Full Length Research Paper
ABSTRACT
Forest elephants are nocturnal and elusive animals, making it difficult to perform direct observations on them. Data on elephants’ diet and feeding habit are lacking despite most forest elephants’ habitats being lost to anthropogenic activities; yet such knowledge may be important for their conservation, particularly in a human dominated landscape. Local ecological knowledge and field investigations were combined to assess diet composition and feeding habit of forest elephants in Campo-Ma’an landscape. The study also aimed to evaluate the level of concordance between the two approaches. The study reports that forest elephants in Campo-Ma’an feed on 87 plants species, including crops. Twenty-two of these plant species were reported by both methods, most of them being potential drivers of human-elephant conflict as they are simultaneously used by humans and elephants. Also, field investigations revealed that, to satisfy their energy requirements, forest elephants relied mostly on leaves and fruits during the wet seasons and mostly on barks from trees during the dry seasons. Overall, the two methods appeared to be complementary, despite field investigations yielding fewer species, as we only covered the park partially. We suggest that combining both methods could be a cost-efficient way to address forest elephants ecological and management questions.
Key words: Indigenous knowledge, Loxodonta cyclotis, plants species consumed, traditional knowledge.
INTRODUCTION
Forest elephants (Loxodonta cyclotis) are now classified as critically endangered (IUCN, 2021) and up to 57.4% of their potential range is found outside protected areas (Wall et al., 2021). Indeed, landscape modification can be critical for wide-ranging elephants whose existence depends on habitat condition (Doumenge et al., 2021; Koirala et al., 2016; Mmbaga et al., 2017). Elephants are generalist feeders (Choudhury et al., 2008) with large body mass, and therefore need large range to collect their food (Biru and Bekele, 2012) and can spend up to 18h per day searching for food (Campos-Arceiz and Blake, 2011; Jin et al., 2006; Leggett, 2009; Sach et al., 2019). It may be a challenge to satisfy their needs in an environment where habitat is increasingly being lost, resulting in reduced food availability for elephants (Koirala et al., 2016). Accordingly, they feed on different biological types of plants ranging from roots/tubers and grasses to trees of different species, depending on the seasons and the ecosystems (Biru and Bekele, 2012; De Boer et al., 2000; Koirala et al., 2016; Kouamé et al., 2011). The bulk of elephant’s diet comes from leaves (Kabigumila, 1993; Short, 1981) and fruits (Blake and Inkamba-Nkulu, 2004; Campos-Arceiz and Blake, 2011; White, 1994). However, various proportions of roots, barks, stems, branches, twigs, and flowers are also consumed by elephants (Biru and Bekele, 2012; Kabigumila, 1993; Koirala et al., 2016; Short, 1981; White et al., 1993). Forest elephants have been reported feeding on more than 500 plant items in Ndoki National Park, Congo (Blake, 2002), 307 food items in the Lopé Reserve, Gabon (White et al., 1993), and on at least 33 fruiting tree species in Odzala National Park, Republic of Congo (Maurois et al., 1997). Moreover, preferences for some key tree species such as Sacoglottis gabonensis (Ngama et al., 2019; White, 1994), Irvingia gabonensis, Pseudospondias macrocarpa, Ballonella toxisperma, Dusboscia macrocarpa, Parinari excelsa (Blake, 2002; Campos-Arceiz and Blake, 2011; Maurois et al., 1997; Ngama et al., 2019) have been reported. In Cameroon, studies on diet and feeding habit of elephant are very limited and mostly described for savanna elephants with Tchamba (1996) and Foguekem et al. (2011) reporting respectively 45 and 20 plant species consumed by savanna elephants in the Waza Logone area. As for forest elephants, Tchamba and Seme (1993) reported 22 fruiting tree species as part of their diet in the Santchou Reserve. Primary data on diet and feeding habit of forest elephants from direct observations can be challenging to obtain due to their low population density, nocturnal lifestyle, and their elusive nature (Kambissi, 2010; Service et al., 2014). However, local people might have knowledge from their long-time interactions with nature (Service et al., 2014). This has prompted the use of alternative and less invasive methods, such as Local Ecological Knowledge (LEK) in elephant ecology (Biru and Bekele, 2012; Buchholtz et al., 2020). LEK provide reliable, timely and cost-effective data from communities living nearby and interacting with nature (Albuquerque et al., 2021; Allendorf et al., 2020; Brittain et al., 2020; Buchholtz et al., 2020; Pan et al., 2016; Service et al., 2014). LEK surveys can reduce the risk of research equipment such as camera traps being stolen (such as, Caravaggi et al., 2017).
