Full Length Research Paper
ABSTRACT
Sorghum is one of the most important cereal crops grown in arid/semi-arid regions of the world. Understanding and utilising the genetic variation in sorghum accessions is essential for improving the crop to adapt to abiotic and biotic constraints. Several authors have reported the loss of sorghum diversity, but there is limited available information on on-farm sorghum diversity in the major sorghum growing areas in Uganda hence limiting the utilization of diversity for improvement of the crop. This study was carried out to determine sorghum diversity on farmers’ fields in Uganda. Phenotypic data was used to assess diversity in 100 sorghum accessions collected from Northern and Eastern regions of Uganda. The accessions were phenotyped using qualitative and quantitative morphological characteristics. Phenotypic evaluation was done using 10×10 Alpha lattice design with two replications at Pakor village in Agago district. Analysis of variance revealed highly significant (p ≤ 0.01) differences for all quantitative traits measured indicating the existence of a wide genetic variation among accessions. The 100 accessions were grouped into two major clusters which were further subdivided into five sub clusters. The largest cluster had 30 accessions and the smallest cluster had five accessions. The five clusters varied with respect to plant height (202.8 to 379.9 cm), days to 50% flowering (77.3 to 163), number of leaves per plant (10.9 to 24.6), 100 grain weight (2.5 to 4.6 g) and yield (1977.4 to 3475.6 kg ha-1). The clustering patterns of accessions was not entirely based on geographic origin and/or breeding status, probably due to gene flow. This study showed the existence of wide genetic diversity within the sorghum accessions, which could be exploited in the improvement of the crop through breeding for high yielding, pest and disease resistant varieties.
Key words: Sorghum bicolor, ward clustering, morphological traits, diversity.
INTRODUCTION
Sorghum [Sorghum bicolor (L.) Moench] is the fifth most important cereal crop following wheat, rice, maize, and barley in both total production and acreage in the world (FAOSTAT, 2022). Sorghum is a staple food crop for millions of the most food-insecure poorest people in the semi-arid tropics of Africa, South Asia and Central America (Abreha et al., 2022; Girma et al., 2019). Sorghum is predominantly grown in marginal environments which are prone to water scarcity. These regions are often too dry for the cultivation of most of the other important cereal crops.
For instance, 60% of the land in Sub-Saharan Africa where sorghum is commonly grown is considered vulnerable to recurrent droughts (Hadebe et al., 2017) and 80% of sorghum cultivated in the US is grown under non-irrigated conditions, where water is a major limiting factor, which substantially reduces yield (Abreha et al., 2022). Therefore, sorghum is important for addressing the current and future food security needs for marginal environments, especially with the looming effect of climate change. In Uganda, sorghum is the third most important staple cereal crop after maize and rice mainly grown by the resource poor farmers in arid and semi-arid regions of the North and East (Olupot, 2011). The crop has multiple uses including but not limited to human consumption in form of bread, porridge and boiled grain, beer production, sugar and biofuel production (stems), feeds (leaves and stems) and as a building material (Mathur et al., 2017; Kigozi et al., 2011). High genetic diversity is vital for the development of climate resilient crop varieties to mitigate the impact of climate change and to meet the multiple uses of the crop.
Most of sorghum diversity both at phenotypic and genotypic level is concentrated from where sorghum was first domesticated (Reddy et al., 2008). Much of the genetic variability is available in Africa and Asia, which are areas of the first domestication and early introduction of the crop respectively (Reddy et al., 2008). Species and varietal diversities in smallholder farming systems are valuable for coping with environmental variability and for specific uses (Altieri, 2004). The study of these processes at the community scale is complementary to large time-space approaches and contributes to the general understanding of the in situ genesis of crop genetic patterns (Labeyrie et al., 2014). Therefore, in the search for diverse breeding materials, farmers’ cultivars or landraces are usually the major sources of genetic variation (Ghebru et al., 2002). Genetic diversity is important because it is useful in breeding programmes to produce superior varieties or hybrids with resistance to abiotic and biotic stresses (Sinha and Kumaravadivel, 2016).
