ABSTRACT
The Azagny National Park represents one of the most important blocks of forest in Côte d’Ivoire, but its liana assemblage has never been characterized. Liana floristics, diversity, species composition and structure were evaluated. Fourteen plots of 1 ha (20 × 25 m) were established in different biotopes of the forest. All individual lianas of dbh ≥ 1 cm were identified, measured and marked. For each 1 ha plot, old growth forest from secondary forest was distinguished. In total, 5,436 lianas, representing 63 species, 47 genera, and 28 families were identified. The average number of species was 18.35, mean basal area was 0.21 m2 and mean Fisher’s alpha, Shannon index and Simpson diversity index values were 19, 2.81 and 0.72, respectively. Ten dominant plant families accounting for 83% of total species richness, 71% of liana abundance and 71% of basal area were also identified. Twiners, zoochorous, light-demanding and microphanerophyte species dominated. There were more large lianas in old growth forests than in secondary forests. The liana assemblage and species floristic composition is generally similar to that in other tropical African forests.
Key words: Lianas, old growth forest, secondary forest, species diversity, Azagny National Park, Côte d’Ivoire
Lianas (woody climbers) are notoriously abundant in the tropics, contributing up to 25% of the woody stem density (Gentry, 1991; Schnitzer and Bongers, 2002), and up to 12 to 40% of the overall species diversity of such forests (Gentry, 1991). Apart from their direct contribution to biodiversity, lianas help maintaining diversity through their effects on forest structure and dynamics (Putz, 1984; Schnitzer et al., 2012), and thus on species composition of both plants and animals.
A number of studies have documented the functional aspects of lianas in tropical forests. Indeed, lianas substantially contribute to canopy closure after tree fall, stabilizing the microclimate underneath, and contributing to whole-forest transpiration (Schnitzer and Bongers, 2002). Lianas contribute also to the carbon budget of tropical forests (Lewis et al., 2009), representing as much as 10% of the fresh above-ground biomass (Putz, 1984), and accounting for up to 40% of the leaf productivity (Hegarty and Caballé, 1991). By colonizing trees, lianas create structural stress on their hosts, compete for light, water and soil nutrients, generally reducing tree growth (Dalling et al., 2012), and reproduction; while increasing rates of tree fall and limb breakage (Putz, 1984).
The varying species composition of lianas, in different forest types, demonstrates that there are large ecological and functional differences across species. Lianas have a similar growth form and are generally light demanding (Putz, 1984), but they do differ in, for example, climbing mechanisms (Putz and Holbrook, 1991) and light requirements (Kuzee and Bongers, 2005). Furthermore, dispersal type varies markedly across liana species (Cai et al., 2009), and are correlated with a wide range of pollinators and propagule distributers. All this enables lianas to occupy a wide range of habitat types (Nabe-Nielsen, 2001). The abundance, species diversity, and distribution of lianas depends on several abiotic factors, including total rainfall, seasonality (DeWalt et al., 2010), soil fertility (Toledo, 2010), landscape topography (Dalling et al., 2012), canopy structure (Ibarra-Manríquez and Martínez-Ramos, 2002) and disturbance regimes.
Although the importance of lianas is broadly recognized in the tropical world, in Ivorian forests, very few liana studies have been undertaken (Etien and Traoré, 2005; Kuzee and Bongers, 2005; Tra Bi et al., 2005; Kouassi, 2007). The present study could help redress this lack. It aims to evaluate the community structure of the liana assemblage in a national park in the South Côte d’Ivoire. Using data from 14 forest plots of 1 ha each, we (1) describe the floristics, diversity and structure of the liana assemblage in this park; (2) characterize liana functional traits (climbing mechanisms, regeneration guilds, stem size and dispersal syndromes); and (3) determine the effect of forest structure on liana species abundance.
Hypotheses were tested the that (1) the liana assemblage in the Azagny National Park (ANP) is similar in diversity and structure with rain forests elsewhere, but precisely similar other such assemblages in West Africa, and (2) there is a correlation between the number of species and of abundance between the two forest types.
Study site
The Azagny National Park, located in the South of Côte d'Ivoire, between 5° 14' and 5° 31' north latitude, and 4° 76' and 5 ° 01' west longitude, covers an area of 21,850 ha (Kouamé et al., 2010) (Figure 1). It covers the Departments of Grand Lahou and Jacqueville, and is part of the phytogeographical region of "Upper Guinea", which stretches from Senegal to Togo.
