ABSTRACT
The African elephant (Loxodonta africana Blumenbach) is a keystone species and ecosystem engineer. Elephants can cause serious damage to important trees, with only certain species being targeted such as Marula (Sclerocarya birrea A. Rich. Hoscht). High levels of elephant utilization may to some extent, compromise the viability of some woody plant populations leading to vegetation changes coupled with a possible loss of species diversity and/or structural diversity. In order to quantify their effect a study was initiated in 2014 to investigate their effect on tree height, degree of branch damage, the extent of debarking, and degree of stem damage. This was done within elephant’s frequently and non–frequently used sites, and a neighbouring enclosure (control site). One hundred and fifty (50 per site) mature S. birrea trees were randomly selected within each site. Tree height was recorded using clinometers, degree of branch damage, extent of debarking (circumference debarked using different percentages of intensity) and degree of stem damage were assessed using different categories. Results indicated that the type elephant damage in both the frequently and non–frequently used sites was different, varied in intensity. A high proportion of Marula trees had been damaged. The size distributions of the trees showed that there was no regeneration. Furthermore, this study also demonstrated that elephants are able to damage Marula trees in several ways, the most destructive being bark stripping and pushing over trees. It is concluded that elephant impact is a powerful mechanism in shaping the structure and composition of Marula woodlands in the Atherstone Collaborative Nature Reserve. The findings of this study provide valuable baseline data and acts as a starting point for the introduction of adaptive management principles in small savanna reserves. This can be achieved by an intensive management programme responding to slight changes in the vegetation and would necessarily involve controlling elephant numbers.
Key words: Crown diameter, damage, elephant, herbivory, marula.
A home range of an animal can be defined as the total area occupied by an individual or group (Schindler, 2005). Habitat diversity in the landscape will influence the location and size of an elephant home range (Okello et al., 2015). Home ranges of elephants in the Atherstone Collaborative Nature Reserve have diversity of habitats, dominated by bushland, woodland and grassland. Elephants roam the landscapes utilizing different habitats and its resources that meet their needs and enhance their survival (Harris et al., 2008). Okello et al. (2015) further indicated that within these broad habitats, different vegetation structures occur, which differ in woody plant density and composition. These different habitats provide diverse resource types needed for elephant survival. Such areas become their core use home range and elephants seem to show preference for such landscapes. Elephants expand their home range in the wet season to find suitable forage, and concentrate near water points in the dry season in regions where water availability is highly seasonal (Okello et al., 2015).
Habitat characteristics and resources within them can determine level of preference and use of different habitats. The level of impact of elephant densities is governed by elephant feeding behavior co–occurring with other ecological and environmental factors (Ferguson, 2014). The effect of herbivory on woody plants depend on the intensity and frequency of damage, plant phenological, resource relationships at the time of herbivory, plant tissue(s) removed (Clegg, 2010), the availability of resources in the environment to support regrowth, and the browsing history of the plant (Gadd et al., 2001). The outcome of herbivore impact on a particular woody species depends on the nature of the damage, the ability of the plant to recover, its demography and role that it plays in a plant community (De Boer, 2015).
Sclerocarya birrea subspecies caffra (Sond.) Kokwaro, also known as Marula is highly selected by the African elephant (Loxodonta africana Blumenbach) (Shannon et al., 2008), and hence heavily utilized (Jacobs and Biggs, 2001). The repeated browsing by elephants causes serious damage through breaking and removing of branches, and by preventing or reducing recruitment and regeneration (Balfour et al., 2007). However, S. birrea trees are resilient to most types of damage (Vogel et al., 2014). They can resprout from the base or epicormically if toppled (i.e. pushed over; the roots can either remain in the soil or the tree can be uprooted to varying degrees), or if the canopy is broken (Jacobs and Biggs, 2002). Hence, it is expected that S. birrea trees are able to sustain relatively high levels of damage before adults die (Vogel et al., 2014). Debarking depends on the ease with which bark can be separated from the underlying wood (Landman et al., 2007). Species with single stems and whose bark has to be chiselled off rather than stripped (e.g. S. birrea) can eventually be ring barked, while species with more than one main stem, but whose bark otherwise strips easily, can usually not be debarked (e.g. A. erubescens) (Loarie et al., 2009). The ease with which Marula trees can be debarked by elephants could ultimatey lead to the mortality of the Marula tree population at the ACNR. It is thus recommended that measures be implemented to protect Marula trees from being debarked. These could be in the form of laying stones around the stem to restrict elephants from coming in contact with the trees. This method has been successfully used to prevent debarking by elephants in the Addo Elephant National Park (Lombard et al., 2001). During 1994, 20 elephants were introduced to the ACNR. According to aerial and ground surveys conducted during 2015, all indications are that elephant numbers are in the vicinity of 106 animals. The total stocking rate equates to 10.35 ha/LSU, with the stocking rate for the grazing component 14.14 ha/LSU, and the number of browser units at 6.19 BU/100 ha. This suggests that the carrying capacity for elephants at the ACNR has been exceeded by far. Reserve management is furthermore of the opinion that the large elephant number has had a significant ecological impact on the vegetation composition of the reserve. This has led to large–scale changes in the demographic structure of Marula, mainly characterized by a reduction in the number of larger trees (Kerley et al., 2008). For the ACNR a much lower portion ranging between 6 and 8% of the total biomass is recommended by LEDET (Kruger, 2013).