The major cereals including sorghum have experienced significant reduction in genetic diversity due to the introduction of improved varieties (Petrovi? and Dimitrijevic, 2012). Other causes of genetic erosion include: emergence of new pests, weeds and diseases, environmental degradation, urbanization, conflicts and land clearing through deforestation and bush fires (Govindaraj et al., 2015). The impact of such genetic reduction has occasionally been witnessed when calamities strike such as the ug99 virus (Puccinia graminis tritici) that is threatening wheat production globally following a breakdown in resistance to stem rust (Joshi et al., 2008). Crop genetic diversity forms a foundation for sustainable agriculture and global food security, now and in future, whether crops are used in traditional farming systems, conventional breeding or in new biotechnologies (FAO,1998). If not well understood and managed, crop genetic diversity of most crops like sorghum most especially on farmers’ fields will be vulnerable to genetic erosion (FAO, 1998). Erosion of these resources results in a severe threat to the world’s long-term food security (Hammer et al., 1999). Since the crop is an important cereal and is cultivated in marginal environments, genetic diversity is very important for crop improvement (Westengen et al., 2014). A good understanding of genetic variability among the accessions on farmers’ fields will allow for selection of desirable parents for breeding programmes, systematic collections, characterisation, documentation and conservation in the national gene bank (Sinha and Kumaravadivel, 2016).
Deliberate efforts therefore, need to be put in place to prevent further loss of genetic diversity. However, to be able to put in appropriate conservation strategies, knowledge of existing diversity on the farmers’ fields is paramount. While knowledge of sorghum diversity remains a key asset for effective implementation of on-farm conservation strategies and for development of new varieties. There is no available information on the status of on-farm sorghum diversity on farmers’ fields in Uganda. Previous studies have reported the existence of significant diversity of sorghum at regional level (Andiku et al., 2021; Akatwijuka et al., 2016; Mbeyegala et al., 2012). Past studies revealed the existence of sorghum diversity at regional level by Mbeyagala et al. (2012) and Akatwijuka et al. (2016). However, in these studies the method of determining sorghum diversity was insufficient, for example Mbeyagala et al. (2012) picked only two panicles per field leaving probably other sorghum types while Akatwijuka et al. (2016) focused only in south western Uganda which is not a major sorghum producing region in Uganda. Relatedly, Andiku et al. (2021), looked at genetic diversity analysis of East African sorghum germplasm collections for agronomic and nutritional quality traits. There are however, no farm level studies that have been done to determine phenotypic diversity of sorghum. In addition, crop genetic diversity on the farmers’ fields varies from time to time. This has limited the use and conservation of sorghum diversity hence increasing the risk of losing them. A good and complete understanding of the genetic basis of target traits, in any crop, is critical to design integral breeding strategies and to implement the necessary steps for the development and release of new improved varieties.
Over the years, a number of studies have dealt with estimating genetic diversity in cultivated sorghum using morphological traits (Adugna, 2014). Measurement of morphological variation is the most easily obtained indicator of genetic diversity and does not require expensive technology (Govindaraj et al., 2015; Ngugi and Maswali, 2010). In this study, an attempt was made to determine phenotypic relationship among sorghum accessions in Northern and Eastern Uganda using both the quantitative and qualitative traits.
MATERIALS AND METHODS
Description of the experimental sites
The evaluation of sorghum accessions was carried out at Makerere University, Agricultural Research Institute, Kabanyolo (MUARIK) in Wakiso district and Pakor village in Agago district in Northern Uganda. MUARIK is located at 0°28’N and 32°37’E, and at an elevation of 1,150 m above sea level, about 17 km north of Kampala city in Wakiso district (Arnold, 1993). The area experiences bi-modal rainfall pattern with two wet seasons, one running from April to May and the other from October to November. However, for 2016, the area experienced a very long dry spell. The dry months are often January to February and July to August although there is variability. The mean annual rainfall of Wakiso district was 1320 mm per annum. The soils are generally sandy clays of high productivity. The dominant soils types are red gravely loams with occasional marram reddish brown sandy loam on red clay loam and yellowish sands with quartz gravel (Yost and Estwaran, 1990). Agago is situated at latitudes 2o 50’N and longitudes 33o 20’E, at an elevation of 1060 m above sea level in Northern Uganda.
The dominant types of soils are sandy loam, clay and clay loam which vary in colour from one location to another. Agago has annual rainfall of 1145 mm (https://en.climate-data.org as accessed on 04/08/2017). The area receives bi-modal type of rainfall with the wet season starting from March to May and a second wet season (September-November). Agago has savannah type of vegetation dominated by short grass with dotted tall trees and shrubs. The dominant grass species are Guinea, star and thatching grasses (Hyparrhenia rufa).