LEK surveys can facilitate the rapid understanding of threats to wildlife, resulting in faster decision-making (Albuquerque et al., 2021; Buchholtz et al., 2020; Haenn et al., 2014). For example, LEK has been used for rapid assessment of the status and threats to pangolin (Manis pentadactyla) (Nash et al., 2016) and to study range shift of grizzly bear (Ursus arctos horribilis) (Service et al., 2014), wildlife presence and abundance, and identification of areas where conservation actions are needed (Allendorf et al., 2020), occupancy and distribution of wildlife (Haenn et al., 2014; Service et al., 2014), and even to study elephant diet in Ethiopia (Biru and Bekele, 2012) and to predict landscape use by elephants in Botswana (Buchholtz et al., 2020). Nevertheless, LEK remains an undervalued source of information for diet and feeding habit of forest elephants. While it has been used in Botswana to model the land use pattern by savanna elephant (Buchholtz et al., 2020), in the entire Congo Basin, study on elephant diet and feeding habit using LEK is limited. In Cameroon, interviews and field investigations were recently used in Nki National Park to study forest elephants feeding pattern (Ndi et al., 2022). LEK has been combined with occupancy analyses to study the reliability and suitability of LEK in rapid assessments of forest elephants’ occupancy in timber logging concessions (Brittain et al., 2020) and LEK studies focused mainly on pangolins. Indeed, Fopa et al. (2020) assessed local ecological and traditional medicine knowledge of pangolins, (Smutsia gigantea, Phataginus tricuspis, Phataginus tetradactyla) as well as the level of conservation awareness amongst local people around Deng-Deng and Mpem et Djim National Parks, whereas Simo et al. (2020) used LEK to tailor camera traps surveys to improve the detectability of pangolin. Similarly, Mouafo et al. (2021) investigated local peoples’ knowledge of pangolin presence, perceptions of population trends, cultural importance, consumptive, and non-consumptive uses, as well as hunting of pangolins. In the CMTOU, studies referring to food for elephants focused on food crop damaged by wildlife including forest elephants using indirect observation methods such as interviews and field visits (Eyebe et al., 2012; Ole, 2011). Much is known about savanna elephant diet, particularly with respect to plant species consumed, their diversity and distribution, their feeding habit, and more importantly the impacts of seasons on their ranging behavior (Blake, 2002). Indeed, forest ecosystem is more diverse, and therefore offers more fruits and other plant items that make up the diet of forest elephants. Moreover, forest habitats are generally not subject to water and mineral shortage as it is the case in savanna (Blake, 2002). To know the elephants’ diet and feeding habit in the perspective of sustainable food supply is an important conservation goal especially in areas where land-use change can cause loss of key resources (Puyravaud et al., 2019). Campo-Ma’an conservation area is plagued by increasing degradation of wildlife habitat, which would be better known to communities living nearby but less understood by scientists and decision makers. To fill this gap, this study combines LEK and field surveys to assess elephant’s diet composition and feeding habit in Campo-Ma’an conservation area. Specifically, we will (1) assess which plant species and parts are reported by local population as being consumed by elephants, (2) determine through field investigations which plant species and plant parts have signs of browsing by elephants, (3) assess the level of co-occurrence in terms of plant species between the two methods as well as their relevance and, (4) identify the influence of the seasons on field surveys of feeding habit. LEK is powerful at the local scale in understanding the resources used by elephants as people interact or share resources with them (Puyravaud et al., 2019). Therefore, the most reported plant species by LEK method are expected to be confirmed or validated by field surveys, or vice versa.
METHOD
Study area
The Campo-Ma’an National Park (CMNP), 264,064 ha, and its peripheral zone covers about 770,000 ha. There are about “111,000” inhabitants from six main native ethic groups and 17 other ethnic groups. This area is located between 2°10’N, 9°50’E and 2°25’N, 10°48’E, in the Southern Region of Cameroon (Figure 1). The climate is coastal equatorial characterized by two dry seasons and two rainy seasons. The mean annual precipitation is about 2500 mm and the mean temperature is 25°C. Many streams, river branches and swampy areas make the study area water rich (MINFOF, 2014; Tchouto, 2004). The vegetation consists mainly of old secondary forest, but patches of primary forest of the dense humid evergreen type still occur and the area has a high level of endemism and plant species diversity. There are about “2,297” vascular plant species and ferns of which 29 species are endemic to the conservation area (MINFOF, 2014; Tchouto, 2004). About 249 plant species are Non-Timber Forest Products and 112 trees species are commercially logged (such as, Lophira alata, Erythrophleum ivorense, Guibourtia ehie, Pterocarpus soyauxii, Piptadeniastrum africanum, Dalium bipindensis, Lovoa trichilioides). Logging opens the forest, giving way to the growth of pioneer tree species such as Alchornea cordifolia, Anthocleista shweinfurthii, Bridelia micrantha, Harungana madagascariensis, Musanga cecropioides, Trema occidentalis and Macaranga spp., species on which herbivores rely for food (Bekhuis et al., 2008; Tchouto et al., 2009). In degraded areas, herbaceous species such as Chromolaena odorata, Lycopodiella cernua, Nephrolepis bisserata, Selaginella myosurus are generally found surrounding woody trees left standing in secondary vegetation. Maranthaceae, Costaceae, and Zingiberaceae families are mostly found along the abandoned logging paths and swamps (Tchouto, 2004). The area harbors threatened wildlife species, among which the forest elephant population, estimated at 544 [425-695] individuals (Nzooh-Dongmo et al., 2015).
Data collection
Open field with high visibility favors direct observation in diet studies (Biru and Bekele, 2012; Sach et al., 2019; Tchamba et al., 2014; Weladji and Tchamba, 2003). In forest area, visibility is limited by the dense vegetation and thick foliage, making it difficult to spot elusive and low-density species such as forest elephant (Blake, 2002; White et al., 1993). Here we use (1) semi structured interviews for LEK and (2) field investigations to assess diet composition and feeding habit.
Local ecological knowledge surveys
LEK data were collected from June through August 2018 using key informant interviews (village chiefs) and questionnaires to villagers. From the 162 villages surrounding the park, 54 village chiefs authorized us to carry out the research in their hamlet. When we later came back to administer the questionnaires, we only found people in 23 villages, from which 98 households were interviewed based on their willingness to take part to the research, which they confirmed by reading and signing the consent form (Supplementary material 1). Efforts were made to interview heads of households, their wives or any adult male and female (>18 years old, the adulthood age in Cameroon (Patrice, 2019)) due to their high likelihood of encountering elephants during wood logging, farming, hunting, and gathering activities (Buchholtz et al., 2020; Tiani et al., 2005) or their ability to learn from their seniors or parents (Gilchrist et al., 2005). Also, women (27%) were interviewed because of their participation in game hunting and gathering activities (Martin et al., 2020; Tiani et al., 2005). Interviews were conducted in French wherever possible, as most people were fluent in French. In one instance, the respondent, a Bagyeli household, did not speak French, and we used a local interpreter. The interview consisted of semi-structured questionnaire similar to Granados and Weladji (2012) during which the respondent answered questions about elephant food habit, local or commercial names of plant species and/or parts consumed (foliage, root/tubers, stems, barks, and fruits), and the corresponding season (wet or dry) (Biru and Bekele, 2012; Koirala et al., 2016). Because the scope of the study was broader, involving human-elephant interactions, each interview lasted about 45 min.
