ABSTRACT
A study on the population structure of small mammals was carried out in Aridtsy Forest, Awi Zone from August 2011 to February 2012 incorporating both wet and dry seasons. Sherman live traps and snap traps in four randomly selected different trapping grids where distinct habitat types, namely, natural forest, bushland, grassland and farmland were employed. During this study a total of 468 individuals, including eight species of small mammals (live traps) and 89 individuals counting six species of small mammals (snap traps) were trapped in a total of 2352 and 1200 trap nights, respectively. From overall trap, seven species of small mammals were under family muridae and a single species was belonging to family Soricidae. There was also a variation in trap success among different habitat types, with the highest in the bushland. There was an affinity to caught more males comprised 59.7% and females 40.3% of the total capture. There was a statistically significant variation in the capture of adults between seasons, but no statistical variation for subadult and young age groups was recorded between seasons.
Key words: Aridtsy Forest, Awi Zone, sex ratio, species, small mammals
Ethiopia is rich in fauna diversity, record of 284 species of mammals. From this overall, about 84 species are rodents (Afework Bekele and Leirs, 1997). This paper addresses the fluctuation of small mammals in terms of reproductive condition, age structure and across habitat trap variations. However, the population structure of small mammals, their occurrence, dispersal and trap rate varies from habitat to habitats. Mulungu et al. (2008) importantly noted the diversity of species, capture probability, and population size varies with vegetation types.
Small mammals are mobile to disperse to suitable sites and leave unsuitable sites; yet they are dependent on resources from a reasonably definitive localized area. They are also ubiquitous and sufficiently fecund to be a useful tool for scientific investigations across landscapes. In addition, responding quickly to disturbances in a particular habitat is well developed (Barnett and Dutton, 1995; Leis et al., 2007).
Among small mammals, rodents range in size from the small African pigmy mouse (Mus minutoides) weighing only 5 g to the capybara (Hydrochaeris hydrochaeris) weighing 50 kg (Vaughan et al., 2000; Tobin and Fall, 2004).
The prolific breeding behaviour of rodents was reported by Kay and Hoekstra (2008). This is especially true for many rodent species which are capable of rapid population growth, especially when conditions are favourable (Wolff and Sherman, 2007). Among rodents, the mating tactics used by sciurids probably are the best known, as their diurnal behaviour and large body size made observations easy. However, many rodent species are small, cryptic and nocturnal, making mating behaviour difficult to observe. The mating patterns of small rodents are usually observed in laboratory or in enclosures (Wolff and Sherman, 2007).
Despite the fact that, litter size for most rodents ranges from one to eight offspring (Kay and Hoekstra, 2008), it can be varied based on seasonal fluctuations of whether condition (Afework Bekele and Leirs, 1997). For example, mole rats can produce as many as 28 young ones in a single litter (Gilbert 1986; Kay and Hoekstra, 2008). It appears that they reproduce throughout the year as long as sufficient food and cover are available (Zerihun Girma et al., 2012).
The fecundity of many rodents is further enhanced by physiological aspects (Kay and Hoekstra, 2008), postpartum or lactational estrus (ovulation immediately following birth), which enables females to be continuously pregnant. As rodents can occupy many types of habitats, consume nearly anything and reproduce rapidly, they have successfully filled almost every niche (Jing-yuan et al., 2008; Kay and Hoekstra, 2008). High reproductive potential and a short period of maturation lead to rapid population growth (Kay and Hoekstra, 2008).
Not all rodent species show a discrete breeding season. However, most of the pest rodents seem to stop breeding during periods of extended fallow, when food is scarce or of low quality (Feldhamer, 1979). As per the study by Afework Bekele and Leirs (1997); Caro (2001);
Jonsson et al. (2002); Vieira (2003); Mohammadi (2010), food limitation has been found to constrain the reproductive success of small mammals. Direct effect of rainfall on reproduction, especially for species with poor ability to conserve water and its influence on population dynamics was revealed by studies of Afework and Leirs (1997) and Linzey and Kesner (1997). Therefore, reproductive success and population dynamics of rodents are greatly influenced by variations in the rainfall patterns. Similar results indicated that, population size increases during the rainy season (Hoffmann and Zeller, 2005; Tilaye Wube, 2005; Workneh Gebresilassie
et al., 2006; Tadesse Habtamu and Afework Bekele, 2008; Happlod and Happold, 2011; Sintayehu Workeneh et al., 2011).