Agago was selected as the site of the field evaluation because of the high diversity observed during the preliminary surveys. In addition, the interest was also to use the field evaluation to learn more from farmers about diversity of sorghum. Field evaluations could not be performed in all the districts due to the resource limitation.
Materials (accessions evaluated)
One hundred accessions were collected from Northern (Agago and Apac) and Eastern (Serere) Uganda during a survey conducted in December, 2015 to January, 2016. The accessions were collected based on grain colour, type of inflorescence, glume colour and plant height using sorghum descriptor guides (IBPGR and ICRISAT, 1993). Each sample was labelled indicating the district, field number and other characteristics like inflorescence type, glume colour and grain colour. The samples were properly dried, threshed and packed in paper bags before evaluation.
Experimental set up
To determine the relationships among accessions from the different districts, field evaluation was done in Agago district and Makerere University, Agricultural Research Institute, Kabanyolo. The evaluation trials were established on 20/03/2016 and 15/03/2016 during the first rain season, respectively. The evaluation was setup using 10 × 10 alpha lattice design replicated twice during the first rainy season on 15/03/2016. The field was first sprayed with glyphosate to kill the weeds. The land was ploughed and then later harrowed. Each of the plots measuring 3 m by 2 m was planted with an accession allocated to it at random at spacing of 60 cm by 20 cm between rows and plants, respectively. Three to four seeds were sown per hill and each stand was later thinned at two weeks after emergence to one plant per hill. Weeding was done twice using hand hoes in both locations.
Data collection
Phenotypic data on various morphological traits were recorded during crop growth. Data on qualitative traits such as midrib, grain and glume colours were collected by observing the help of sorghum descriptor guides (IBGR and ICRISAT, 1993). Data on quantitative traits such as flag leaf length (FL/cm) was determined by measuring the length of the flag leaf from the sheath to the apex, flag leaf width (FW/cm) was determined by measuring the width of the flag leaf from the broadest part. Panicle length (PL/cm) and width (PW/cm) were determined by measuring the length of the panicle from the base where the branches originate up to the tip whereas width was determined by determining the width from the broadest part of the panicle. Number of leaves (No. L) and nodes were determined by counting the leaves and nodes (No. N) from each plant sampled from the base up to the last leaf or flag leaf. Internode length (IL/cm) was determined by measuring the distance between the different internodes. Three internodes were measured from both the bottom and upper most parts of each plant. Days to 50% flowering (DFL/days) was determined by counting the number of days taken by plants in a given plot from the time of planting to when half of them had flowered. Plant height (PHT/cm) was determined both at 50%flowering and at physiological maturity. Height at 50% flowering was determined by measuring the height from the base of the plant to the tip of the panicle when it has just emerged or when half of the plants had flowered whereas height at maturity was taken from the base up to the tip of the panicle at physiological maturity. For the accessions with goose neck panicles (Head shape 10), the height was measured for the straight part and added to the length of the curved part to get overall plant height at maturity. Panicle weight (PWt/g) was determined by weighing 10 panicles that were dried under sunshine for 6 days and 100 grain weight was determined by counting 100 grains and they were then weighed at 13% moisture content. Yield (kg/ha) was determined by weighing the total amount of grains harvested per plot divided by the size of the plot and multiplying it by 10000 m2
Data analysis
Data collected on both quantitative and qualitative traits during phenotypic evaluation was entered into excel and subjected to various types of analyses. Both descriptive and inferential statistical analytical tools were used in the study. Summary statistics (means, variances) were used to compare sorghum accessions from different geographical locations based on quantitative and qualitative traits. Analysis of variance was performed on quantitative traits such as plant height, flag leaf length and width, days to 50% flowering and yield using GenStat 14th Edition statistical package; and significant means were separated using Least Significant Difference (LSD) at the 0.05 probability level. Gower’s distances were used to compare the relationship among the accessions from different areas. Gower’s distance was chosen because of its ability to combine both quantitative and qualitative traits. Ward cluster analysis was performed using R-statistical software to determine the relationship among accessions from different geographical locations.