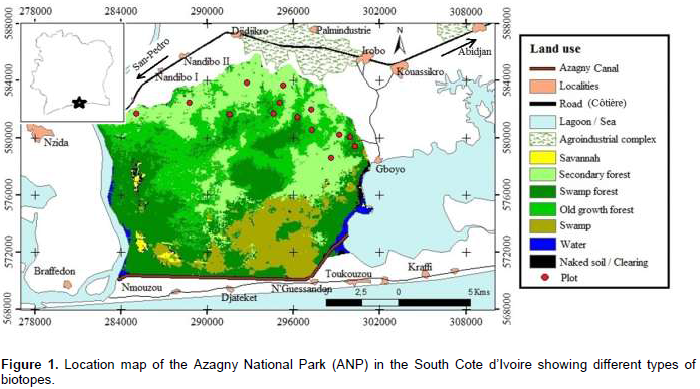
Azagny National Park is a low lying and composed of vast swamp areas (about 9,000 ha), made of salt water, which does not show any sign of natural sinking (Kouamé et al., 2008). The water regime of the park gives its ecological specificity. Three quarters of its area are formed by the complex Bandama River, the Azagny Canal and the Ebrié lagoon. Finally, the park is flooded to 45% throughout the year and 62% during the main rainy season. The climate of the region is sub-equatorial (Avenard et al., 1971). Few studies have been undertaken in this park concerning its diversity. In the same way, the history of the occupation of the park allows a variety of habitats to meet the objectives of this study.
Data collection
From September 2013 to December 2014, data was collected in the Azagny National Park. Fourteen plots of 1 ha, sub-divided into smaller plots (20 × 25 m), were surveyed. Seven different plots have been selected in old growth forests and seven in secondary forests, which are old fallows to allow comparisons.
In each 1 ha plot, a grid of 20 contiguous 20 × 25 m2 quadrats was demarcated. All liana individuals of dbh ≥ 1 cm were identified and measured. Liana dbh was measured at 1.3 m distance along the stem from their rooting point. To facilitate comparison with other liana studies, true liana species was only included: woody climbing plants that germinate on the ground but lose their ability to support themselves as they grow, so they have to rely on external physical support to ascend to the canopy (Gerwing et al., 2006; Schnitzer et al., 2008).
All botanical identifications were based on both reproductive (flowers or fruits) and vegetative (leaves, bark and trunk) characteristics of specimens collected or observed in the field. In most cases, either fertile or sterile material was collected for identification at the reference Herbarium of the Botanic Garden in Félix Houphouët-Boigny University. Family and species nomenclatures follow Lebrun and Stork (1991–1997).
Data analysis
Liana floristic and structural components at three scales were characterized: fine (20 × 25 m quadrat), plot (7 ha) and community (14 ha). All analyses are based on identifications at three taxonomic ranks: species, genus, and family. Morphogroups not identified to a named taxon (10.31% of all recorded stems) were excluded from further analyses.
To describe the liana community structure, for each taxon, the Importance Value Index (IVI), was calculated, that is, the average percentage of relative density, its occurrence frequency (based on 20 × 25 m plots) and its basal area (Mueller-Dombois and Ellenberg, 1974). The total number of species, genera, and families were tallied for each plot (1 ha) and for the whole community (14 ha).
Three indices were used: Fisher’s alpha, the Shannon index, and Simpson diversity (1/D, where D is the standard Simpson Dominance), to calculate liana diversity in the 14 ha community. These indices were selected based on their discriminant ability, sensitivity to sample size, and popularity. Estimate S 8.0 was used to compute the abundance-based coverage estimator (ACE) and Coleman non-parametric estimators of species richness (Chazdon et al., 1998).
In each 20 × 25 m quadrat, the lianas were categorized as small (dbh ≤ 5 cm) or large (dbh > 5 cm). The hypothesis that the number of liana species would affect the liana abundance and that the abundance of species, by diameter class, depends on the type biotope, was tested using Wilcolson test (R 2.15). Old growth forests and secondary forests subplots were compared for abundance of liana stems using t tests.Functional traits/ecological characteristics (climbing mechanism, stem size, regeneration light requirements and primary dispersal syndrome) were assigned to each species, either by direct field observations and/or using data available in the primary literature (Aké-Assi, 2001, 2002; Adou Yao, 2005; Vroh, 2013). Climbing mechanisms for all liana species were categorized as (1) stem twiner, (2) hook climber, (3) root climber, and (4) tendril climber. Stem size was classified following Raunkiær (1934) as: mega-phanerophyte (˃ 30 m), meso-phanerophyte (8 to 29 m), micro-phanerophyte (2 to 7 m), and nano-phanerophyte (Ë‚ 2 m). Regeneration light requirements were grouped into two classes: light demanding and shade tolerant. Three primary dispersal syndrome classes were used: anemochory, zoochory and barochory.