Many small mammal populations undergo seasonal and multiyear fluctuations in numbers. Different scientific papers, for example, Afework and Leirs (1997), Kingdon (1997), Macdonald (1984), Nowak (1999) and Kaminski et al. (2007) indicated that, their distribution is strongly influenced by microhabitat factors, It is also widely accepted that the distribution of rodents and insectivores is not uniform in all habitat types (Yalden, 2008; Tilahun Chekol et al., 2012). Different studies hypothesized that habitat complexity and heterogeneity at the different altitudes influence diversity and distribution of small mammals (Castiglia and Caporioni, 2005; Mohammed Kasso et al., 2010).
Ecologically, Small mammals are the chief elements of forest ecosystems. They affect the structure, composition and dynamics of forest communities through activities such as, seed dispersal (Mugatha, 2002; Solari et al., 2002; Lobo et al., 2009; Samuels, 2009), pollination (Richardson et al., 2000), impacts on insect populations (Bernard et al., 1997), and as food for carnivorous animals (Linzey and Kesner, 1997; Aschwanden et al., 2007; Avenant and Cavallini, 2007).
In many instances, small mammals provide major benefits to the environment as bio-engineers (Apline and Singleton, 2003). However, some rodent species (less than 5%) are pests and cause significant losses to agricultural crops and stored food grains in many regions of the world. Therefore, this study reports small mammal population structure, exclusively those able to be captured by Sherman live-traps and snap-traps in Aridtsy Forest, to respond the relationship of seasonality and habitat variations on population structure of small mammals. Thus, the objective of this paper is to present important information on variation of small mammal population structures with seasonal variation and in different habitats as parameters. Additionally, category of age and sexual differences between seasons are presented here.
Study area description
Our study was conducted in Aridtsy Forest in Awi Zone, northwestern Ethiopia, Amhara Regional State.
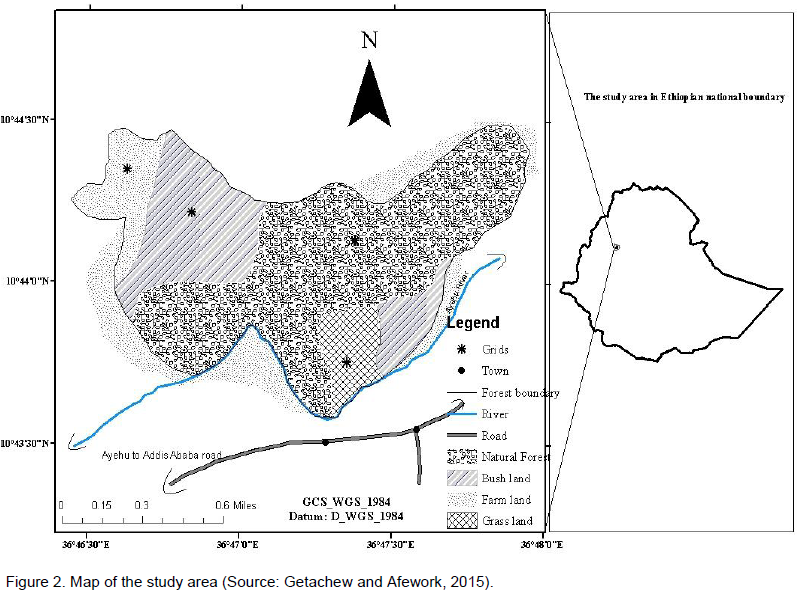
Aridtsy Forest is located in Ankesha Guagusa Woreda at about 30 km from the main administrative Zone (Getachew and Afework, 2015). This Forest is wet and dominated by some tree species such as Cordia africana, Acacia abyssinaa, Croton macrostachyus, Ficus vasta, Albizia schimperiana and Millettia ferrginea (Figure 1). The area coverage of the forest is around 127 ha, and it is a natural forest. Geographically, the study area was located between 10°43’40” - 10°44’20” N and 36° 46’40” - 36° 48’0” E (Figure 2).
The climatic condition of the study area is in the warm agro-climatic zone. The area has one long rainy season mainly from early May to late October. Average rainfall in the area varies between 6.47 mm in February to 240.84 mm in August (Getachew and Afework, 2015). The driest season is from January to March. The mean monthly temperature ranges between 9.7 to 32.0°C. Suitable agro-climatic conditions made the region to be endowed with the production of different commercial and food crops.

Sampling design and data collection
To study the population structure of small mammals, preliminary survey was conducted in Aridtsy Forest and nearby farmlands during the first week of August 2011. During this survey, all the available and relevant information about the study area was gathered. Different vegetation types and representative habitat sites were observed. Grids were selected randomly based on different criteria like altitudinal differences and vegetation cover. The four selected habitats were natural forest, bushland, grassland and farmland. The total area of the study was classified into 4 grids. Grid code was given, one for each habitat type as, NF, BL, GL and FL, for natural forest, bushland, grassland and farmland, respectively.