RESULTS
Quantitative traits
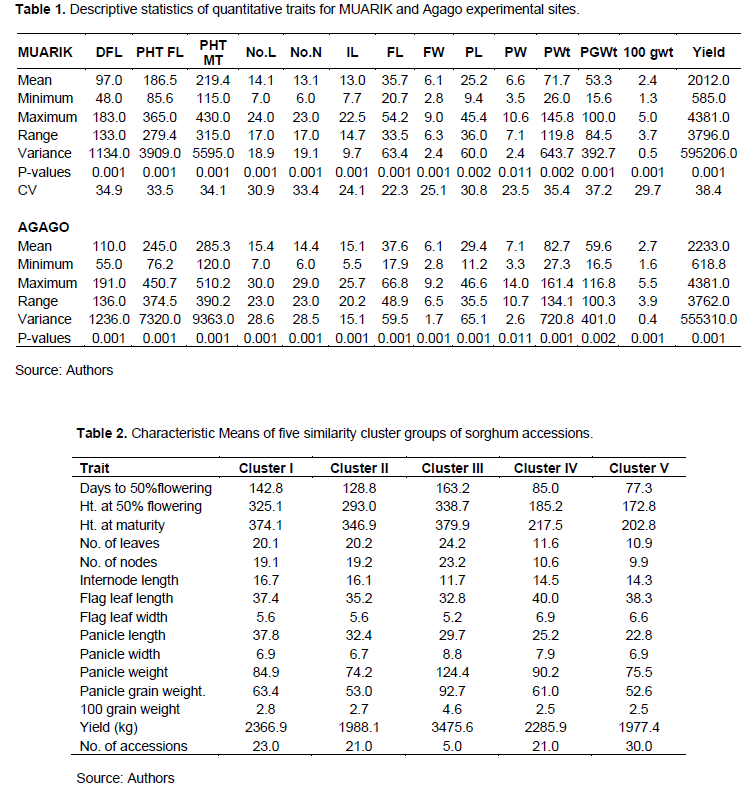
A wide range of variation (p<0.05) was observed for different quantitative traits. The results of the quantitative traits collected on the accessions such as days to 50% flowering (p<0.001), height at 50% flowering (p<0.001), height at maturity (p<0.001), number of leaves (p<0.001), number of nodes (p<0.001), internode length (p=0.011), flag leaf length (p<0.001) and width (p<0.001), panicle length and width, panicle weight, 100 grain weight (p<0.001) and yield (p<0.001) for the two locations are presented in Table 1.
The analysis for each experimental site showed that the accessions had relatively similar performance in most quantitative traits, although there were slight variations in traits such as days to 50% flowering, plant height at both 50% flowering and at maturity, panicle length, panicle width, one hundred grain weight and yield.
Qualitative traits
Morphological variation among sorghum accessions was high with regard to midrib colour, panicle compactness, glume and grain colour. Four different colours were observed for midrib ranging from white (87%), green (9%) tan (3%) and red (1%). In terms of glume colour, the majority of sorghum accessions had black (53.2%), red (46.9%) and brown (6.3%). There was wide variation in grain colour among the accessions where brown was predominant (51.7%), red (19%), white (16.8%), cream (4.2%), yellow (3.2%) and purplish-brown (3.2%). Panicle compactness (inflorescence type) varied greatly among sorghum accessions where majority had very loose drooping primary branches (40.8%) and compact elliptic type (23.5%). Other types of inflorescence observed included semi-loose drooping primary branches (12.2%), semi-compact elliptic (7.1%), half broom corn branches (7.1%), loose erect primary branches (4.1%), compact oval (2.04%), loose drooping primary branches (1%) semi-loose erect primary branches and broom corn (1%).
Phenotypic relationships and distances
Analysis using Gower’s distance and Ward clustering methods grouped the 100 accessions into two major groups. The two groups were further sub divided into five sub groups/clusters (Figure 1). In general, sorghum accessions clustered according to geographical locations with some overlaps where accessions cut across all the locations.
Among the different clusters, the cluster size varied from 5 to 30 accessions. The maximum number of accessions was included in cluster V (30) and the minimum number of accessions was included in cluster III having five accessions (Figure 1).
Cluster I consisted of 23 accessions, 21 were from Agago and two from Apac and were characterized by late flowering and second tallest (Table 2). Furthermore, accessions in this cluster had panicles with very loose drooping primary branches and the grain colour varied from brown, red and white.