Field surveys
Twenty transects of 2.5 ha each (500 x 50 m) were surveyed for a total coverage of 50 ha/month during 12 consecutive months from June 2019 to May 2020. Transects were delimited with discrete markers and all woody plant species examined for bark-stripping. Following Koirala et al. (2016), opportunistic surveys on food plants were also carried out each month along the tracks leading to the transect locations. Elephant feeding sites can be identified by tracks or food scraps. Conspicuous feeding such as uprooting or breaking plants stems and branches, pulling down climbing plants or stripping leaves are some characteristics of elephants feeding sites (Biru and Bekele, 2012; Campos-Arceiz and Blake, 2011; Koirala et al., 2016; Short, 1981; White et al., 1993). However, it was not always easy to disentangle elephant browsing signs from those of other herbivores. Therefore, additional steps were taken, such as assessing the presence of elephants’ footprints, identifying fresh elephants’ dung piles near leaves, stems, and fruits with signs of consumption, or by visually assessing and characterizing the impact on the damaged plant (Koirala et al., 2016). Visual and physical investigations of dung piles using a stick were also performed whenever possible to identify undigested seeds (Biru and Bekele, 2012). Caution was taken to avoid reconsideration of debarking signs during consecutive monitoring of transects whereas all other observations were considered independent from the previous visits. Plant parts were identified with local, commercial and/or scientific names to at least the genus level with the help of field assistants when necessary and recorded along with parts eaten, the day and month of the observation. Where specimens could not be identified in the field, they were collected and later identified at the Cameroon National Herbarium.
Validation of some consumed plants
In addition to transects, 9 camera traps were placed under identified fruiting trees (such as, 5 Saccoglottis gabonensis, 2 Tieghemella africana and 2 D. macrocarpa) reported as preferred fruit trees during LEK surveys and were active from May 2019 to July 2020, 24 hours/day. Stations were chosen based on prior knowledge of the area by a team of four field assistants (3 local trackers/hunters, and 1 forest warden) able to identify trees and areas potentially or known to be used by elephants. Herbivores in forest are likely to use road verges to browse (Bekhuis et al., 2008), or fruiting trees as feeding sites (Blake and Inkamba-Nkulu, 2004). Accordingly, camera traps were set 80 to 150 cm in height, angled horizontal and approximately 5 to 15 m away from target features (such as, roads, fruiting trees). The quiet period was set to three seconds for photos (that is the trigger delay between consecutive photos) and a maximum of 60 seconds for videos. Camera trap photos and videos were date and time stamped.
Data analysis
Data from LEK surveys and field investigations were verified for spelling of local names and cross tabulated with one plant part per row. Botanists were consulted to identify unknown species. The local or commercial names of the plants reported eaten by elephants were searched for scientific names using Vivien and Faure (2011) or the Plant Resources of Tropical Africa database (https://www.prota4u.org/database/) mostly for non-commercial species. Scientific names were reported following Angiosperm Phylogeny Group classification system (The Angiosperm Phylogeny Group, 2009). For species that were still unidentified, we consulted the National Herbarium using dried plant samples or images. After this stage, any remaining unidentified species was removed from the list. Data were grouped into taxonomic family, scientific names, local name, and parts consumed (Biru and Bekele, 2012), and biological types. For field investigations, plant parts with signs of elephant browsing during each monthly field visit were considered independent observations. In this study, while elephants’ diet refers to the plant species known as consumed by elephants, feeding habit refers to the variety of plant parts and proportions, on which elephants rely for food on a seasonal basis. Diet composition, which refers to different plant species providing food for elephants were identified and grouped by taxa, biological types and feeding habit, which is the distribution of different plant parts eaten over time (stems, leaves, barks, fruits, tubers). Data were grouped into a contingency table and relative frequency of feeding signs was calculated for each plant part, and subject to chi-square analysis. When a cell in the contingency table had only a small number of counts, Fisher exact tests were used instead. We also assessed co-occurrence between the LEK and the field survey approaches by comparing the diet and the pattern of feeding habit obtained from each method. Images or videos of elephants feeding on plant species were examined for identification and validation of plant species and parts eaten (such as, barks or fruits), or sampled during the next field trip for further identification or validation. All statistical analysis were performed using R v. 3.6.3 (R Core Team, 2020), with a 95% level of significance.
SUPPLEMENTARY MATERIAL 1
CONSENT FORM
_________________________________________________________________________
HUMAN-WILDLIFE CONFLICT IN THE CAMPO-MA’AN TECHNICAL OPERATIONAL UNIT, SOUTHERN CAMEROON
By: ______________, PhD student, ___________ University. Contact: __________
Preamble:
This questionnaire is designed for research on “the human-wildlife conflicts” in your community, carried out by me, _________________________.
The research aims are to: (1) assess the socio-economic impact of the human-wildlife interactions around CMNP; (2) study the relationship between different stakeholders that is park staff, local people, the private organizations as well as the non governmental organizations operating in the area; (3) Assess people’s attitudes and perceptions towards wildlife, the park and the wildlife legislation; (4) Study some ecological aspects of the elephants including testing some mitigation measures; and finally (5) Propose plans to mitigate conflicts and promote ecosystem-based management for the park.
If you accept to participate, you will be asked several questions (see questionnaire), and eventually we will visit your farm to assess the level of damage caused by elephants to your crops. The answers that you will provide us on the following questionnaire, which lasts approximately 45 minutes, will remain confidential and will be used exclusively by the researchers for the study.
There is no risk in participating in this study. However, by providing your name, we may use this information in the events of a compensation program that is retroactive. There is no guaranty for this, however. You are free to decline or accept that your name be disclosed for this purpose.