Floristic and taxonomic diversity
A total number of 5,436 stems were recorded in the fourteen 1 ha plots. Of these stems, 89.69% (4,876 stems) were identified to species level, and represented 63 species in 47 genera and 28 families. Secondary forest (41 species, 32 genera and 25 families) was slightly richer than old growth forest (39 species, 34 genera and 21 families). The ten most abundant species collectively accounted for 46.3% (2,258 stems) of all stems, and 57.6% (1.33 m²) of all basal area (Table 1). Adenia lobata (Passifloraceae) had the highest Importance Value Index (19.2%): it accounted for 6.9% of all liana stems and 5.4% of the total basal area, and was distributed in 6.9% of the quadrats. The ten most important genera included 16 species (25.4%), and contributed 54.7% of all stems and 58.3% to the basal area. Adenia (Passifloraceae) was the most abundant genus, but Strophantus (Apocynaceae) had the highest basal area (8.8%). Cissus (3 species) was the most speciose genus but contributed only 3.8% to abundance, and 2.5% to basal area. Ten of 28 families contained 27 genera, and contributed 71.0% to the number of stems, 70.9% to basal area, and 90.0% to total Importance Value Index. The most speciose families were Fabaceae (7 species), Apocynaceae (5 species), and Icacinaceae (5). Calamus deerratus (Arecaceae) was the only palm liana in the Azagny National Park liana assemblage.
Species richness and diversity
An average of 18.35 species, 13 genera and 11.28 families were recorded per hectare (Table 2). Fisher’s α was 19.0 ± 2.7 ha-1, Shannon index was 2.81 ± 0.25 ha-1, and Simpson index was 0.72 ± 0.01 ha-1. In the whole community (14 ha), we found that hectare-based species number estimates ranged from Coleman = 50.3 to ACE = 74.8. At the smaller scale of 20 × 25 m quadrats, all values were considerably lower.
Liana assemblage structure
Based on the fourteen 1 ha plots, mean stem density was 388.3 ± 143.9 stems ha-1, and mean basal area was 0.21 ± 0.13 m2 ha-1. Taxonomic abundances (Figure 2), at the 14 ha level, varied greatly. Six species (6%) were only known as two individuals; while 56% of all stems were represented by species with less than sexteen liana stems (Figure 2A). In contrast, genera and families exhibited lognormal-like distributions (Figure 2B and C), indicating that taxa vary markedly in their abundances. Most liana individuals were small: nearly 78% were smaller than 3 cm in diameter; while only 1% of stems had a dbh of more than 10 cm (Figure 2E). On average, stems measured between 2 and 3 cm in dbh. The largest stem measured was 17 cm dbh (Leptoderris ledermannii, Fabaceae). Species richness (Figure 2D), abundance (Figure 2E), and total basal area (Figure 2F) decreased with increasing stem size. Large lianas (> 11 cm dbh) contributed 8% to the total liana basal area and included 6 species.