The aim of this study was to investigate and compare the damage by elephants to Marula trees occurring in different landscapes in the ACNR, in order to obtain a detailed assessment of the current Marula population status in the reserve. The damage was investigated in terms of: (a) height of damage, (b) branch herbivory, (c) debarking damage, (d) stem damage and uprooting by elephants. The population structure of Marula on the reserve proved to be unstable. Due to the fact that no regeneration of Marula trees is evident, the current generation of Marula trees is under severe pressure from a too large population of elephants. Such degradation could lead to a loss in ecosystem function, which not only implies a loss in ecosystem productivity and resilience, but also the need for ecosystem restoration.
Study area
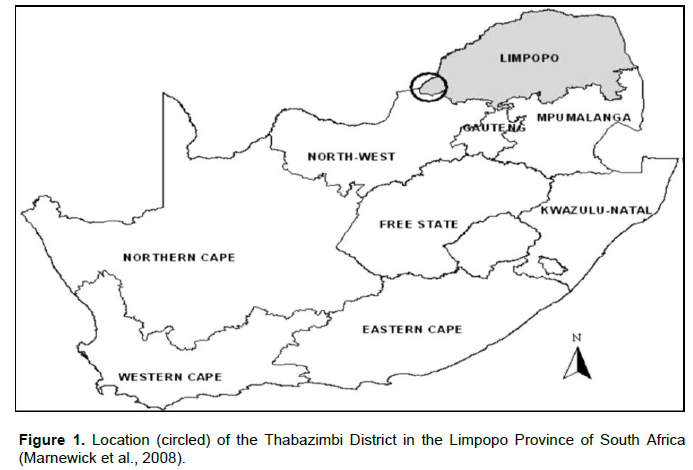
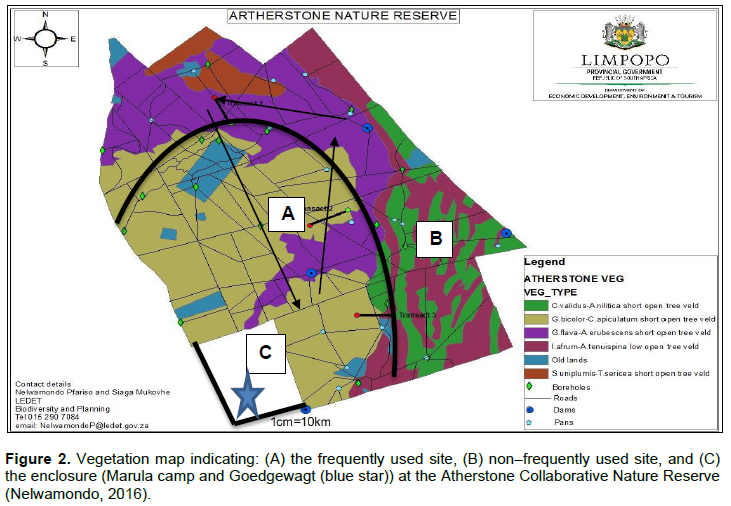
The Atherstone Collaborative Nature Reserve (24Ëš34.491’S and 26Ëš47.11’E) is situated in the Thabazimbi District of the Limpopo Province’s Bushveld Region in South Africa (Figure 1). The reserve covers an area of approximately 22688,163 ha (Pretorius, 2011). Vegetation and landscape features vary from tall open woodland to low woodland. The study commenced in May to December. The study was conducted within three areas (Figure 2): the Mixed Bushveld and Turf Thornveld veld types, which dominates the reserve, and the Marula camp and Goedgewag area (an enclosure).