After the relevant information was gathered during the preliminary survey, continuous field work on an ecological study of small mammals in the study area was carried out. During the study, both wet and dry seasons were included. The first wet period data collection was carried out in August 2011 and the second wet season data collection was in October 2011. The first dry period data collection was carried out in December 2011 and the second dry season data collection was from January 22 - February 9, 2012.
Grid in the farmland area was in the maize plantation, which covered an area of around 2 ha. The same grids were used during the whole study periods. Both Sherman live-traps and snap-traps, 49 and 25 traps, respectively, were used during the study periods. In addition to this, data were also gathered by direct observation in the area during the daytime throughout the trip periods during both wet and dry seasons.
A 4900 m2 (0.49 ha) live-trapping grid was established, one in each habitat type. A total of 49 live traps were set over an area of 70 m x 70 m positioned at 10 m intervals for each grid and 25 snap traps at an interval of 20 m were used at least 200 m away from the live-trapping sites.
Trapping stations were marked with red plastic tap approximately one meter above the traps. The traps were baited with peanut butter and checked twice a day, early in the morning (06:00 - 08:00 hours) and late in the afternoon (17:00 - 18:00 h) and repeated as necessary. The trapped specimens were transferred from the trap into a polyethylene bag. For each individual trapped, grid and trap station number, toe-clipping, body mass and sexual conditions were recorded. Further, trapped specimens were also distinguished as adults, subadults and juveniles on the basis of weight, pelage colour (usually very grey in juveniles). Reproductive condition of males as scrotal and abdominal testes, and females as pregnant or with suckling nipples, perforate or imperforate vagina were recorded. They were released with the same site from where they were trapped.
Snap-traps were also baited with peanut butter and checked twice a day, late in the afternoon and in the next morning, immediately after Sherman live-traps were checked. Trapping was done for three consecutive nights for a total of 75 trap nights per grid. Records on date, location, habitat and species type were noted for each individual trapped. Digital caliper was used during measurement processes. Sex, weight, head and body length, hind foot length, ear length and tail length were also recorded for each individual. Dissection was carried out on the snap trapped specimens in pregnant females for embryo count. Skins of sample specimen of each species were prepared for identification at the species level.
Species identification was carried out based on the taxonomic characteristics listed in Nowak (1999), Yalden et al. (1976) and Kingdon (1997). Since, identification of the sexes, particularly young groups either male or female was very challenging, important references on Kay and Hoekstra (2008) was used during sexual identification activities. In addition to this, Zoological Museum referencing was also carried out for species identification. For this purpose, voucher skins were prepared and compared with the specimens deposited in the Zoological Natural History Museum of Addis Ababa University through morphological identification methods.
Data analysis
An abundance of small mammals in each habitat was assessed by the percentage of trap success between the seasons. Trap success was calculated by using the number of caught individuals and trapping nights. The Chi-square test was used to interpret variations of small mammal species in different trapping seasons and grids. Data were analyzed using SPSS (15.0) computer program.
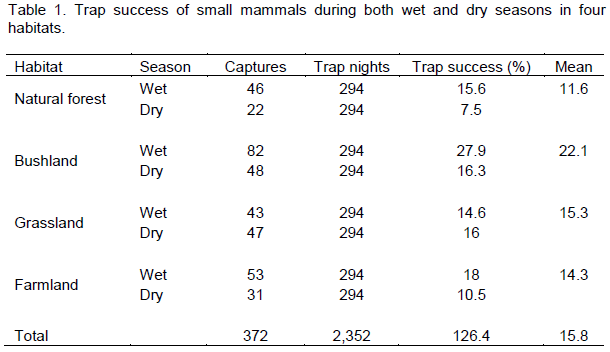
During this study we caught 468 individuals belonging to eight species of small mammals within a total of 2352 trap nights. The reveled species list was the following, Lophuromys flavopuntatus, Arvicanthis dembeensis, Stenocephalemys albipes, Mastomys natalensis, Pelomys harringtoni, Acomys cahirinus and Lemniscomys zebra. The eight was an insectivore Crocidura flavescens. We faced fluctuation in species richness during wet and dry seasons. The varied success of trapping was found among habitats during the study periods spent. Trap success for different habitats and for the wet and dry seasons are given in Table 1. The highest trap success was in bushland, followed by grassland and the lowest trap success was in the forest habitat. There was variation in the trap success between the different habitat types during the study periods, but this was not significant (χ2= 4.92, df=3, p>0.05).
Mean capture success in the study sites in 2352 trap nights was 15.8%. During the wet season, trap success was highest for bushland (27.9%), followed by farmland (18%), natural forest (15.6%) and grassland (14.6%). During the dry season, the trap success was highest for bushland (16.3%), followed by grassland (16%), farmland (10.5%) and 7.5% for natural forest. Capture success varied by seasons, trap success was significantly higher in the wet season (χ2=5.69, df=1, p<0.05).