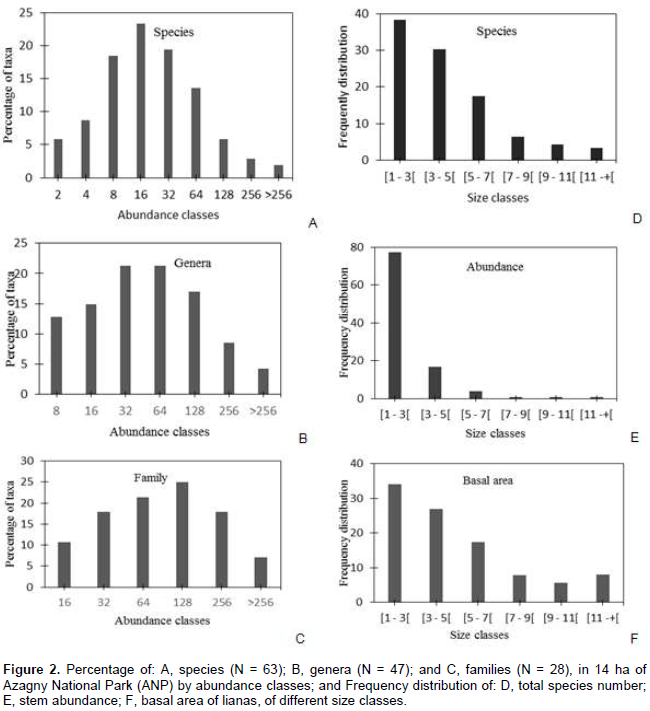
The correlation test shows that the number of species is correlated positively with the abundance (N = 0.86, p Ë‚ 0.0001). When the number of liana species increases, abundance also increases. There is no difference between the proportion of small lianas (dbh ≤ 5 cm) patches of old growth forest (average 17.67 ± 6.58), and those of the secondary forest (average 18.92 ± 9.01). By contrast, for large lianas (dbh ˃ 5 cm), there is a difference between old growth forest (mean 1.92 ± 2.37) and secondary forest (average 0.30 ± 0.8). There are more large lianas in old growth forest than in secondary forest. Secondary forest plots had more liana stems (mean 8.59 ± 1.90) than old growth forest plots (mean 9.31 ± 2.19), possibly partly because the secondary forest fallows are recent and continue to be pressured.
Liana characteristics
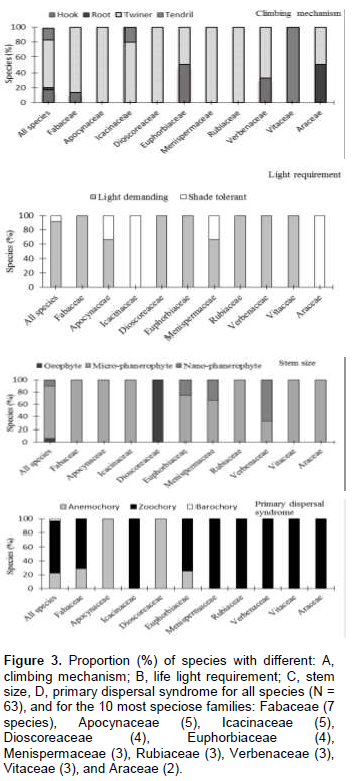
The functional and ecological characteristics are summarized for the total species assemblage, as well as for the 10 most important families separately (Figure 3). Most liana species were stem twiners (63%): Secamone afzelii and Baissea baillonii, followed by hook climbers (18%): Salacia debilis and Clerodendrum formicarum, and tendril climbers (16%): A. lobata and Cissus aralioides. Liana species were predominantly micro-phanerophyte (84%) or nano-phanerophyte (10%), in stem size. Most species were light demanding (92%); only a few were either shade tolerant. The seeds of most species were animal dispersed (75%), followed by wind dispersed (22%). Only a few species were barochorous (3%). With few exceptions, individual families generally exhibited similar trends in functional characteristics to the complete liana assemblage. Notable exceptions are as follows: Fabaceae are mostly twiner-climbers and Vitaceae are only tendril climbers. Icacinaceae are only shade-tolerant. Icacinaceae, Menispermataceae, Araceae, Verbenaceae, Vitaceae and Rubiaceae species are entirely dispersed by animals; while all of the Apocynaceae and Dioscoreaceae lianas are wind-dispersed.
Floristic composition
Nearly all individuals (89.69% of all stems) were identified to family, genera, or species. The same result has been
found by Ewango et al. (2015), who identified 90.2% of all individuals in a 20 ha plot in the Congo Basin. Our results are lower than those of Schnitzer et al. (2012), who identified 98.5% of all individuals in a 50 ha plot in Panama, and Kuzee and Bongers (2005), who identified 94% of their lianas in Côte d’Ivoire, but are generally well above the results reported in most other studies. The liana flora, in our study plots, was dominated by only a few widespread and more generalist species, among them, A. lobata. Such dominance may be the result of effective dispersal capacity, prolific vegetative sprouting, lack of specific habitat requirements, and low abundance of seed predators, or combinations of these. Although this species is generally thought to be light-demanding (pioneer), it was also observed in shady environments (Kouamé et al., 2005).