Western Sandy Bushveld (Figure 3) varies from tall open woodland to low woodland with broad–leaved and microphyllous tree species being prominent (Mucina and Rutherford, 2006). The Vegetation Unit was further devided into Mixed Bushveld and Turf Thornveld (Acocks, 1954), in an area that was classified by Pauw (1988) as Grewia bicolor – Combretum apiculatum Short Open Tree Veld. This veld type was again was reclassified in 2004 by De Klerk (2004) as Red Bushwillow–veld. Dominant tree species include A. erubescens on flat areas, Combretum apiculatum on shallow soils of gravelly upland sites and Terminalia sericea on deep sands. Other tree and shrub species include; Acacia erioloba (E. Mey.), A. nigrescens and Sclerocarya birrea (tall trees); A. mellifera subsp. detinens, A. nilotica and Combretum zeyheri (small trees); C. hereroense, Euclea undulata and Coptosperma supra–axillare (tall shrubs); and Clerodendrum ternatum, Indigofera filipes and Justicia flava (low shrubs). The field layer comprises grass species such as Anthephora pubescens, Digitaria eriantha subsp. eriantha, Eragrostis pallens, E. rigidior and Schmidtia pappophoroides. Herbs that occur in the vegetation include; Blepharis integrifolia, Chamaecrista absus, Evolvulus alsinoides and Geigeria burkei (Mucina and Rutherford, 2006). Sclerocarya birrea stand out in this veld type, with a number of large trees towering above other trees.
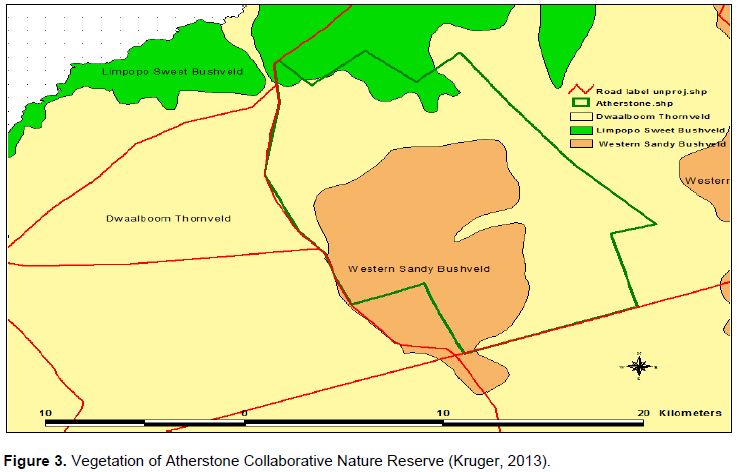
They give a special character to this veld type, but are poorl represented (Mucina and Rutherford, 2006).
The Marula camp and Goedgewagt enclosure is Western Sandy Bushveld (Mucina and Rutherford, 2006). The Goedgewagt is an area outside the reserve which mainly caters for livestock, the Marula trees found in the area are not damaged, and it was selected together with Marula camp as a way of comparison measure between the different landscapes. The enclosure is part of the bigger Combretum apiculatum/S. birrea veld type. The only animals that can get access to the Marula Camp are Vervet monkeys (Cercopithecus aethiops Linnaeus) and warthogs (Phacochoerus aethiopicus Pallas). Frequently used sites can be defined as areas that become elephant’s core use as seem to show preference for such landscapes. So, habitat characteristics and resources within them can determine level of preference. Non–frequently used sites are defined as those areas that are not used more frequently as other areas.
Data collection
One hundred and fifty mature Marula trees were randomly selected, with 50 each in the three study areas. The following data was collected under damage classification:
i) Tree height was recorded, determined by measuring it with a 2 m survey clinometer, using the following height classes: 8–10, 10–12, 12–14, 14–16, and 16–18 m. Height was then totalled and expressed in percentage.
ii) Degree of branch damage (Figure 4A) was assessed, using five categories: 1 = no utilization, 2 = minor utilization (a few minor branches broken), 3 = moderate utilization (many minor branches broken), 4 = high utilization (main branches broken), and 5 = main stem utilization (main meristem broken off).