It remains at your discretion to determine whether you wish to answer the questionnaire in whole or in part, or if you do not wish to participate at all. If this study is published, the anonymity and confidentiality of this questionnaire will always apply. You must also be at least 18 years old to participate.
If you have any questions, please do not hesitate to ask me during the interview or later by email at “ ” or by phone at“ ”.
Do you agree to participate in the study under the conditions described above?
If yes, say YES
If no, say NO
Thank you!”
RESULTS
Plant species and plant parts consumed reported by local ecological knowledge
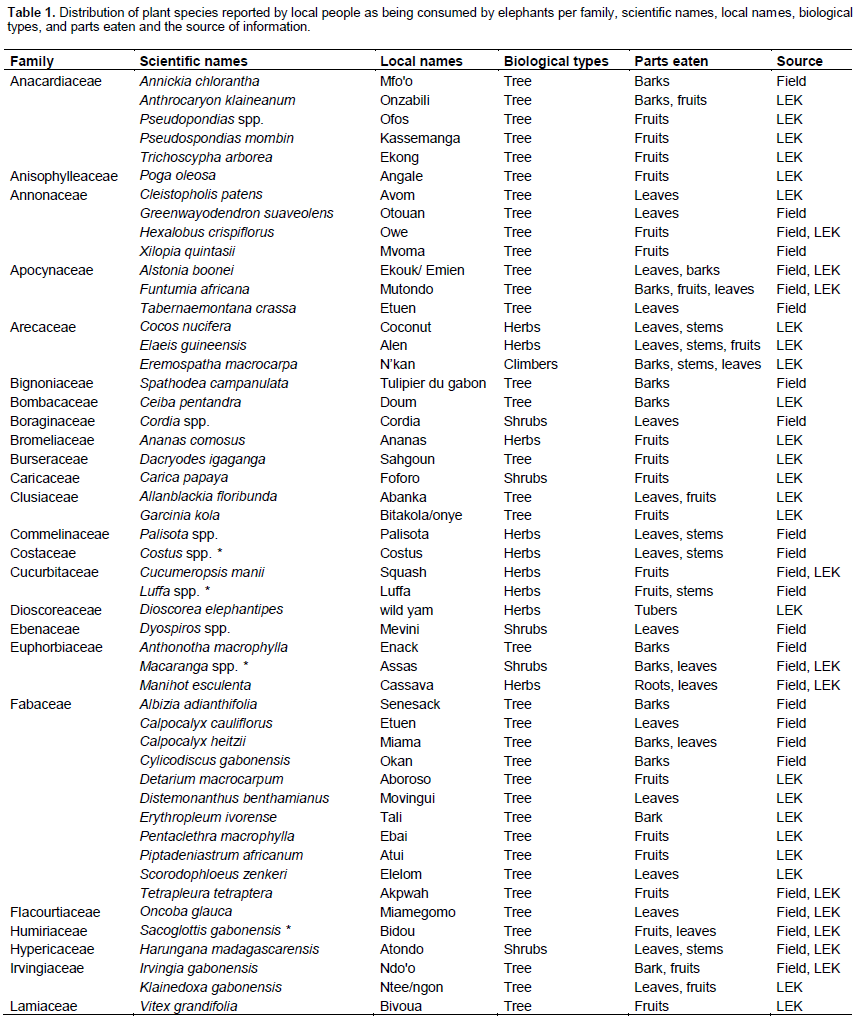
LEK data revealed that 62 plant species from 36 taxonomic families were part of the Campo-Ma’an elephants’ diet, of which 10 were cultivated food crops (Table 1). The plants parts most reported included fruits, leaves, stems, roots, and barks, with a significant difference in frequencies of the parts reported (Chi spare test, χ² = 104.200, df = 4, p < 0.001; Figure 2A). According to local knowledge, elephants consumed significantly more fruits as compared to leaves (χ² = 10.714, df = 1, p = 0.001), stems (χ² = 34.909, df = 1, p < 0.001), roots (χ² = 46.049, df = 1, p < 0.001), and tree barks (χ² = 48.600, df = 1, p < 0.001). Also, leaves were significantly more reported being consumed than stems (χ² = 9, df = 1, p = 0.003), roots (χ² = 17.065, df = 1, p < 0.001), and tree barks (χ² = 19.200, df = 1, p < 0.001). No significant difference in reported consumption was observed between barks, stems and roots (χ² = 3.875, df = 2, p = 0.144).

Plant species and plant parts consumed reported by field surveys
Field investigations showed that 47 plants species from 29 taxonomic families, of which 4 are food crops, were consumed by elephants (Table 1). Elephants’ diet included 8 herbs, 6 shrubs and 33 trees. Plant parts eaten included fruits, leaves, barks, stems, and roots with a significant difference in their distribution (Chi square test, χ² = 35.500, df = 4, p < 0.001; Figure 2B). Fruits were more consumed than stems (χ² = 7.043, df = 1, p = 0.008) and roots (χ² = 29.121, df = 1, p < 0.001). Similarly, more leaves were consumed than roots (χ² = 30.118, df = 1, p < 0.001) and stems (χ² = 7.680, df = 1, p < 0.001). Signs of consumption from stems and barks were comparable (χ² = 1.060, df = 1, p = 0.303). No significant difference was observed between barks, leaves, and fruits consumption signs (χ² = 3.694, df = 2, p = 0.157). As compared to signs of roots consumption, there were significantly more stems (χ² = 11.267, df = 1, p < 0.001) and barks (χ² = 17.190, df = 1, p < 0.001) consumption signs.