Species composition and family dominance of lianas in Azagny are largely the same as those found in most
West African tropical forests Côte d’Ivoire (Etien and Traoré, 2005); Benin (Natta and Sinsin, 2005), and Togo (Kokou and Caballé, 2005). The most abundant families of Azagny (Apocynaceae, Passifloraceae, Euphorbiaceae, Fabaceae, Menispermaceae, Verbenaceae and Piperaceae) are widely distributed in West Africa (Kuzee and Bongers, 2005; Addo-Fordjour et al., 2008), even in Central Africa Cameroon (Parren, 2003) and Democratic Republic of Congo (Ewango et al., 2015), suggesting a strong similarity among West and Central African lowland forests. The speciose families Apocynaceae, Euphorbiaceae, and Fabaceae (which also include many non-lianas) and Vitaceae, Passifloraceae, Dioscoreaceae (only including climbers) are important liana families in both Old and New World floras (Gentry, 1991). The most common liana families in Asian forests are Menispermaceae, Fabaceae, Rubiaceae, Dilleniaceae and Icacinaceae (Appanah et al., 1993) suggesting a closer affinity to the Azagny Forest assemblage. However, the Azagny National Park is poor in palm lianas compared to the Neotropics and continental Asian forests.
Diversity and community structure
Species richness and species diversity (Shannon index and Fisher α) indices increased with sample sizes, as predicted. The Azagny National Park contains a few highly abundant lianas. A. lobata accounts for 7% of all liana stems, with 376 individuals, in 14 ha. These results are lower when compared with those of certain authors. Strophanthus barteri (Apocynaceae) represents 12% of stems in Ghana (Addo-Fordjour et al., 2008); while Maripa panamensis (Convolvulaceae) represents 11% of stems in both secondary and primary forests in Panama (DeWalt et al., 2000).
Family dominance, however, was in accord with other studies in Africa (Gentry, 1991; Bongers et al., 2005), with Apocynaceae, Passifloraceae, Euphorbiaceae, Fabaceae, Menispermaceae, Verbenaceae, Piperaceae, Aristolochiaceae, Vitaceae and Icacinaceae being the most important families. The 10 most abundant liana species (of 63) represent 71% of the stems, which may be characteristic of the Azagny National Park.
Small lianas account for the highest species richness, abundance, and basal area (Figure 2D to F) and, compared to other tropical forests, the Azagny National Park is particularly poor in large liana stems. Again, it was speculated that this is the result of disturbances in the recent past (Konan, 2008). Alternatively, its high liana density may be related to the climate seasonality (DeWalt et al., 2010) under which large-diameter lianas are few (nutrient-poor soils have fewer lianas (Gentry, 1991).
Within the Azagny National Park, liana density (388.3 ± 143.9 stems ha-1) is lower compared to other African forests (Parren, 2003; Ewango et al., 2015). Some Bolivian Amazonian forests showed exceptionally high liana density (mean of 2,471 lianas ha-1 ≥ 2 cm dbh), and lianas accounted for as much as 44% of the total woody species (Pérez-Salicrup et al., 2001).
As predicted, the abundance of lianas was positively correlated with the number of species, which means that the number of species increases with abundance. The proportion of small vines (dbh ≤ 5 cm) is the same in both habitats. By contrast, there is a big difference in terms of large lianas (dbh ˃ 5 cm) in both habitats. Forest disturbance (Hegarty and Caballé, 1991) and seasonality (Gentry, 1991; DeWalt et al., 2010) are factors most strongly controlling the abundance, species richness, and distribution of lianas in other forests. Abundance was higher in secondary forests plots than in old growth forests plots. This is caused by the human activities that take place in secondary forests. These activities include agriculture, hunting and gathering of plant species (Konan, 2008).
Functional characteristics of the liana community
In Azagny, twining is the dominant climbing mode (63% of the species, Figure 3A). Our findings corroborate that of many other studies in tropical forests: Laurance et al. (2001) in Brazil; Sridhar Reddy and Parthasarathy (2003) in India; and Addo-Fordjour et al. (2008) in Ghana. Because of their ability to ascend trees directly, twining species indiscriminately colonize a wide range of trees and species. There seems to be an association between stem mechanical architecture and climbing mechanism; some families with heavy stems are exclusively twining (e.g. Fabaceae, Asclepiadaceae, Menispermaceae, and Apocynaceae) or hook-climbing (e.g. Verbenaceae); while other families that tend to have flexible stems, also rely on tendrils (e.g. Passifloraceae, like some Adenia and most Vitaceae species, Cissus).