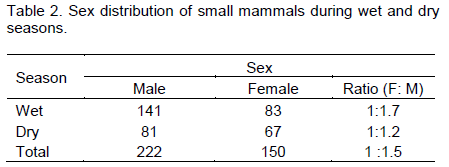
Of the total captured small mammals, the number of males and females was 222 and 150, respectively (Table 2). Higher numbers of males were trapped during the wet season (141) than during the dry season (81). Similarly, female to male sex ratio of rodents showed variation between seasons (1:1.7 for wet and 1:1.2 for dry season). Males showed significant difference in population size between seasons (χ2=16.22, df=1, p<0.05), but no significant difference in females between seasons (χ2=1.71, df=1, p>0.05).
The proportion of male and female individuals varied among species (Table 3). Males outnumbered females in all habitats and trapping sessions. The sample contained significantly more males than females. Except, L. zebra (no captured male), each had the highest proportion of males from all seasons and habitats. Female to male sex ratio of L. flavopuntatus was (1:1.6).
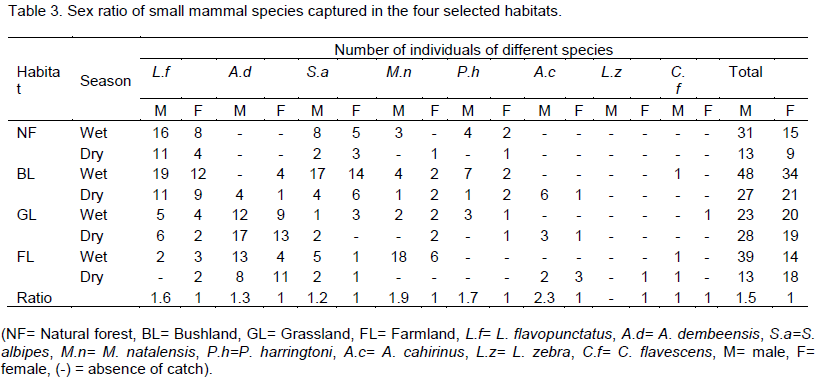
Males contributed a higher proportion in A. dembeensis with female to male ratio of 1:1.3 and 1:1.2 (female to male) ratios were observed in S. albipes. Female to male ratio of M. natalensis, P. harringtoni and A. cahirinus were 1:1.9, 1:1.7, and 1:2.3, respectively. The overall male to female sex ratio during the study sites for the whole study period was 1:1.5 statistically, there was significant difference between sex ratio (χ2=13.94, df=1, p<0.05).
During the wet season, adult species of small mammals accounted for 39%, whereas subadult and young ones accounted for 15.9 and 5.4%, respectively. During the dry season, adults, subadults and young accounted for, 25.3, 11.5 and 2.9%, respectively (Figure 3). The number of adult individuals was high in both seasons compared to the other age groups. There was seasonal variation in the total capture of adult individuals (χ2=10.88, df=1, p<0.05), but, subadult as well as young individuals showed no seasonal variations in their captures (χ2=2.5, df=1, p>0.05 and χ2=2.62, df=1, p>0.05, respectively).
All age groups (adult, subadult and young) were represented in both seasons for L. flavopunctatus, S. albipes, A. dembeensis, M. natalensis and P. harringtoni (Table 4). However, M. natalensis and P. harringtoni had no capture of young ones during the dry season. Young were more numerous in the population during the wet
season and accounted for a trap success of 0.7% for females and 0.6% for males. However, statistically, there was no significant difference among adult females (χ2=0.712, df=1, p>0.05), subadult males (χ2=2.06, df=1, p>0.05), subadult females (χ2 =0.58, df =1, p>0.05) and both females (χ2=0.53, df=1, p>0.05) and males (χ2=2.57, df=1, p>0.05) of young categories between seasons. But, adult males showed significant difference between seasons (χ2 =12.4, df=1, p<0.05).
Among the adult female rodents, 42.2% were pregnant and 23.3% were lactating (Table 5). Pregnants were captured only from rodents, except L. zebra. More number of pregnant females were trapped during the wet season (65.8%) than during the dry season (34.2%) (χ2=3.78, df=1, p<0.01). However, among pregnant rodents, L. flavopunctatus (36.8%) and A. dembeensis (28.9%) contributed the highest number. The least number of pregnant females was from A. cahirinus (2.6%). One L. flavopuntatus gave birth to four youngs inside the trap during the second wet season. During the dry season, the number of pregnant rodents was less in all species except, A. dembeensis. The proportion of lactating females was not significantly different during the wet (47.6%) and the dry seasons (52.4%). High number of lactating females were registered from L. flavopuntatus (38.1%), followed by A. dembeensis (28.6%). Alterna-tively, the least percentage of lactating females was recorded in M. natalensis (4.8%). There was no significant variation in lactating females between seasons (χ2=0.048, df=1, p>0.01).