Degree of co-occurrence in plant species between the LEK and field surveys
Overall, 47% (n = 47) species seen with signs of feeding in the field were reported during LEK surveys. There were significant differences between the feeding habit patterns reported from LEK and field surveys (Fisher exact test, two-sided, p < 0.001). The proportion of barks consumed were significantly greater than reported by local communities (χ² = 12.565, df = 1, p < 0.001). More fruits were reported than observed in the field (χ² = 12.565, df = 1, p < 0.001), whereas the contribution of leaves (χ² = 0.600, df =1, p = 0.439) and stems (χ² = 1.087, df = 1, p = 0.297) in feeding habit were comparable for both methods. The pattern of root consumption also appeared to be similar for LEK and field surveys (χ² = 1.800, df = 1, p = 0.180). Of the nine targeted trees (from three different species), camera trap confirmed that forest elephants fed on their fruits, barks, and leaves. In additional, 5 other plant species were seen being consumed through video and photos (Table 1).
Influence of seasons on field surveys feeding habit
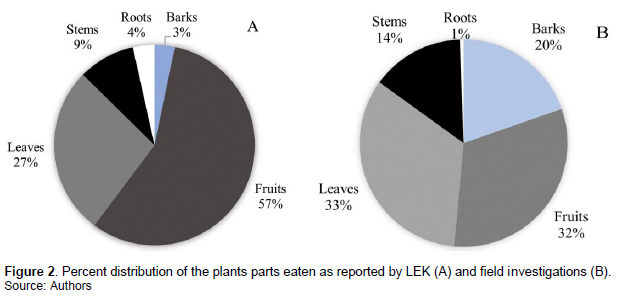
Field surveys’ feeding habit patterns differed between dry and wet seasons (Fisher exact test, two-sided, p < 0.001, Figure 3A and B). More barks were seen stripped by elephant during dry as compared to wet seasons (Chi square test, χ² = 15.680, df = 1, p < 0.001) whereas more leaves (χ² = 9.931, df = 1, p < 0.001) and more stems (χ² = 4.840, df = 1, p < 0.001) were browsed during wet as compared to dry seasons. Fruit and root consumption was comparable between wet and dry seasons (all p > 0.05) and no sign of root consumption by elephant was observed during the dry season.
DISCUSSION
Knowledge of diet and feeding habit of elephant is important for developing human elephant conflict mitigation strategies (Koirala et al., 2016). A total of 87 plants species which forest elephants relied on for food were found. The LEK surveys reported 62 plant species, while the field surveys found 47 plant species as part of the forest elephant diet, with 22 co-occurring plant species and eight plant species (fruit tree, herbs, and shrubs trees) validated for a total of 44 families (Table 1). Their food items came from trees, shrubs, herbs, and climbers. Elephants relied on a variety of plant parts such as roots, stems, barks, leaves, and fruits, consumed at varying proportions and seasons (Figures 2 and 3). These results are higher than the 43 species of plants from 24 families reported by Ndi et al., (2022) in Nki National Park but close to the 95 plant species found by De Boer et al. (2000) in a mosaic of forest and savanna in Mozambique, and 106 plants species consumed by Asian elephants in Shangyong National Natural Reserve in China (Jin et al., 2006). The total number of plants potentially consumed by elephants appeared to be lower than the 351 plants species found in Congo within the Ndoki National Park (Blake, 2002) and 230 species reported in Gabon within the Lopé Reserve (White et al., 1993). However, these researchers obtained those results using different approaches by combining direct observations, dungs, and food scraps resulting from long-term studies. The forest elephants of Campo ate mostly leaves, especially during wet seasons. Although the vegetation is described as evergreen, the wet period corresponds with the emergence of buds and the production of new leaves which are less lignified and more tender. Herbaceous plants (Costus spp., Palisota spp., Luffa spp.) or shrubs (Leea guineensis, Harungana madagascarensis, Macaranga spp.) and crops were reported by field investigations during the wet seasons. Also, signs of browsing on leaves, twigs, or young stems of M. cecropioïdes, Macaranga spp. and Harungana madasgarensis saplings were generally observed in disturbed areas such as logging trails, logging decks, edges of farms, and felling gaps. Leaves have been reported as an important part of elephant’s diet in Lopé Reserve in Gabon (White et al., 1993). We found that fruits play an important role in elephant diet in both dry and wet seasons in Campo-Ma’an. This can be explained by the fact that the area is dominated by a variety of tree species with different phenology schedules, thereby securing the availability of fruits on a continuous basis, although patchily distributed. Also, fruits have high concentrations of minerals, proteins and sugars needed for metabolism. Indeed, we saw the following trees producing fruits during both dry and wet seasons Sacoglottis gabonensis, Duboscia macrocarpa, Irvingia gabonensis, T. africana, Uapaca guineensis, and all were reported by both the LEK and field survey methods. Seasonal movement of elephants has been related to such fruits in Ndoki National Park in Congo (Blake, 2002) and Lopé reserve in Gabon (Beirne et al., 2020; Mills et al., 2018; White, 1994). Consumption of bark by forest elephants increased during dry seasons whereas the proportion of leaves eaten decreased. This variation in proportions may suggest that key minerals needed by elephants during the dry season might be greater in concentration in the barks of trees as compared to the leaves. Key minerals (calcium, iodine, iron, and zinc) have been documented for savanna and Asian elephants (Sach et al., 2019) but they remain unknown for forest elephants. Although debarking is not generally severe for trees, their contribution, with roots and stems in providing minerals to elephants has been reported in Tanzania (Kabigumila, 1993) and Gabon (White et al., 1993). The low concentration of food resources in minerals of over seasons (Rode et al., 2006; Sach et al., 2019) is often compensated by water and soil from bais. In the CMNP, four potential bais and a lick have been monitored for ecotourism by WWF (MINFOF, 2014) but none of them was consistently used by forest elephants. Therefore, the feeding strategy of forest elephants is based on their ability to select foods that best meet their nutritional needs among the available resources (Sach et al., 2019). Root/tubers and stems appeared to be consumed at varying proportions over seasons. Roots have been seen hollowed out during the rainy season when soil is moist in area other than swampy areas.