Herbaceous climbers are generally light-demanding, since they establish and grow particularly well in large clearings (Putz, 1984). In contrast, woody lianas often occur in very heterogeneous light habitats such as in old gaps, forest margins, and under irregular and broken forest canopies (Putz, 1984; Hegarty and Caballé, 1991). Most liana species can start their life as a seedling in the understorey, and wait for a long time until they find support and get access to the canopy. Liana abundance in old-growth forest is, therefore, not so much determined by light availability, but rather by trellis availability. Eight per cent of the Azagny National Park liana species were classified as being shade tolerant. These species have the ability to remain self-supporting for a longer period, and can grow several meters tall before they have to rely on trees for support, for instance Piper guineense, Cercestis afzelii, Flagellaria guineensis and Xylopia acutiflora.
The prevalence of zoochory shows the importance of animals in the liana’s dissemination (Figure 3). The same result has been found by some authors as Bullock (1995) and Addo-Fordjour et al. (2008). This is important for conservation: most lianas rely on animals for their seed dispersal and/or pollination, whilst animals rely on them for food and habitat (Ødegaard, 2000; Schnitzer and Bongers, 2002).
The authors have not declared any conflict of interests.
The authors would like to thank the population around the ANP and Ivorian Office of Parks and Reserves for the authorizations and the agents of that office for their help on field. They would like to thank PASRES for helping in the funding of this study.
REFERENCES
|
Addo-Fordjour P, Anning AK, Atakora EA, Agyei PS (2008). Diversity and distribution of climbing plants in a semi-deciduous rain forest, KNUST botanic garden, Ghana. Int. J. Bot. 4(2):186-195.
Crossref
|
|
|
|
Adou Yao CY (2005). Pratiques paysannes et dynamiques de la biodiversité dans la forêt classée de Monogaga (Côte d'Ivoire). Thèse Doctorat unique, Département Hommes Natures et Société, Université MNHN, Paris. pp. 233.
|
|
|
|
Aké Assi L (2001). Flore de la Côte d'Ivoire 1, catalogue, systématique, biogéographie et écologie. Genève, Suisse : Conservatoire et Jardin Botanique de Genève; Boisseria 57:396.
|
|
|
|
Aké Assi L (2002). Flore de la Côte d'Ivoire 2, catalogue, systématique, biogéographie et écologie. Genève, Suisse : Conservatoire et Jardin Botanique de Genève; Boisseria 58:441.
|
|
|
|
Appanah S, Gentry AH, Lafrankie JV (1993). Liana diversity and species richness of Malaysian rain forests. J. Trop. For. Sci. 6:116-123.
|
|
|
|
Avenard JM, Eldin M, Girard G, Sircoulon J, Touchebeuf P, Guillaumet JL, Adjanohoun E, Perraud A (1971). Le Milieu Naturel de Côte d'Ivoire. Mémoire Orstom: Paris, France. pp. 392.
|
|
|
|
Bongers F, Parren MPE Traoré D (2005). Forest climbing plants of West Africa: diversity, ecology and management. Wallingford, Oxfordshire, CAB International.
|
|
|
|
Bullock SH (1995). Plant reproduction in neotropical dry forests. In: Bullock SH, Mooney HA, Medina E (Eds) Seasonally dry tropical forests: 277-303.
Crossref
|
|
|
|
Cai Z-Q, Schnitzer SA, Wen B, Chen YJ, Bongers F (2009). Liana communities in three tropical forest types in Xishuangbanna, South-West China. J. Trop. For. Sci. 21:252-264.
|
|
|
|
Chazdon RL, Colwell RK, Denslow JS, Guariguata MR (1998). Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of NE Costa Rica. In: Dallmeier F, Comiskey JA (Eds) Forest biodiversity research, monitoring and modeling: conceptual background and Old World case studies: 285-309.
|
|
|
|
Dalling JW, Schnitzer SA, Baldeck C, Harms KE, John R, Mangan SA, Lobo E, Yavitt JB Hubbell SP (2012). Resource-based habitat associations in a neotropical liana community. J. Ecol. 100:1174-1182.
Crossref
|
|
|
|
DeWalt SJ, Schnitzer SA Denslow JS (2000). Density and diversity of lianas along a chronosequence in a Central Panamanian lowland forest. Journal of Tropical Ecology 16: 1–19.
