berkelman, 1997). Rand (1936); Milon et al. (1973); Brown and Amadon (1989) and Langrand (1990) reported the same, as well as the existence of carrion in the diet of the Madagascar Buzzards. These previous records and our study showed that two prey categories are the most taken from several localities by Madagascar Buzzards, reptiles and birds, then followed by others prey types.
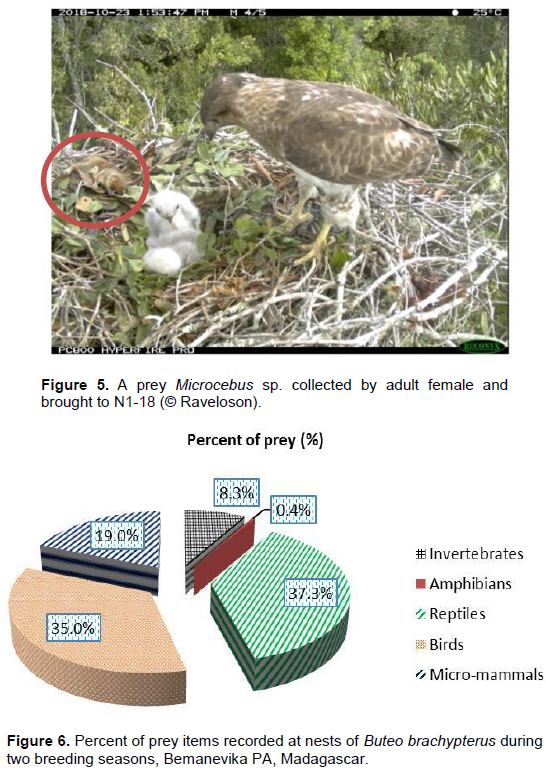
Thus, our result confirms that Buteo brachypterus is a dietary generalist species. In addition, the specific distribution of each prey species from one to another locality in Madagascar may explain this diversity of prey types for B. brachypterus. During this study, reptiles (37.3%) were predominantly chameleons (98%), and birds (35%) made up the greatest portion of the diet of Madagascar Buzzards at Bemanevika PA. Berkelman (1993) found that birds (33.2%) were taken more than chameleons (29.1%). This difference appears to be explained by prey availability and forest types at these two sites and the breeding period of chameleons seems to coincide to those of Madagascar Buzzard in Bemanevika PA (Angelinah Rene de Roland, comm.pers). In October and November, chameleons are known to lay their eggs on the ground, making them susceptible to predators (J Rabearivony, pers. obs.) like Madagascar Buzzards. Like chameleons, small fledgling birds were among the most consumed prey as they are easier to capture because of their lack of experience and flying skills. This was similar to what was observed in the Frances’s Sparrowhawk Accipiter francesiae, especially taking small fledgling passerines on Masoala (Rene de Roland, 2000b). We suggest the agility of each individual’s hunting skills and methods used could explain these differences in prey types taken by Madagascar Buzzards.