iii) The extent of debarking (Figure 4B) focused on debarking only (focusing on the main stem) and was evaluated using five categories: 1 = no bark removal, 2 = 1–25% of circumference debarked, 3 = 25–50% debarked, 4 = 50–75% debarked and 5 = 75–100% debarked.
iv) Degree of stem damage was assessed (the degree of the stem damage mainly focused on the intensity of the damage), using six categories: 1 = no damage, 2 = main stem completely ring-barked, 3 = whole tree pushed over, main stem broken but still partly attached, 4 = whole tree uprooted, 5 = whole tree pushed over, main stem still intact and 6 = canopy and one of main stems removed.
v) Feeding modes whereby the main stem was pushed over or broken was considered to represent 100% damage. Uprooting events in which all the stems were removed or flattened were also classified as 100% damage. Damage by factors other than elephant (other large mammalian browsers such as giraffe or old age, disease or lightning) was classified as unknown damage in accordance with Ben–Shahar (1993).
Data analysis
Data were analysed via a two–tailed t–test for independent samples, using SPSS software (SPSS, 2013). The total numbers of counts per category were compared.
Height
The results show no significant difference in tree height between frequently and non–frequently used sites and the enclosure. Only mature trees occurred at all three sites. The average tree height in the non–frequently used site was 12.50 m, compared to 11.58 m in the frequently used site, while in the enclosure, it was 12.38 m. According to Figure 5, 45% of the frequently used site trees occurred in the 8 to 10 m height class, 32% in the 10 to 12 m and 23% in the 14 to 16 m height class in the frequently used site. In the non–frequently used site, 25% of the trees occurred in the 8 to 10 m height class, whereas 31% in the 10 to 12 m and 44% in the 14 to 16 m height class. In the enclosure, 25% of the trees occurred in the 8 to 10 m height class, whereas 36% occurred in the 10 to 12 m, and 34% in the 14 to 16 m height class. No trees lower than 8 m and between 12 to 14 m were encountered.
Branch damage
In terms of branch damage, a significant difference (p<0.05, t–value = -4.748, df=88.215) was found between frequently used sites (56%, n= 50) and non–frequently used sites (37.2%, n= 50) (Table 1). Elephant impact on branches seemed to decrease with increases in tree height. In the frequently used site, only 6% of Marula trees were not damaged, whereas 24% of Marula trees were not damaged in the non–frequently used site. No damage occurred in the enclosure. In the frequently used site, 42% of Marula trees were classified as those that had minor branch utilization, 79% as moderate branch utilization and 75% as high branch utilization, respectively, compared to 58% of Marula trees within minor branch utilization , 21% moderate branch utilization, and 25% high branch utilization classes in the non–frequently used site (Figure 6).
Debarking damage
In terms of debarking damage, a significant difference (p<0.05, t–value = -.161, df=97.907) was found between frequently used sites (73.6%, n= 50) and non–frequently used sites (72.8%, n= 50) (Table 1). In total, including frequently and non–frequently used sites, 96% of all the surveyed Marula trees had debarking damage (Figure 7), while no damage occurred in the enclosure. Of these, more than 50% had undergone major damage in the form of ringbarking. Only 4% of Marula trees had no bark removal in the frequently used site, in the non–frequently used site all recorded trees were debarked. In the minor debarking damage by elephants, while major frequently used site, 33% of surveyed trees experienced damage was encountered on 52% of the surveyed trees. In the non–frequently used site, 67% of surveyed trees had minor debarking damage, while major debarking damage was observed on 52% of trees.
Stem damage
In terms of stem damage, a significant difference (p<0.05, t–value = -3.176, df=61.864) was found between frequently used sites (37.7%, n= 50) and non–frequently used sites (28.8%, n= 50) (Table 1). In the frequently used site, 23% of Marula trees were recorded with no stem damage, 43% of trees were dead but the main stem was still intact, 11% of trees were pushed over, while all trees were pushed over but main stem was still intact, 50% of trees had their canopy or one of the main stem removed and all trees were uprooted. In the non–frequently used site, Marula trees were all damaged, 57% of trees were dead but the main stem was still intact, 89% of trees were pushed over, while none of the trees were pushed over but main stem was still intact, 50% of trees had their canopy or one of the main stem removed and none of the trees were uprooted. In the enclosure, the trees were not affected by elephants (Figure 8).