Out of the total 89 snap-trapped rodents, 48 were males and 41 were females. Of the total adult females, 20 pregnant female rodents among the four species and 14 non-pregnant were captured. The number of embryos counted ranged between three and twelve among the females trapped (Figure 4). The number of embryos of pregnant females varied from species to species and season to season. The higher number of embryos (8-12) was recorded from M. natalensis and the least (3) was for L. flavopunctatus. There was wide variation in embryo count in individuals of M. natalensis unlike individuals of L. flavopunctatus and S. albipes.
There was variation in trap success among the different habitat types and seasons. We found high trap success in the bushalnd habitat during survey time; this might be due to dense vegetation ground cover, which directly provides good shelter for small mammals and accounts more capture rate. The lowest trap success was in the natural forest, here traps were disturbed all time and we were unable to effectively sample, due to negative interaction or activities of hyenas at night time and baboons in day time. These situations lead to low capture in this habitat. Trap success was significantly higher in wet seasons than in the dry season. This could be associated with the influence of rainfall, which facilitates the growth of ground cover and availability of food, which in turn, enhances breeding. The overall trap success in the present study was 15.8%. The present trap success was low compared to the study by Demeke et al. (2007), who obtained 17.6% from Arbaminch Forest and Farm-lands, Tadesse Habtamu and Afework Bekele (2008) with trap success of 36.8% from Alatish National Park and Mohammed (2010) who recorded 44.1% from Chilalo–Galama Mountain range. Relative to these studies, unlimited human activities and cattle moving in the habitats resulted in low trap success in the present study. In addition to human related factors, the bait used in the current study may have attracted the attention of ants and other invertebrates; as a result of this the bait was consumed. These factors might have led to low trap success. Small mammal communities are negatively affected by grazing and species diversity is low in an area that is heavily altered by human activities (Ogada et al., 2009)
Results from the present study were consistent with the findings of Smith et al. (1975) and Tilahun Chekol et al. (2012) who have recorded higher capture frequency of males. According to Ku and Lin (1980), the higher capture frequency of males might be due to the fact that males are more active than females and their ability to consume novel bait. The present study showed largely a male biased sex ratio. This is probably due to the males traveling over greater distances. Therefore, they have a higher probability of being trapped. Phelps (2006) reported that significantly high proportions of males were trapped from Tar Greek. According to D’Andrea et al. (1999), differences in movement behaviour rather than unequal reproductive effort by parents lead to a male biased sex ratio. However, Miller and Miller (1995) and Nicolas and Colyn (2003) have noted no significant difference in the sex ratio of rodents. Fluctuation in small mammal populations can be attributed to changes in reproductive parameters, such as the proportion of reproductively active females among trapping seasons. The proportion of reproductively active females within L. flavopunctatus was significantly different among trapping seasons. Pregnant females were more in number during the wet season than in the dry season. Makundi et al. (2006) reported that reproductively active L. flavopunctatus were present in the population almost throughout the year, but there was a seasonal peak during the wet season. Numerous studies have reported that reproductive characteristics of populations of small mammals were correlated with the rainy season (Hubert, 1978; Leirs et al., 1989; Afework and Leris, 1997; Linzey and Kesner, 1997; Nicolas and Colyn, 2003; Tilaye Wube, 2005). More pregnant individuals of small mammals were recorded during the wet season than during the dry season. This is supported by previous investigations which explained that reproduction is mostly linked with the rainy season and with the availability of sufficient resources for rearing the young. It has been suggested that seasonal variations in weather, particularly rainfall, influences the nutritional aspects, which affects the life strategies of rodents (Makundi et al., 2006). However, this is exceptional to A. dembeensis, which had more pregnant individuals during the dry season than the wet season. This might be due to the presence of nutritious food during the dry season, which is favoured by this species. This contradicts the study carried out by Afework and Leris (1997) in Central Ethiopia where, breeding activity of A. dembeensis was during the rainy season.