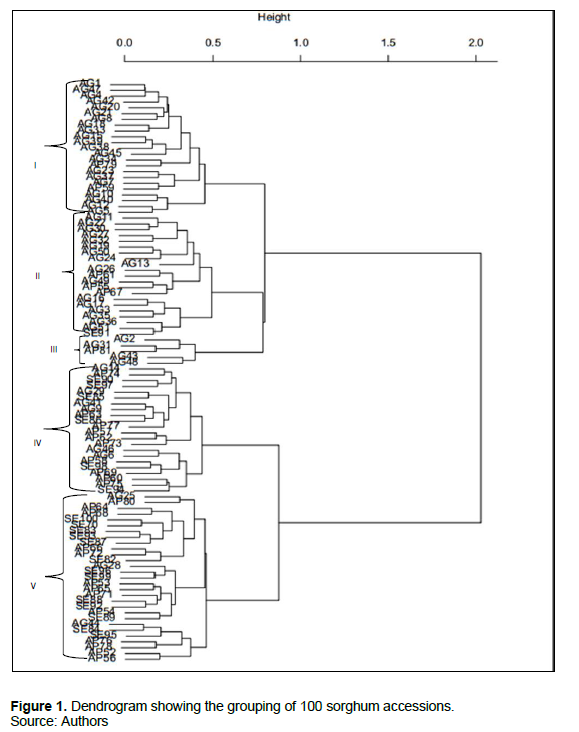
Cluster II had 21 accessions (Figure 1) out of which 17 were from Agago, three from Apac and one from Serere with varied morphological characters. All accessions in this cluster were characterized by late flowering, medium plant height, varied grain shape and colour, and very loose drooping primary branches type of panicles.
Cluster III consisted of five accessions, four were from Agago, one was from Apac and six were from Serere (Figure 1). All accessions in this cluster were characterized by early flowering, moderate height and less number of leaves (Table 2).
Cluster IV consisted of 21 accessions from all the three study areas almost in equal proportions (Figure 1) out of which seven were from Agago, six were from Serere and eight were from Apac. The cluster was characterized by moderate days to 50% flowering, largest flag leaves and lowest 100-grain weight (Table 2). Cluster V consisted of the largest number of accessions (30) out of which two were from Agago, 12 were from Apac and 16 were from Serere. The cluster was characterized by early flowering, shortest plant height and lowest number of leaves.
The cluster mean of the five similarity cluster groups in the 100 sorghum accessions are presented in Table 2. Sub cluster V had the lowest mean values for height at maturity (202.8), days to 50% flowering (77.3), lowest number of leaves per plant (10.9) and yield (1977.4). Sub clusters I, II and IV showed moderate mean values for leaf length, leaf width, height, yield, days to 50% flowering, and 100-grain weight. Sub cluster III had the highest mean values for plant height (379.9), panicle weight (124.4), panicle width (8.8), grain weight per panicle (92.7), 100-grain weight (4.6) and yield (3475.6). Based on the cluster means, the important cluster is cluster III that had the highest mean values for 100-grain weight and yield. Hence, the accessions falling under these clusters could be used as the parents for hybridization program. However, other clusters also had special attributes that can be explored in the improvement of the crop for example early flowering observed in sub clusters IV and V.
DISCUSSION
The objective of this study was to determine the phenotypic relationship among the accessions. There was wide variability in both quantitative and qualitativetraits across all the sites. Ward clustering using Gower’s distances grouped accessions into two main clusters that were further sub divided into five sub clusters with the cluster sizes ranging from five to thirty accessions.
Overall, the cluster analysis confirmed the presence of variation among accessions both in quantitative and qualitative traits. Qualitative traits such as pigmentation are very useful in differentiating accessions and selection for different breeding purposes. High significant differences, hence, high phenotypic diversity among accessions in all the traits tested have also been reported in Kenya (Ngugi and Maswili, 2010). Sinha and Kumaravadivel (2016) reported significant variation for all 14 quantitative traits investigated on 40 accessions in Tamil Nadu, India. The Agago accessions were tallest, had the highest number of leaves and they took too long to reach to 50% flowering and they formed the biggest number of sub clusters I, II, III, IV, whereas sub cluster V was dominated by accessions from Apac and Serere and were mostly short, had lowest number of leaves and also reached 50% flowering early. Ngugi and Maswili (2010) observed that the Turkana accessions that were tallest had more leaves, nodes and took longest to mature.