The study aimed at investigating the interactions between elephants and vegetation; assess the long–term impact of elephant damage on selected vegetation types, and extent of damage on certain species for browse, such as S. birrea. The results revealed that sites with high elephant density had been detrimentally impacted with regard to the height of Marula trees. Thus it came as no surprise that distributional differences existed on tree height on both the frequently and non–frequently used sites, and as expected in the enclosure. Teren and Owen–Smith (2010) speculated that this could be because these Marula trees have outgrown the size threshold for pollarding by elephants. The absence of Marula trees in lower tree strata (8 m and lower) indicated that either little or no regeneration of the Marula population occurred during the last decade or that these strata of Marula trees are being targeted by elephants. This needs further investigation. It is therefore predicted that further loss of individuals in the 8 to 10 m height classes is to increase significantly.
Branch damage appeared to be lesser on taller trees, where elephants could not reach, which explains the low percentage recorded in all the branch damage class which showed a decreasing trend. Where trees were shorter, more branches appeared to be broken, as elephants attempted to reach utilizable plant parts, which explains a higher percentage recorded on minor utilization class within the non–frequently used site. Overall, elephants did not target Marula tree branches per se, but that the upper parts of trees were mostly targeted when fruits were available or where leaves were out of reach, which explains the high percentage recorded (on high utilization class). An important aspect is the time during which browsing occurs. This is important because it determines the plant responses to browsing (Gadd et al., 2001). None of the Marula trees were observed to have died as a result of branches being broken. Breaking of branches or harvesting of leaves is considered much less damaging to a plant.
Debarking damage
The study has indicated that almost all trees that were surveyed were ringbarked (70%+) irrespective of where they occurred (frequently and non–frequently used site). Elephants did not restrict ringbarking to any specific area in the reserve (excluding enclosure). The zero percent recorded in the non–frequently used site (for the no bark removal) was because the trees were exposed and available to the mega–herbivores. However, the higher intensity of debarking (50 to 75% and 75 to 100%) was commonly observed. Tree species vary considerably in the degree to which they are debarked. Although elephants do feed on trees by debarking, trees show an ability to recover by scar ridges formation, although the process is slow and scar tissue seldom covers the entire exposed area. However, the bark regenerates infrequently and usually a small number of trees regenerated. Hence it is expected that Marulas are able to sustain relatively high levels of damage before adults die.
Stem damage occurred, irrespective of whether it was in the frequently or non–frequently used site. There was a decreasing trend on the graph on the non–frequently used site. The highest trend noted within the dead but still intact main stem within the non–frequently used site, was due to the debarking that was followed by wood borer infestation. It has been suggested by Van Aarde et al. (2005) that some of the tree felling insidents may be a social display unrelated to feeding (especially by the male groups). Furthermore, Fritz et al. (2002) indicated that elephants are so much larger than most co–existing herbivores, which lend them to have greater impacts, such as tree, felling on vegetation.
Immune–contraception is regarded as the most effective means of controlling elephant populations using reproductive control measures as it is safe, reliable, effective, easily administered and reversible (Bertschinger et al., 2008). The primary objective for a contraceptive program will be to manage the growth rate of the population by simulating natural disturbance cycles; thus it will promote an indefinite period of zero growth. Only if translocation is unsuccessful could selective culling of individual bulls, and specific herds to reduce the overall population to within the recommended guidelines, be followed (Delsink, 2009). However, an important point is that culling programme will not prevent the disappearance of mature Marula trees from the ACNR. Elephants will still have an impact on their favoured plant species, even at low densities. Culling can therefore not be seen as a way of prevention measure for elephants in selecting for favoured species, but merely an attempt to slow the process down.
The demography of S. birrea at the ACNR could also be increased by re–stocking the population with plants from other populations (augmentation) (e.g. 100 individual seedlings of S. birrea that can be grown in a greenhouse). Adult Marula trees can be secured for the time being by surrounding them with stones, so that they cannot be affected and consumed by elephants. With introduced saplings, the saplings can be secured by restricting the presence of elephant bulls where Marula trees are prevalent. This will assist in reducing damage to Marula trees especially during the dry season. Vegetation plots can also be monitored. These sites should be photographed and examined at the end of the dry season and during the peak flowering/seeding period. A series of photographs must be taken from the same point at the same time every year. This will provide a visual reference point of the impact of various external influences on the vegetation, such as excessive grazing and fire practices (Bothma and Van Rooyen, 2002).