In the present study, all age categories of small mammals were constituted through all trapping periods. Seasonally, there was variation in the age distribution in population of most species. Similar observations were made in Gabon (Nicolas and Colyn, 2003). The capture frequency of adults outnumbered the other age categories. This might be due to the movement and faster trapability of adults than the other age groups. Smith et al. (1975) have noted that older animals frequently rank higher in social level and may be caught first and more often than other age group individuals. Comparatively, there were more young individuals during the wet season than the dry season. This may be due to the correlation between rainfall and seasonality in reproduction. This is also reflected by other investigators. For instance, Leirs et al. (1989), Afework and Leirs (1997), Tilaye Wube (2005) and Makundi et al. (2006) have revealed the effect of rainfall on population dynamics. Similarly, Fichet-Calvet et al. (2009) stated that during the rainy season, young individuals represent the basis of the pyramid. The observations made in the current study suggest that breeding of most of the small mammal species in the study area was during the wet season. According to Linzey and Kesner (1997), rainfall could indirectly govern reproductive success by affecting the supply of insects.
No pregnant females were trapped in the case of S. albipes and P. harringtoni during the dry season. This clearly indicates that the reproduction of these species is seasonal. However, Serekebirhan Takele et al. (2011) have noted that there is a continuous breeding of these species throughout the year. Besides rainfall, which is linked to reproduction, dietary requirement of small mammals obviously change in quality and quantity within seasons, increasing during the wet season. Such variations certainly determine the peak period of pregnancy and survival of the small mammal species.
The recorded data from the snap-trapped rodents revealed that the number of dissected embryos observed varied from species to species and season to season. A higher embryo count was made during the wet season than the dry season. The embryo count of A. dembeensis and M. natalensis showed fluctuation, even though for both species, the highest record was during the wet season. Seasonal fluctuation of embryos may be related to availability of food. Similarly, Afework and Leris (1997) and Nicolas and Colyn (2003) described that litter size varied among species of rodents and seasons. Based on the results obtained, this finding suggests a further study as necessary to seasonal differences and physiological reproduction of small mammals. Habitats of small mammals should not be influenced by the activities of humans, and further study is required on the impacts grazing if small mammals and other biodiversity are to be maintained in this area.
Authors did not declare any conflict of interest.
We would like to thank Addis Ababa University for the facilities and financial support. Officials of Ankesha Guagusa Woreda Administration, National Meteorological Agency and Zoological National History Museum of Addis Ababa University are highly acknowledged for the provision of important services.
REFERENCES
|
Afework B, Leirs H (1997). Population ecology of rodents of maize fields and grassland in central Ethiopia. Belg. J. Zool. 127: 39-48.
|
|
|
|
Apline KP, Singleton GR (2003). Balancing Rodent Management and Small Mammal Conservation in Agricultural Landscapes: Challenges for the Present and the Future. In: Rats, Mice and People: Rodent Biology and Management, pp.80 — 88, (Singleton, G. S., Hinds, L. A., Krebs, C. J. and Spratt, D. M. eds). Australian Centre for Intl. Agric. Res., Canberra.
|
|
|
|
|
Aschwanden J, Holzgang O, Jenni L (2007). Importance of ecological compensation areas for small mammals in intensively farmed areas. Wildl. Biol. 13: 150-158.
Crossref
|
|
|
|
|
Avenant NL, Cavallini P (2007). Correcting rodent community structure with ecological integrity, Tussen-die-rivere nature reserve, Free State Province, South Africa. Integr. Zool. 2: 212-219.
Crossref
|
|
|
|
|
Barnett A, Dutton J (1995). Expedition Field Techniques: Small Mammals (Excluding Bats). Royal Geographical Society with IBG, London, pp. 1-120.
|
|
|
|
|
Bernard JB, Allen ME, Ullry DE (1997). Feeding Captive Insectivorous Animals: Nutritional Aspects of Insects as Food. Michigan State University, Michigan, pp. 7.
|
|
|
|
|
Caro TM (2001). Species richness and abundance of small mammals inside and outside an African National Park. Biol. Conserv. 98:251-257.
Crossref
|
|
|
|
|
Castiglia R, Caporioni M (2005). Altitudinal distribution and outdoor occurrence in chromosomal races of the house mouse (Mus musculus domesticus) in central Italy. Folia Zool. 54:225-239.
|
|
|
|
|
D'Andrea PS, Gentile R, Cerqueira R, Grelle CE, Horta C, Rey L (1999). Ecology of small mammals in a Brazilian rural area. Revta Bras. Zool. 16:611-620.
Crossref
|
|
|
|
|
Demeke D, Afework B, Gurja B (2007). Feeding ecology of pest rodents from Arbaminch forest and farmlands, Ethiopia. SINET: Ethiop. J. Sci. 30:127-134.
|
|
|
|
|
Feldhamer GA (1979). Vegetative and edaphic factors affecting abundance and distribution of small mammals in southeast Oregon. Gre. Basi. Nat. 39:207-218.
|
|
|
|
|
Fichet-Calvet E, Audenaert L, Barriere P, Verheyen E (2009). Diversity, dynamics and reproduction in a community of small mammals in Upper Guinea, with emphasis on pygmy mice. Afr. J. Ecol. 48: 600-614.