The results show both similarities and dissimilarities between LEK and field surveys in reported plant species and parts eaten by forest elephants. Twenty-five species were reported exclusively by field investigations and 40 exclusively by LEK surveys. Moreover, among species reported by the two methods, differences were still observed for parts eaten. Thirty-two percent of parts reported by LEK surveys were consistent with field investigations whereas 68% were found to be partially consistent to field observations. For example, LEK reported fruits from D. macrocarpa were the only part eaten by elephants, whereas field investigations showed evidence of barks and leaves with forest elephant feeding signs. Also, for S. gabonensis, the most reported specie for fruits consumption (about 13% of reports), we did not obtain evidence of leaves consumption during field investigations as reported by LEK surveys. Therefore, we argue that discordance between the two methods may be due to the influence of food selection related to seasonal availability (Jin et al., 2006). As such, the two methods might be seen as complementary, and not mutually exclusive, if we are to gather timely and inexpensive information on wildlife (Gilchrist et al., 2005; Service et al., 2014). Elephants’ feeding habit may generate conflict with humans, as I. gabonensis, Hexalobus crispiflorus, Coula edulis, reported by both methods, are also food items used by local communities. The understories of some tree species are used as hunting sites since fruit from those trees attract several wildlife species, thereby exposing them to hunters. For example, we have noticed that Ongokea gore fruits are used as bait on traps for small sized mammals. Those plants have been reported by both methods as part of the forest elephant diet, suggesting that local populations have important and reliable knowledge about the diet of forest elephants in their surroundings. Field investigations were limited to the southwestern tip of the conservation area. Therefore, as compared to data from the LEK, we may have only covered a limited number of species available in the conservation area. Tchouto (2004) reported 15 different types of vegetation in Campo-Ma’an conservation area with most fruitful plant species being distributed in limited spaces. Given that direct observation of forest elephants is difficult and costly due to forest elephants being elusive and mostly nocturnal (Kambissi, 2010), relying on LEK could be beneficial for providing information on some aspects of elephant ecology, including their diet, and feeding habits. For example, LEK surveys have been recommended as a tool to be used when doing research on elusive and threaten species such as pangolins (Fopa et al., 2020; Nash et al., 2016). Local communities are likely to know a great deal about their local environment and the species with which they have interacted over time, either in competition for shared resources or when dealing with crop damage from wildlife.
CONCLUSION
This study has shown considerable overlap in plant species consumed by forest elephants as reported by LEK and field surveys. LEK approach provided valuable information that was confirmed by field surveys of elephant diet composition as well as their feeding habits. Some differences were nevertheless observed between the two methods used, and we believe further investigations are needed before one can better understand what can explain the observed disparities. The findings suggest therefore that LEK can effectively give information on species that can provide important food items to forest elephants. Furthermore, this study gives an overview of the level of interactions that LEK surveys participants have with forest elephants. The combination of LEK and field surveys could be a cost-effective way to collect relevant information on species, while helping to improve the awareness of populations on the potential impacts or threats their activities could pose to forest elephants. Moreover, knowledge of elephants’ diet composition can be useful for habitat restoration in a human induced habitat losses and habitat fragmentation.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors are grateful to the Zoo de Granby, MITACS Accelerate, Quebec Centre for Biodiversity Sciences (QCBS), and Concordia University for their financial support. They also appreciate Valerie Michel of Zoo de Granby and Bolivar Kuate for helping with data collection and data entry and the Ministry of Forestry and Wildlife (MINFOF) of Cameroon, particularly Benjamin Sock and Albert Mounga Abana, former Conservators of the Campo-Ma’an National Park, for facilitating the research to take place in the Campo-Ma’an conservation area. The field assistants Louis Desiré Dontégo Kafack, Lucien Ngongo, Pierre Bambe, Charles Eyoh, Virtinus Nwalichoi Nanganoa, David Engon Engon, Dieudonné Assembe, Philémon Atouba, Patrick Mba, and Henri Nkoh are also appreciated.
REFERENCES
|
Albuquerque UP, Ludwig D, Feitosa IS, Moreno J, Moura B, De Henrique P, Gonçalves S, Henriques R, Cristina T, Gonçalves-souza T, Soares W, Júnior F (2021). Integrating traditional ecological knowledge into academic research at local and global scales. Regional Environmental Change, pp. 1-11. |
|
|
Allendorf TD, Gurung B, Poudel S, Dahal S (2020). Using community knowledge to identify potential hotspots of mammal diversity in southeastern Nepal. Biodiversity and Conservation 29(3):933-946. |
|
|