Elephants, like any herbivore, do not forage randomly but usually exhibit a hierarchy of selection from landscape, through vegetation type, to species and plant part. As such, the elephants will exhibit hierarchy of foraging in that palatable landscape. This explains the reason behind the damage difference on both the frequently and non–frequently used site. Elephants may be avoiding certain vegetation types and therefore not much damage found on Marula trees in those areas. Management practices such as increased elephant population have contributed to the decline of Marula trees in the reserve, though other contributing factors such as biotic or abiotic factors should never be discounted when considering vegetation change. To prevent the extinction of Marula trees in the reserve, it is imperative that the reduction of the elephant population needs to be addressed. Security measures should be adopted to protect the threatened tree species from developing even age population structures. A necessary measure would be to monitor the structural diversity of the Marula population.
The authors have not declared any conflict of interests.
REFERENCES
|
Acocks JPH (1954). Veld Types of South Africa. First edition. Pretoria. South Africa. Mem. Bot. Survey South Afr. 7:1-129.
|
|
|
|
Asner GP, Levick SR (2012). Landscape–scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15:1211-1217.
Crossref
|
|
|
|
|
Balfour D, Dublin HT, Fennessy J, Gibson D, Niskanen L, Whyte IJ (eds) (2007). Review of Options for Managing the Impacts of Locally Overabundant African Elephants. IUCN, Gland, Switzerland, P 80.
|
|
|
|
|
Ben–Shahar R (1993). Patterns of elephant damage to vegetation in northern Botswana. Biol. Conserv. 65:249-256.
Crossref
|
|
|
|
|
Bertschinger H, Delsink A, Van Altena JJ, Kirkpatrick J, Killian H, Ganswindt A, Slotow A, Castley G (2008). Reproductive control of elephants. In: Elephant Management: A Scientific Assessment of South Africa. Scholes, R.J. and Mennell, K.G. (eds). Witwatersrand University Press, Johannesburg, pp. 357-428.
|
|
|
|
|
Bothma J, Du P, Van Rooyen N (2002). Ecological Monitoring. In: Bothma, J. Du P. (ed.). Game Ranch Management. Van Schaik, Pretoria, pp. 30-33.
|
|
|
|
|
Clegg BW (2010). Habitat and Diet Selection by African Elephant at the Landscape Level: A Functional Integration of Multi–scale Foraging Processes. PhD Thesis, University of the Witwatersrand, Johannesburg.
|
|
|
|
|
De Boer WF, Van Oort JWA, Grover M, Peel MJS (2015). Elephant–mediated habitat modifications and changes in herbivore species assemblages in Sabi Sands, South Africa. Eur. J. Wildl. Res. 61:491-503.
Crossref
|
|
|
|
|
De Klerk JN (2004). Bush Encroachment: in Namibia. Report on Phase 1 of the Bush Encroachment Research, Monitoring and Management Project. Ministry of Environment and Tourism, Government of the Republic of Namibia.
|
|
|
|
|
Delsink AK (2009). Elephant Management Plan – Atherstone Nature Reserve. Hoedspruit: Wildlife Management Solutions.
|
|
|
|
|
Ferguson AJ (2014). High Elephant Impact is Capable of Converting Tall Mopane Woodland to Shrubland in the South East Lowveld of Zimbabwe. Honours Degree. University of Cape Town, Cape Town.
|
|
|
|
|
Fritz H, Duncan P, Gordon IJ, Illius AW (2002). Mega–herbivores influence trophic guilds structure in African ungulate communities. Oecologia, 131:620-625.
Crossref
|
|
|
|
|
Gadd ME, Young TP, Palmer TM (2001). Effects of simulated shoot and leaf herbivory on vegetative growth and plant defense in Acacia drepanolobium. Oikos 92:515-521.
Crossref
|
|
|
|
|
Harris GM, Russel GJ, Van Aarde RI, Pimm SL (2008). Rules of habitat use by elephants Loxodonta africana in southern Africa: Insights for regional management. Oryx 42:66-75.
Crossref
|
|
|
|
|
Jacobs OS, Biggs R (2001). The effect of different fire treatments on the population structure and density of the Marula, Sclerocarya birrea (A. Rich.) subsp. caffra (Sond.) kokwaro (Kokwaro and Gillet 1980) in the Kruger National Park. Afr. J. Range Forage Sci. 18:13-23.