The significant mean values for quantitative traits such as plant height, 100 grain weight, yield and panicle weight obtained in Agago were generally much higher than at MUARIK although the accessions that performed well or poorly maintained the same performance across the two experimental sites. The differences for some of the characters indicated that the conditions in the two locations were not similar in many cases. For example, differences in rainfall, fertility of the soil as well as pests such as birds that could have reduced the yield of the accessions at MUARIK. Flowering is largely influenced by prevailing temperature and it occurs soon after panicle emergence (booting stage). However, despite the influence of the environment on the quantitative traits, the qualitative traits such as glume colour, colour of the seed, midrib colour and type of inflorescence remained the same throughout the two locations indicating that the environment less affects the qualitative traits. Therefore, the expressions for most of these characters such as yield, days to 50% flowering, height, panicle weight and pigmentation were genetic which can be explored in the improvement of sorghum through breeding.
The higher variance values obtained in both locations on the measurement of the quantitative traits can be attributed to high variability among the accessions. The large difference in quantitative characters among sorghum accessions in two locations is attributed to the differences in rainfall recorded in the growing seasons. Water demand is high for plants at germination, booting and flowering compared to during grain filling and ripening. Dry spells at the beginning and during the growing season are usually detrimental on sorghum and millet (Kudadjie et al., 2007). Bello et al. (2007) observed that high error or environmental variance estimate for some characters similar to what was obtained similar to this study. One way of increasing precision and reducing error could be by increasing the sample size and performing multi-locational trials, although this may be costly. Comparative performance among the sorghum accessions across two experimental sites proved that some accessions were superior to others in some attributes such as yield, 100-grain weight but other accessions were also more superior as far as attaining 50% flowering was concerned. Good scope exists among the accessions ranging from high yielding, early maturing as well as for forage. Depending on the breeding objectives, there was a wide range of accessions/ cultivars to choose from.
The clustering pattern of 100 accessions showed the existence of significant amount of variability among the sorghum accessions grown on farmers’ fields in northern and Eastern Uganda. The wide variability exhibited by the accessions in both quantitative and qualitative traits on farmers’ fields in Northern and Eastern Uganda is an indication of wide opportunities for improvement of sorghum in Uganda. The results in this study suggest that diversity of the sorghum accessions were structured more on geographical locations and based on quantitative traits like plant height, days to 50% flowering, yield, panicle length, width and 100 grain weight and qualitative traits like grain colouration than agro-ecological conditions. For example, sorghum accessions from Agago were grouped together in cluster I, while cluster V had a majority of accessions from Serere and Apac, which was an indication of their similarity in characteristics. The clustering of sorghum accessions based on geographical locations was distinct with some overlaps where accessions cut across the locations. These overlaps among sorghum accessions during clustering indicate that, there was relatedness among these accessions. Several factors could have contributed to the detected patterns for example seed exchanges among farmers and on-farm participatory trials conducted by National Agricultural Research Organisation. The predominantly autogamous breeding system can contribute towards explaining patterns of genetic diversity and structure observed. Secondly, environmental, biological, cultural, and socio-economic factors all play a role in farmer’s decisions to choose or keep a particular sorghum cultivar at any given time. Farmers make decisions on how much of each accession to plant, the percentage of seed or germplasm to save and the percentage to buy or exchange from other sources. Each of these decisions affects the genetic diversity of crop cultivars and is linked to a complex set of environmental and socio-economic influence.
There was a close relationship among the Agago accessions that can be attributed to sharing of accessions. This pattern of genetic relationships where accessions from the same region were genetically similar could also be attributed to existence of variety exchange patterns of such accessions between relatives or friends in the communities. Reports of other studies in sorghum accessions have shown grouping primarily on the basis of origin and clustering within groups as driven by racial classification (Sharma et al., 2010). The morphological diversity was also observed within each region and was distributed with geographical origin using Sudanese sorghum landraces (Grenier et al., 2004). The main evolutionary forces responsible for producing genetic structure in plant populations are gene flow, selection associated with environmental heterogeneity and/or farmer preferences and random genetic drift (Muui, 2014).