Beirne C, Meier AC, Brumagin G, Jasperse-sjolander L, Lewis M, Masseloux J, Myers K, Fay M, Okouyi J, White LJT, Poulsen JR (2020). Climatic and Resource Determinants of Forest Elephant Movements. Frontiers in Ecology and Evolution 8:1-14. |
|
|
Bekhuis PDBM, De Jong CB, Prins HHT (2008). Diet selection and density estimates of forest buffalo in Campo-Ma'an National Park, Cameroon. African Journal of Ecology 46(4):668-675. |
|
|
Biru Y, Bekele A (2012). Food habits of African elephant (Loxodonta africana) in Babile Elephant Sanctuary, Ethiopia. Tropical Ecology. © International Society for Tropical Ecology. Volume 53. View |
|
|
Blake S (2002). The ecology of forest elephant distribution and its implications for conservation. University of Edinburgh, PhD Thesis. |
|
|
Blake S, Inkamba-Nkulu C (2004). Fruit, minerals, and forest elephant trails: Do all roads lead to Rome? Biotropica 36(3):392-401. |
|
|
Brittain S, Bata MN, De Ornellas P, Milner-Gull EJ, Rowcliffe M (2020). Combining local knowledge and occupancy analysis for a rapid assessment of the forest elephant loxodonta cyclotis in Cameroon's timber production forests. Oryx 54(1):90-100. |
|
|
Buchholtz EK, Fitzgerald LA, Songhurst A, Mcculloch GP, Stronza AL (2020). Experts and elephants?: local ecological knowledge predicts landscape use for a species involved in human-wildlife conflict. Ecology and Society 25(4):26. |
|
|
Campos-Arceiz A, Blake S (2011). Megagardeners of the forest - the role of elephants in seed dispersal. Acta Oecologica 37(6):542-553. |
|
|
Caravaggi A, Banks PB, Burton AC, Finlay CMV, Haswell PM, Hayward MW, Rowcliffe MJ, Wood MD (2017). A review of camera trapping for conservation behaviour research. Remote Sensing in Ecology and Conservation 3(3):109-122. |
|
|
Choudhury A, Lahiri Choudhury DK, Desai A, Duckworth JW, Easa PS, Johnsingh AJT, Fernando P, Hedges S, Gunawardena M, Kurt F, Karanth U, Lister A, Menon V, Riddle H, Rübel A, Wikramanavake F (2008). Elephas maximus. The IUCN Red List of Threatened Species 2008: e.T7140A12828813. The IUCN Red List of Threatened Species 2008 8235:17. |
|
|
De Boer WF, Ntumi CP, Correia AU, Mafuca JM (2000). Diet and distribution of elephant in the Maputo Elephant Reserve, Mozambique. African Journal of Ecology 38(3):188-201. |
|
|
Doumenge C, Palla F, Itsoua Madzous GL (2021). Aires protégées d'Afrique Centrale - État 2020. OFAC-COMIFAC (eds.), Yaoundé, Cameroun, UICN, Gland, Suisse. |
|
|
Eyebe AJ, Dkamela GP, Endamana D (2012). Overview of human wildlife conflict in Cameroon. Poverty and conservation learning group discussion. Paper (5):1-26. |
|
|
Foguekem D, Tchamba MN, Gonwouo LN, Ngassam P, Loomis M (2011). Nutritional status of forage plants and their use by elephant in Waza national park, Cameroon. Scientific Research and Essays 6(17):3577-3583. |
|
|
Fopa GD, Simo F, Kekeunou S, Ichu IG, Ingram DJ, Olson D (2020). Understanding local ecological knowledge, ethnozoology, and public opinion to improve pangolin conservation in the Center and East Regions of Cameroon. Journal of Ethnobiology 40(2):234-251. |
|
|
Gilchrist G, Mallory M, Merkel F, Gilchrist G, Mallory M, Merkel F (2005). Can local ecological knowledge contribute to wildlife management?? Case studies of migratory birds. Ecology and Society 10(1):5. |
|
|
Granados A, Weladji RB (2012). Human-Elephant Conflict around Bénoué national park, Cameroon: Influence on local attitudes and implications for conservation. Human Dimensions of Wildlife 17(2):77-90. |
|
|
Haenn N, Schmook B, Reyes Y, Calmé S (2014). Improving conservation outcomes with insights from local experts and bureaucracies. Conservation Biology 28(4):951-958. |
|
|
IUCN (2021). African elephant species now Endangered and Critically Endangered - IUCN Red List. View (visited March 25th, 2021). |
|
|
Jin C, Xiaobao D, Ling Z, Zhilin B (2006). Diet composition and foraging ecology of Asian elephants. Acta Ecologica Sinica 26(2):309-316. |
|
|
Kabigumila J (1993). Feeding habits of elephants in Ngorongoro Crater, Tanzania. African Journal of Ecology 31(2):156-164. |
|
|
Kambissi ZG (2010). Etude des réponses des éléphants de forêt (Loxodonta africana cydotis) aux activités humaines à l'aide des enregistreurs acoustiques à la grande saline et au petit baï de la Compagnie Equatoriale de Bois (CES), au sud-est du Gabon. |
|
|
Koirala RK, Raubenheimer D, Aryal A, Pathak ML, Ji W (2016). Feeding preferences of the Asian elephant (Elephas maximus) in Nepal. BMC Ecology pp. 1-9. |
|
|
Kouamé D, Yao C, Nandjui A, N'guessan E (2011). Le rôle de l'éléphant dans la germination des graines de Irvingia gabonensis (Irvingiaceae), Balanites wilsoniana (Balanitaceae), Parinari excelsa (Chrysobalanaceae) et Sacoglottis gabonensis (Humiriaceae) en forêt tropicale: cas du parc national d'Azagn. International Journal of Biological and Chemical Sciences 4(5):1442-1454. |
|
|
Leggett K (2009). Diurnal activities of the desert-dwelling elephants in northwestern Namibia. Pachyderm 45:20-33. |
|
|
Martin EA, Brull GR, Funk SM, Luiselli L, Okale R, Fa JE (2020). Wild meat hunting and use by sedentarised Baka Pygmies in southeastern Cameroon. PeerJ. pp. 1-27. |
|
|
Maurois C, Chamberlan C, Maréchal C (1997). Aperçu du régime alimentaire de l'éléphant de forêt, Loxodonta africana cyclotis, dans le parc national d'Odzala, République du Congo. Mammalia 61(1):127-130. |
|
|
Mills EC, Poulsen JR, Fay JM, Morkel P, Clark CJ, Meier A, Beirne C, White LJT (2018). Forest elephant movement and habitat use in a tropical forest-grassland mosaic in Gabon. PLoS One 13 (7):1-17. |
|
|