Crossref
|
|
|
|
|
Jacobs OS, Biggs R (2002). The impact of the African elephant on Marula trees in the Kruger National Park. South Afr. J. Wildl. Res. 32:13-22.
|
|
|
|
|
Kerley GIH, Landman M (2006). The impacts of elephants on biodiversity in the Eastern Cape Subtropical Thickets. South Afr. J. Sci.102:9-10.
|
|
|
|
|
Kerley GIH, Landman M, Kruger L, Owen-Smith N, Balfour D, De Boer WF, Gaylard A, Lindsay K, Slotow R (2008). Effects of elephants on ecosystems and biodiversity In: Scholes, R.J. and Mennell, K.G. (eds). Elephant Management – A Scientific Assessment for South Africa. Wits University Press, Johannesburg, South Africa. pp. 1462-1467.
|
|
|
|
|
Kohi EM, De Boer WF, Peel MJS, Slotow R, Van der Waal C, Heitkoenig IMA, Skidmore A, Prins HHT (2011). African elephants Loxodonta africana browse heterogeneity in African savanna. Biotropica 43:711-721.
Crossref
|
|
|
|
|
Kruger JW (2013). Elephant Management Plan for Atherstone Nature Reserve (ANR) 2014-2018. Limpopo Department of Economic Development, Environment and Tourism (LEDET), Internal Report.
|
|
|
|
|
Landman M, Kerley GIH, Schoeman DS (2007). Relevance of elephant herbivory as a threat to important plants in the Addo Elephant National Park, South Africa. J. Zool. 274:51-58.
Crossref
|
|
|
|
|
Loarie SR, Van Aarde RJ, Pimm SL (2009). Elephant seasonal vegetations preferences across dry and wet savannas. Biol. Conserv. 142:3099-3107.
Crossref
|
|
|
|
|
Lombard AT, Johnson CF, Cowling RM, Pressey RL (2001). Protecting plants from elephants: Botanical reserve scenarios within the Addo Elephant National Park, South Africa. Biol. Conserv. 102:191-203.
Crossref
|
|
|
|
|
Marnewick K, Funston PJ, Karanth KU (2008). Evaluating camera trapping as a method for estimating cheetah abundance in ranching areas. South Afr. J. Wildlife Res.38:59-65.
Crossref
|
|
|
|
|
Mucina L, Rutherford MC (2006). The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria.
|
|
|
|
|
Okello MM, Njumbi SJ, Kiringe JW, Isiiche J (2015). Habitat use and preference by the African elephant outside of the protected area, and management implications in the Amboseli Landscape, Kenya. Int. J. Biodivers. Conserv. 7:211-236.
Crossref
|
|
|
|
|
Pauw JC (1988). Guidelines for Wildlife Management in the Bushveld Communities of the Atherstone Nature Reserve in the Northwest Transvaal. MSc. Dissertation. University of Pretoria. Pretoria.
|
|
|
|
|
Pretorius Y, De Boer WF, Van Der Waal C, De Knegt HJ, Grant RC, Knox NM, Kohi EM, Mwakiwa E, Page BR, Peel MJS, Skidmore AK, Slotow R, Van Wieren SE, Prins HHT (2011). Soil nutrient status determines how elephant utilize trees and shape environments. Afr. J. Ecol. 80:875-883.
Crossref
|
|
|
|
|
Schindler D (2005). Determining Woodland Caribou frequently used sites and habitat use in Eastern Manitoba. Preliminary Analysis and Interim Report. University of Winnipeg, Canada, P 72.
|
|
|
|
|
Shannon G, Druce DJ, Page BR, Eckhardt HC, Grant R, Slotow R (2008). The utilization of large savanna trees by elephant in southern Kruger National Park. J. Trop. Ecol. 24:281-289.
Crossref
|
|
|
|
|
Teren G, Owen–Smith N (2010). Elephants and riparian woodland changes in the Linyanti. Pachyderm, 47:18-25.
|
|
|
|
|
Van Aarde RJ, Grainger M, Whyte I (2005). Landscape heterogeneity and the use of space by elephants in the Kruger National Park, South Africa. Afr. J. Ecol. 43:369-375.
Crossref
|
|
|
|
|
Vogel SM, Henley MD, Rode SC, Van De Vyver D, Meares KF, Simmons G, De Boer WF (2014). Elephant (Loxodonta africana) impact on trees used by nesting vultures and raptors in South Africa. Afr. J. Ecol. 52:458-465.
Crossref
|
|