Crossref
|
|
|
|
|
Getachew B, Afework B (2015). Diversity and habitat association of small mammals in Aridtsy forest, Awi Zone, Ethiopia. Zool. Res. 36(2): 88-94.
|
|
|
|
|
Gilbert AN (1986). Mammary number and litter size in rodentia: the "one-half rule". Proc. Natl. Acad. Sci. 83: 4828-4830.
Crossref
|
|
|
|
|
Happold D, Happold M (2011). The Mammals of Africa: progress and problems. In: The 11thAfrican Small Mammal Symposium "African's Small Mammal Biologists Trackling Africa's Big Problems Scientific Program.
|
|
|
|
|
Hoffmann A, Zeller U (2005). Influence of variations in land use intensity on species diversity and abundance of small mammals in the Nama Karoo, Namibia. Belg. J. Zool. 135: 91-96.
|
|
|
|
|
Hubert B (1978). Modern rodent fauna of the Lower Omo Valley, Ethiopia. Bull. Carnegie Nat. Hist. Mus. 6: 109-112.
|
|
|
|
|
Jing-vuan L, Hong D, Geng-bai T, Pin-hong Y, Shen-wen W, Hong P (2008). Community structure and diversity distributions of small mammals in different sample plots in the eastern part of Wuling Mountains. Zool. Res. 29: 637- 645.
|
|
|
|
|
Jonsson P, Hartikainen T, Koskela, E, Mapper T (2002). Determinants of reproductive success in voles: space use in relation to food and litter size manipulation. Evol. Ecol. 16: 455-467.
Crossref
|
|
|
|
|
Kaminski JA, Davis ML, Kelly M (2007). Disturbance effects on small mammal species in a managed Appalachian forest. Am. Midl. Nat. 157: 385-397.
Crossref
|
|
|
|
|
Kay EH, Hoekstra HE (2008). Rodents. Curr. Biol. 18: 1-5.
Crossref
|
|
|
|
|
Kingdon J (1997). The Kingdon Field Guide to African Mammals. Acadamic Press, London, pp. 443- 445.
|
|
|
|
|
Ku TY, Lin CC (1980). Abundance and distribution of field rodents in Taiwan. Plant Prot. Bull. 22: 397- 420.
|
|
|
|
|
Leirs H, Verhegen W, Michiels M, Verhagen R, Stuyck J (1989). The relation between rainfall and breeding season of Mastomys natalensis (Smith, 1834) in Morogoro, Tanzania. Belg. J. Zool. 119: 59-64.
|
|
|
|
|
Leis SA, Leslie, DM, Engle DM and Fehmi JS (2007). Small mammals as indicators of short-term and long-term distribution in mixed prairie. Environ. Monit. Assess. 36: 849-861.
|
|
|
|
|
Linzey AV, Kesner MH (1997). Small mammals of a woodland-savannah ecosystem in Zimbabwe, density and habitat occupancy patterns. J. Zool., Lond. 243: 137-152.
Crossref
|
|
|
|
|
Lobo N, Duong M, Millar JS (2009). Conifer seed preferences of small mammals. Can. J. Zool. 87: 773-780.
Crossref
|
|
|
|
|
Macdonald D (1984). The Encyclopedia of Mammals, 2nd edn. Oxford Ltd, London, pp. 446-447.
|
|
|
|
|
Makundi RH, Massawe AW, Mulungu LS (2006). Breeding seasonality and population dynamics of three rodent species in the Magamba Forest Reserve, Western Usambara Mounains, north-east Tanzania. Afr. J. Ecol. 45: 17-21.
Crossref
|
|
|
|
|
Miller CJ, Miller TK (1995). Population dynamics and diet of rodents on Rangitoto Island, New Zealand, including the effect of a 1080 poison operation. J. Ecol. 19: 19-27.
|
|
|
|
|
Mohammadi S (2010). Microhabitat selection by small mammals. Advan. Biol. Res. 4: 283-287.
|
|
|
|
|
Mohammed K, Afework B, Graham H (2010). Species composition, abundance and habitat association of rodents and insectivores from Chilalo-Galama Mountain range, Arsi, Ethiopia. Afr. J. Ecol. 48: 1105-1114.
Crossref
|
|
|
|
|
Mugatha SM (2002). Influence of Land Use Patterns on Diversity, Distribution and Abundance of Small mammals in Gachoka Division, Mbeere District, Kenya. LUCID Project Working, International Livestock Research Institute, Nairobi, pp. 1-46.
|
|
|
|
|
Mulungu LS, Makundi RH, Massawe AP, Machangu RS, Mbjie NE (2008). Diversity and distribution of rodent and shrew species associated with variations in altitude on Mount Kilimanjaro, Tanzania. Mammalia 72:178—185.