Sorghum is primarily a self-pollinated crop resulting in a low level of observed heterozygosity, but the gene pool as a whole maintains a high level allelic variation. The low heterozygosity was a clear indication that the accessions were homozygous and thus high level of stability within the population. Although sorghum is self-pollinated, it also out crosses 7 to 30%, cross pollination could have occurred between different accessions within farmers’ fields resulting into crosses that have related similarities in different locations. The accessions within a certain region also showed variability within themselves, for example accessions from Agago, Serere and Apac were clustered into more than one group. This is an indication of variability among the accessions. According to Sinha and Kumaravadivel (2016) citing Geleta and Labuschagne (2005), morphological variation was found among sorghum accessions collected from Eastern parts of Ethiopia using 10 morphological traits and variation among the sorghum germplasm.
In general, the findings of this study reveal the existence of great diversity among the sorghum accessions grown on farmers’ fields in Northern and Eastern Uganda. Introduction of new sorghum varieties and landraces through breeding and human transportation may have caused higher sorghum diversity in Northern and Eastern Uganda. This is particularly true as more diversity was observed among Agago accessions and Agago being located closer to the border with South Sudan that is one of centers of domestication of Sorghum, there is a possibility that, through human movement some landraces were introduced into the country hence contributing to high variability. On the other hand, Serere being close to the National Research Station, less variability of sorghum accessions was observed and this can be attributed to replacement of traditional varieties with improved varieties.
CONCLUSION
High levels of diversity were observed which provide farmers and plant breeders with options to develop, through selection and breeding, new and more productive varieties that are adapted to changing environments.
Sorghum accessions generally clustered based on their geographical regions. Most of the diversity resided in individuals within a population. The results from this study suggest need for collection strategies of accessions for conservation with possible focus on the Agago and Serere accessions because of the high levels of diversity and uniqueness.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The Regional Universities Forum for Capacity Building in Agriculture (RUFORUM) funded this study (Grant No: RU 2015 GRC-134).
REFERENCES
|
Abreha KB, Enyew M, Carlsson AS, Vetukuri RR, Feyissa T, Motlhaodi T, Ng'uni D, Geleta M (2022). Sorghum in dryland: morphological, physiological, and molecular responses of sorghum under drought stress. Planta 255(1):1-23. |
|
|
Adugna A (2014). Analysis of in situ diversity and population structure in Ethiopian cultivated Sorghum bicolor (L.) landraces using phenotypic traits and SSR markers. SpringerPlus 3(1):1-14. |
|
|
Akatwijuka R, Rubaihayo PR, Odong TL (2016). Genetic diversity among sorghum landraces of southwestern highlands of Uganda. African Crop Science Journal 24(2):179-190. |
|
|
Altieri MA (2004). Linking ecologists and traditional farmers in the search for sustainable agriculture. Frontiers in Ecology and the Environment 2(1):35-42. |
|
|
Andiku C, Shimelis H, Shayanowako AI, Gangashetty PI, Manyasa E (2021). Genetic Diversity Analysis of East African Sorghum (Sorghum bicolor [L.] Moench) Germplasm Collections for Agronomic and Nutritional Quality Traits. Heliyon 8(6):e09690. doi:10.1016/j.heliyon.2022.e09690. |
|
|
Arnold C (1993). Agro-meteorological Data for Makerere University Agricultural Research Institute, Kabanyolo (MUARIK), Uganda. |
|
|
Bello D, Kadams AM, Simon SY, Mashi DS (2007). Studies on genetic variability in cultivated sorghum (Sorghum bicolor L. Moench) cultivars of Adamawa State Nigeria. American-Euriasian Journal Agricultural & Environment Science 2(3):297-302. |
|
|
Food and Agriculture Organization (FAO) (1998). The Second Report on the State of the World's Plant Genetic Resources for Food and Agriculture Organization of the United Nations, Rome, Italy. (Accessed on 14th March 2016). Available at: |
|
|