Crossref
|
|
|
|
DeWalt SJ, Schnitzer SA, Chave J, Bongers F, Burnham RJ, Cai Z, Chuyong G, Clark DB, Ewango CEN, Gerwing JJ, Gortaire E, Hart T, Ibarra-Manríquez G, Ickes K, Kenfack D, Macía MJ, Makana J-R, Martínez-Ramos M, Mascaro J, Moses S, Muller-Landau HC, Parren MPE, Parthasarathy N, Pérez-Salicrup DR, Putz FE, Romero-Saltos H, Thomas D (2010). Annual rainfall and seasonality predict pan-tropical patterns of liana density and basal area. Biotropica 42:309-317.
Crossref
|
|
|
|
Etien DT, Traoré D (2005). Climbing plants of Haut-Sassandra forest: species and biomorphology. In Bongers F, Parren MPE, Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 137-145.
|
|
|
|
Ewango CEN, Bongers F, Makana J-R., Poorter L, Sosef MSM (2015). Structure and composition of the liana assemblage of a mixed rain forest in the Congo Basin. Pl. Ecol. Evol. 148(1):29-42.
Crossref
|
|
|
|
Gentry AH (1991). The distribution and evolution of climbing plants. In: Putz FE, Mooney AH (Eds) The biology of vines: pp. 3-50. Cambridge, Cambridge University Press.
|
|
|
|
Gerwing JJ, Schnitzer SA, Burnham RJ, Bongers F, Chave J, De Walt SJ, Ewango CEN, Foster R, Kenfack D (2006). A standard protocol for liana censuses. Biotropica 38: -256-261.
|
|
|
|
Hegarty EE, Caballé G (1991). Distribution and abundance of vines in forest communities. In: Putz FE, Mooney AH (Eds) The biology of vines: pp. 313-336. Cambridge, Cambridge University Press.
|
|
|
|
Ibarra-Manríquez G Martínez-Ramos M (2002). Landscape variation of liana communities in a Neotropical rain forest. Plant Ecol. 160:91-112.
Crossref
|
|
|
|
Kokou K, Caballé G (2005). Climbers in forest fragments in Togo. In Bongers F, Parren MPE, Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 109-121.
|
|
|
|
Konan KE (2008). Conservation de la diversité végétale et activtés humaines dans les aires protégées du sud forestier ivoirien : l'exemple du Parc National d'Azagny. Thèse, Université de Cocody-IGT. pp. 270.
|
|
|
|
Kouamé D, Adou Yao CY, Kouassi KE, N'Guessan KE, Akoi K (2008). Preliminary Floristic Inventory and Diversity in Azagny National Park (Côte d'Ivoire). European Journal of Scientific Research, 23(4): 537-547.
|
|
|
|
Kouamé D, Adou Yao CY, Nandjui A, N'Guessan KE (2010). Le rôle de l'éléphant dans la germination des graines de Irvingia gabonensis (Irvingiaceae), Balanites wilsoniana (Balanitaceae), Parinari excelsa (Chrysobalanaceae) et Sacoglottis gabonensis (Humiriaceae) en forêt tropicale : cas du Parc National d'Azagny en Côte d'Ivoire. Int. J. Biol. Chem. Sci. 4(5):1442-1454
|
|
|
|
Kouamé FN', Traoré D, Bongers F, Poorter L (2005). Climbers after logging: the case of Haut-Sassandra. In Bongers F, Parren MPE, Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 109-121.
|
|
|
|
Kouassi KE (2007). Flore de la forêt classée de la Haute Dodo, Sud-Ouest de la Côte d'Ivoire. Étude de quelques espèces commercialisées : cas de Garcinia afzelii (Clusiaceae), des rotins (palmiers lianes) des genres Calamus, Eremospatha et Laccosperma (Arecaceae). Thèse unique de botanique, option foresterie, Unité de Formation et de Recherche de Biosciences, Université de Cocody-Abidjan. pp. 214.
|
|
|
|
Kuzee ME, Bongers F (2005). Climber abundance, diversity and colonization in degraded forests of different ages in Côte d'Ivoire. In Bongers F, Parren MPE, Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 73-91.
|
|
|
|
Laurance WF, Pérez-Salicrup D, Delamônica P, Fearnside PM, D'Angelo S, Jerozolisnki A, Pohl L, Lovejoy TE (2001). Rain forest fragmentation and the structure of Amazonian liana communities. Ecol. 82:105-116.