MINFOF (2014). Plan d'aménagement du Parc National de Campo-Ma'an et de sa zone périphérique. Période 2015-2019, Ministère des Forêts et de la Faune, Parc National de Campo-Ma'an. Campo - Cameroun. |
|
|
Mmbaga NE, Munishi LK, Treydte AC (2017). How dynamics and drivers of land use/land cover change impact elephant conservation and agricultural livelihood development in Rombo, Tanzania. Journal of Land Use Science 12(2-3):168-181. |
|
|
Mouafo ADT, Ingram DJ, Pagning RT, Constantine I, Ngwayi N, Mayaka TB (2021). Local knowledge and use of pangolins by culturally diverse communities in the forest-savannah transition area of Cameroon. Tropical Conservation Science 14, Art. No.: 194008292110281. |
|
|
Nash HC, Wong MHG, Turvey ST (2016). Using local ecological knowledge to determine status and threats of the Critically Endangered Chinese pangolin (Manis pentadactyla) in Hainan, China. Biological Conservation 196:189-195. |
|
|
Ndi FC, Fonkwo NS, Kinge TR (2022). Feeding pattern of forest elephants in the Nki National Park and its environs, East Region, Cameroon. International Journal of Biodiversity and Conservation 14(1):26-34. |
|
|
Ngama S, Bindelle J, Poulsen JR, Hornick JL, Linden A, Korte L, Doucet JL, Vermeulen C (2019). Do topography and fruit presence influence occurrence and intensity of crop-raiding by forest elephants (Loxodonta africana cyclotis)? PLoS One 14(3):1-15. |
|
|
Nzooh-Dongmo Z, Goran Kouamé PN, Fondja C, Nkono J (2015). Evaluation de la dynamique des populations de grands et moyens mammifères dans le domaine forestier permanent de l'unité technique opérationnelle Campo-Ma'an. |
|
|
Ole CMD (2011). Etat des lieux et perspectives de gestion durable des conflits homme-faune à la périphérie du parc national de Campo-Ma'an. Université de Dschang. |
|
|
Pan Y, Wei G, Cunningham AA, Li S, Shu C, Milner-Gulland EJ, Turvey ST (2016). Using local ecological knowledge to assess the status of the critically endengered chinese giant salalmander Andrias davivianus in Guizhou province, China. Oryx 50:257-264. |
|
|
Patrice FN (2019). El tratamiento penal de la delincuencia juvenil: el cuestionable statu quo del legislador penal camerunes / The criminal point of view of juvenile delinquency: the dubious status quo of cameroonian penal legislator. Misión Jurídica: Revista de Derecho y Ciencias Sociales 12(16):97-112. |
|
|
Puyravaud J, Gubbi S, Poornesha HC, Davidar P (2019). Deforestation Increases Frequency of Incidents With Elephants (Elephas maximus). Tropical Conservation Science 12(1). |
|
|
Rode KD, Chiyo PI, Chapman CA, McDowell LR (2006). Nutritional ecology of elephants in Kibale National Park, Uganda, and its relationship with crop-raiding behaviour. Journal of Tropical Ecology 22:441-449. |
|
|
Sach F, Dierenfeld ES, Langley-evans SC, Watts MJ, Yon L (2019). African savanna elephants (Loxodonta africana) as an example of a herbivore making movement choices based on nutritional needs. PeerJ 7(e6260):1-27. |
|
|
Service CN, Adams MS, Artelle KA, Paquet P, Grant LV, Darimont CT (2014). Indigenous knowledge and science unite to reveal spatial and temporal dimensions of distributional shift in wildlife of conservation concern. PLoS One 9(7):e101595. |
|
|
Short J (1981). Diet and feeding behaviour of the forest elephant. Mammalia. 45(2):177-185. |
|
|
Tchamba MN (1996). Seasonal forage utilization by elephants in the Waza-Logone Region, Cameroon. In. Elephant and their interactions with people and vegetation in the Waza-Logone Region, Cameroon pp. 127-139. |
|
|
Tchamba MN, Seme MP (1993). Diet and feeding behaviour of the forest elephant in the Santchou Reserve, Cameroon. African Journal of Ecology 31(2):165-171. |
|
|
Tchamba MN, Weladji RB, Foguekem D, Loomis M (2014). Plant biomass density as an indicator of food supply for elephants (Loxodonta africana) in Waza National Park, Cameroon. Tropical Conservation Science 7(4):747-764. |
|
|
Tchouto MGP (2004). Plant diversity in a central African rain forest: Implications biodiversity conservation in Cameroon [PhD thesis, Wageningen University]. Wageningen University Theses and Dissertations Archive. |
|
|
Tchouto MGP, De Wilde JJFE, De Boer WF, Van Der Maesen LJG, Cleef AM (2009). Bio-indicator species and Central African rain forest refuges in the Campo-Ma'an area, Cameroon. Systematics and Biodiversity 7(1):21-31. |
|
|
The Angiosperm Phylogeny Group. (2009). An update of the Angiosperm phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161(2):105-121. |
|
|
Tiani AM, Akwah G, Nguiébouri J (2005). Women in Campo-Ma'an national park: Uncertainties and adaptations in Cameroon, in: Colfer, C. (Ed.), The Equitable Forest. Resources for the future and CIFOR, Washington, DC., Washington, DC, pp. 131-149. |
|
|
Vivien JJ, Faure JJ (2011). Arbres des forêts denses d'Afrique Centrale. Ediprint, France. |
|
|
Wall J, Wittemyer G, Klinkenberg B, LeMay V, Blake S, Strindberg S, Henley M, Vollrath F, Maisels F, Ferwerda J, Douglas-Hamilton I (2021). Human footprint and protected areas shape elephant range across Africa. Current Biology 31(11):2437-2445. |
|
|
Weladji RB, Tchamba MN (2003). Conflict between people and protected areas within the Bénoué Conservation Area, North Cameroon. Oryx 37(1):72-79. |
|
|
White LJT (1994). Sacoglottis gabonensis Fruiting and the Seasonal Movements of Elephants in the Lope. Journal of Tropical Ecology 10(1):121-125. |
|
|
White LJT, Tutin CG, Fernandez M (1993). Group composition and diet of forest elephants, Loxodonta africana cyclotis Matschie 1900, in the Lopé Reserve, Gabon. African Journal of Ecology 31(3):181-199. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0