FAOSTAT (2022). Database of agricultural production. Rome: Food and Agriculture Organisation of the United Nations. (Accessed on 4th July 2022). Available at: |
|
|
Ghebru B, Schmidt R, Bennetzen J (2002). Genetic diversity of Eritrean sorghum landraces assessed with simple sequence repeat (SSR) markers. Theoretical and Applied Genetics 105(2):229-236. |
|
|
Girma G, Nida H, Seyoum A, Mekonen M, Nega A, Lule D, Dessalegn K, Bekele A, Gebreyohannes A, Adeyanju A, Tirfessa A (2019). A large-scale genome-wide association analyses of Ethiopian sorghum landrace collection reveal loci associated with important traits. Frontiers in Plant Science P 691. |
|
|
Govindaraj M, Vetriventhan M, Srinivasan M (2015). Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genetics Research International 2015:431487. |
|
|
Grenier C, Bramel PJ, Dahlberg JA, El-Ahmadi A, Mahmoud M, Peterson GC, Rosenow DT, Ejeta G (2004). Sorghums of the Sudan: analysis of regional diversity and distribution. Genetic Resources and Crop Evolution 51(5):489-500. |
|
|
Hadebe ST, Modi AT, Mabhaudhi T (2017). Drought tolerance and water use of cereal crops: a focus on sorghum as a food security crop in Sub-Saharan Africa. Journal of Agronomic Crop Science 203(3):177-191. |
|
|
Hammer K, Diederichsen A, Spahillari M (1999). Basic studies toward strategies for conservation of plant genetic resources. In Technical Meeting on the Methodology of the FAO World Information and Early Warning System on Plant Genetic Resources, Prague (Czech Republic), 21-23 Jun 1999. |
|
|
IBPGR, ICRISAT (1993). Descriptors for sorghum [Sorghum bicolor (L.) Moench]. International Board for Plant Genetic Resources, Rome, Italy. |
|
|
Joshi AK, Mishra B, Prashar M, Tomar SMS, Singh RP (2008). Ug99 race of stem rust pathogen: challenges and current status of research to sustain wheat production in India. Indian Journal of Genetics and Plant Breeding 68(3):231. |
|
|
Kigozi J, Byaruhanga Y, Kaaya A, Banadda N (2011). Development of the production process for sorghum ice-cream cones. Journal of food Technology 9(6):143-149. |
|
|
Kudadjie CY, Struik PC, Richards P, Offei SK, Atengdem P (2007). Understanding variation in sorghum through with-farmer experimentation. International Journal of Agricultural Sustainability 5(2-3):124-139. |
|
|
Labeyrie V, Deu M, Barnaud A, Calatayud C, Buiron M, Wambugu P, Manel S, Glaszmann JC, Leclerc C (2014). Influence of ethnolinguistic diversity on the sorghum genetic patterns in subsistence farming systems in Eastern Kenya. PLoS One 9(3):e92178. |
|
|
Mathur S, Umakanth AV, Tonapi VA, Sharma R, Sharma MK (2017). Sweet sorghum as biofuel feedstock: recent advances and available resources. Biotechnol Biofuels 10:146. |
|
|
Mbeyagala EK, Kiambi DD, Okori P, Edema R (2012). Molecular diversity among sorghum (Sorghum bicolor (L.) Moench) landraces in Uganda. International Journal of Botany 8(3):85-95. |
|
|
Muui CW (2014). Identification and characterization of sorghum (Sorghum bicolor (L.) Moench) landraces and improvement of on-farm seed production in eastern Kenya (Doctoral dissertation, Kenyatta University). |
|
|
Ngugi K, Maswili R (2010). Phenotypic diversity in sorghum landraces from Kenya. African Crop Science Journal 18(4):165-173. |
|
|
Olupot JR (2011). Genetic analysis of Striga hermonthica resistance in sorghum (Sorghum bicolor) genotypes in Eastern Uganda. PhD thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa. |
|
|
Petrovi? S, Dimitrijevi? M (2012). Genetic erosion of diversity in cereals. Genetika 44(2):217-226. |
|
|
Reddy BV, Kumar AA, Reddy PS, Elangovan M (2008). Sorghum germplasm: diversity and utilization pp. 153-169. |
|
|
Sharma R, Deshpande SP, Senthilvel S, Rao VP, Rajaram V, Hash CT, Thakur RP (2010). SSR allelic diversity in relation to morphological traits and resistance to grain mould in sorghum. Crop and Pasture Science 61(3):230-240. |
|
|
Sinha S, Kumaravadivel N (2016). Understanding genetic diversity of sorghum using quantitative traits. Scientifica 2016:8. |
|
|
Westengen OT, Okongo MA, Onek L, Berg T, Upadhyaya H, Birkeland S, Khalsa SDK, Ring KH, Stenseth NC, Brysting AK (2014). Ethnolinguistic structuring of sorghum genetic diversity in Africa and the role of local seed systems. Proceedings of the National Academy of Sciences 111(39):14100-14105. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0