Crossref
|
|
|
|
Lebrun J-P, Stork AL (1991-1997). Énumération des plantes à fleurs d'Afrique tropicale. Vols 1-4. Genève, Conservatoire et Jardin botaniques de la Ville de Genève.
|
|
|
|
Lewis SL, Lopez-Gonzalez G, Sonké B, Affun-Baffoe K, Baker TR, Ojo LO, Phillips OL, Reitsma JM, White L, Comiskey JA, Djuikouo MK, Ewango CEN, Feldpausch TR, Hamilton AC, Gloor M, Hart T, Hladik A, Lloyd J, Lovett JC, Makana J-R, Malhi Y, Mbago FM, Ndangalasi HJ, Peacock J, Peh KS-H, Sheil D, Sunderland T, Swaine MD, Taplin J, Taylor D, Thomas SC, Votere R, Wöll H (2009). Increasing carbon storage in intact African tropical forests. Nature 457:1003-1006.
Crossref
|
|
|
|
Mueller-Dombois D, Ellenberg H (1974). Aims and methods of vegetation ecology. New York, John Wiley & Sons.
|
|
|
|
Nabe-Nielsen J (2001). Diversity and distribution of lianas in a neotropical rain forest, Yasuní National Park, Ecuador. J. Trop. Ecol. 17:1-19.
Crossref
|
|
|
|
Natta AK, Sinsin B (2005). Taxonomic diversity of climbers of riparian forests in Benin. In Bongers F, Parren MPE, Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 123-136.
|
|
|
|
Ødegaard F (2000). The relative importance of trees versus lianas as host for phytophagous beetles (Coleoptera) in tropical forests. J. Biogeogr. 27:283-296.
Crossref
|
|
|
|
Parren MPE (2003). Lianas and logging in West Africa. Tropenbos-Cameroon Series 6. Wageningen, Tropenbos International.
|
|
|
|
Pérez-Salicrup DR, Sork V, Putz FE (2001). Lianas and trees in a liana forest of Amazonian Bolivia. Biotropica 33:34-47.
Crossref
|
|
|
|
Putz FE (1984). The natural history of lianas on Barro Colorado Island, Panama. Ecol. 65:1713-1724.
Crossref
|
|
|
|
Putz FE, Holbrook NM (1991). Biomechanical studies of vines. In: Putz FE, Mooney HA (Eds) The biology of vinesn Cambridge, Cambridge University Press: pp. 73-98.
|
|
|
|
Raunkiær C (1934). The life forms of plants and statistical plant geography. Oxford, Clarendon Press.
|
|
|
|
Schnitzer SA, Bongers F (2002). The ecology of lianas and their role in forests. Trends Ecol. Evol. 17:223-230.
Crossref
|
|
|
|
Schnitzer SA, Mangan SA, Dalling JW, Baldeck CA, Hubbell SP, Ledo A, Muller-Landau H, Tobin MF, Aguilar S, Brassfield D, Hernandez A, Lao S, Perez R, Valdes O, Rutishauser Y.S. (2012). Liana abundance, diversity, and distribution on Barro Colorado Island, Panama.
|
|
|
|
Schnitzer SA, Rutishauser S, Aguilar S (2008). Supplemental protocol for liana censuses. For. Ecol. Manag. 255(2008):1044-1049.
Crossref
|
|
|
|
Sridhar Reddy M, Parthasarathy N (2003). Liana diversity and distribution in four tropical dry evergreen forests on the Coromandel coast of south India. Biodivers. Conserv. 12:1609-627.
Crossref
|
|
|
|
Toledo MV (2010). Neotropical lowland forests along environmental gradients. PhD Thesis, Wageningen University, Wageningen, The Netherlands.
|
|
|
|
Tra Bi F H, Kouamé FN', Traoré D (2005). Utilisation of climbers in two forest reserves in west Côte d'Ivoire. In: Bongers F, Parren MPE Traoré D (Eds.), Forest Climbing Plants of West Africa: diversity, ecology and management. CABI Publishing, Cambridge (UK), pp. 167-181.
|
|
|
|
Vroh BTA (2013). Evaluation de la dynamique de la végétation dans les zones agricoles d'Azaguié (Sud-est Côte d'Ivoire). Université Félix-Houphouët-Boigny, UFR Biosciences, Thèse Unique de Botanique. P 131.
|