Crossref
|
|
|
|
|
Nicolas V, Colyn M (2003). Seasonal variation in population and community structure of small rodents in a tropical forest of Gabon. Can. J. Zool. 81: 1034-1046.
Crossref
|
|
|
|
|
Nowak RM (1999). Walker's Mammals of the World, The Johns Hopkins University Press, Baltimore, pp. 6(2):1609-1639.
|
|
|
|
|
Ogada D, Kerbis J, Agwanda B (2009). Preliminary report of shrews and rodents in and around Lake Bogoria National Reserve, Kenya. J. East Afri. Nat.l History 98(1): 129—139.
|
|
|
|
|
Phelps KL (2006). Ecological Characteristics of Small Mammal Communities Inhabiting Tar Creek Superfund Site, Oklahoma. M.Sc. Thesis, Oklahoma State University, Oklahoma.
|
|
|
|
|
Richardson DM, Allsopp N, Dantonio CM, Milton J, Rejmanek M (2000). Plant invasions the role of mutualisms. Biol. Rev. 75: 65-93.
Crossref
|
|
|
|
|
Samuels JX (2009). Cranial morphology and dietary habits of rodents. Zool. J. Linn. Soc. 156: 864-888.
Crossref
|
|
|
|
|
Serekebirhan T, Afework B, Gurja B, Balakrishnan M (2011). A comparison of rodent and insectivore communities between sugarcane plantation and natural habitat in Ethiopia. Trop. Ecol. 52: 61-68.
|
|
|
|
|
Sintayehu W, Afework B, Balakrishnan M (2011). Species diversity and abundance of small mammals in Nechisar National Park, Ethiopia. Afr. J. Ecol. 50: 102-108.
|
|
|
|
|
Smith MH, Gardner RH, Gentry, JB, Kaufman, DW, Ofarrell MJ (1975). Density estimations of small mammal populations. In: Small Mammals: Their Productivity and Population Dynamics (Golley, F. B., Petrusewicz, K. and Ryszkowski. L. eds). Cambridge University Press, Cambridge. pp. 25-53
|
|
|
|
|
Solari S, Rodriguez JJ, Vivar E, Velazco PM (2002). A framework for assessment and monitoring of small mammals in a lowland Tropical Forest. Environ. Mon. Assess. 76: 89-104.
Crossref
|
|
|
|
|
Tadesse H, Afework B (2008). Habitat association of insectivores and rodents of Alatish National Park, northwestern Ethiopia. Trop. Ecol. 49: 1-11.
|
|
|
|
|
Tilahun C, Afework B, Balakrishnan M (2012). Population density, biomass and habitat association of rodents and insectivores in Pawe area, northwestern Ethiopia. Trop. Ecol. 53: 15-24.
|
|
|
|
|
Tilaye W (2005). Reproductive rhythm of the grass rat, Arvicanthis abyssinicus, at the Entoto Mountain, Ethiopia. Belg. J. Zool. 135: 53-56.
|
|
|
|
|
Tobin ME, Fall MW (2004). Pest Control: Rodents. National Wildlife Research Center, U. S. Department of Agriculture, Colorado, pp. 2-22.
|
|
|
|
|
Vaughan TA, Ryan JM, Czaplewski NJ (2000). Mammalogy, 4th edn. Thomson Learning, Inc., New York, pp. 565.
|
|
|
|
|
Vieira MV (2003). Seasonal niche dynamics in coexisting rodents of the Brazilian Cerrado. Stud. Neotro. Faun. Environ. 38: 7-15.
Crossref
|
|
|
|
|
Wolff JO, Sherman PW (2007). Rodent Societies: An Ecological and Evolutionary Perspective. The University of Chicago Press, Chicago, pp. 605.
Crossref
|
|
|
|
|
Workneh G, Afework B, Gurja B, Balakrishnan M (2006). Home range and reproduction of rodents in Maynugus Irrigation Field, Northern Ethiopia. SINET: Ethiop. J. Sci. 29: 57-62.
|
|
|
|
|
Yalden DW, Largen MJ, Kock D (1976). Catalogue of the mammals of Ethiopia insectivora and rodentia. Ital. J. Zool. 15: 1-118.
|
|
|
|
|
Yalden DW (2008). Small mammals of the Bale Mountains, Ethiopia. Afr. J. Ecol. 26: 281-294.
Crossref
|
|
|
|
|
Zerihun G, Afework B, Hemson G (2012). Small mammals of Kaka and Hunkolo, southeast Ethiopia. Trop. Ecol. 53: 33-41.
|
|