Full Length Research Paper
ABSTRACT
Concern about maintaining the biodiversity of lichen communities’ species has been an issue with lichenologists for many years. Many of the understudied regions face increasing threats from urban development, pollution, and potentially climate change, among other factors. The objective of this study was to examine the diversity of lichens on Mt. Cameroon. To achieve this objective, eight collection sites were surveyed on two flanks of the mountain at elevations ranging from 3 to 2178 m above sea level. The visual estimate sampling method using circular plots was adopted for the survey. Voucher specimens were collected in triplicate and deposited in the herbaria in Limbe and the University Buea. Lichens were identified by studying the morphology and chemical spot test. The morphology of the thallus and reproductive structures were examined under the stereomicroscope at 10×. The K-test, C-tests and KC-spot test were performed for each specimen with KOH and Ca(OCl)2. The abundance rating scale, species diversity, similarity and richness indices were computed. Identification by molecular, morphological and chemical spot tests produced a total of 89 species, 22 site-specific species, 52 genera belonging to 27 families and 11 orders. Four lichen specimens were identified to genus level and eighty-five to species level. According to the Cameroon lichen database, 82 of these are new discoveries. Parmeliaceae, Heterodermia, Usnea and Dirinaria applanata dominated the area. The identified species occurred in six growth forms and from nine substrates types. Foliose and corticolous lichens were most represented. Among the sites surveyed, Upper Buea situated on the leeward flank at high altitude >1000 m, recorded the highest diversity and site-specific species.
Key words: Lichens diversity, Mt. Cameroon, Upper Buea Leeward flank.
INTRODUCTION
The lichen mycobiota in several areas of the world especially in the tropics is still under-explored (Feuerer and Hawksworth, 2007). Many of the understudied regions face increasing threats from urban development, pollution, and potentially climate change, among other factors (Bjerke, 2011). Concern about maintaining the biodiversity of lichen communities’ species has been an issue with lichenologists for many years (Will-Wolf et al., 2006). This is observed over 100 years ago with effects of air pollution and expanding in the last 50 years to include effects of climate change (Ellis, 2019), deforestation, land management, fragmentation of natural habitats and loss of biodiversity. With the loss of sensitive lichen species from Europe and America due to the high rate of deforestation, global climate change and air pollution (Rocha et al., 2019), lichenologists are emphasizing the need to catalogue lichen species before their natural diversity is lost and to create ‘protected areas’ for their conservation (Jovans, 2008).
Accurate identification of lichen species is crucial to effectively assess biotic diversity, species’ response to disturbance in monitoring programs and implementation of effective conservation management strategies in an area (Giordani and Brunialti, 2015). An effective monitoring program depends on complete and accurate inventory information about the full extent of the species assemblage within an area (McClenahen et al., 2007). Thus, rapid inventories of lichen taxonomic diversity assessment achieved by morphological, anatomical, chemical, and molecular identification (Lumbsch et al., 2011) are particularly relevant. Nevertheless, while DNA-based molecular identification may be successful in areas where lichens are well studied (Kelly et al., 2011), it is not for underexplored areas like Mt. Cameroon (Orock et al., 2012).
Compared to tropical America and South East Asia, the lichen flora of tropical Africa is insufficiently known and that of western Africa is largely unknown (Feuerer and Hawksworth, 2007). Urgent survey is required in the countries within these regions to obtain data on lichen status with focus on rare and declining species found in the world red- list of lichens. Currently, only 101 lichenized fungal species are listed for the Republic of Cameroon, West Africa (Feuerer, 2011). However, floristic surveys of lichenized fungi in an important ecological area like Mt. Cameroon are lacking. Mount Cameroon area with extensive biodiversity is often threatened by volcanic eruptions and deforestation. This volcano with a return period of 20 years (Suh et al., 2003), has had the most frequent eruptions than any other West African volcano with eight eruptions in the last 100 years (1909, 1922, 1925, 1954, 1959, 1982, 1999, and 2000). Also, human-related activities are fragmenting, degrading, and isolating the remaining forest patches despite conservation efforts. It is therefore necessary to know the different lichen species remaining around Mt. Cameroon after various eruptions and alarming deforestation rates. The objectives of this work are to assemble a checklist of lichens at Mt. Cameroon.
MATERIALS AND METHODS
Study area and sites
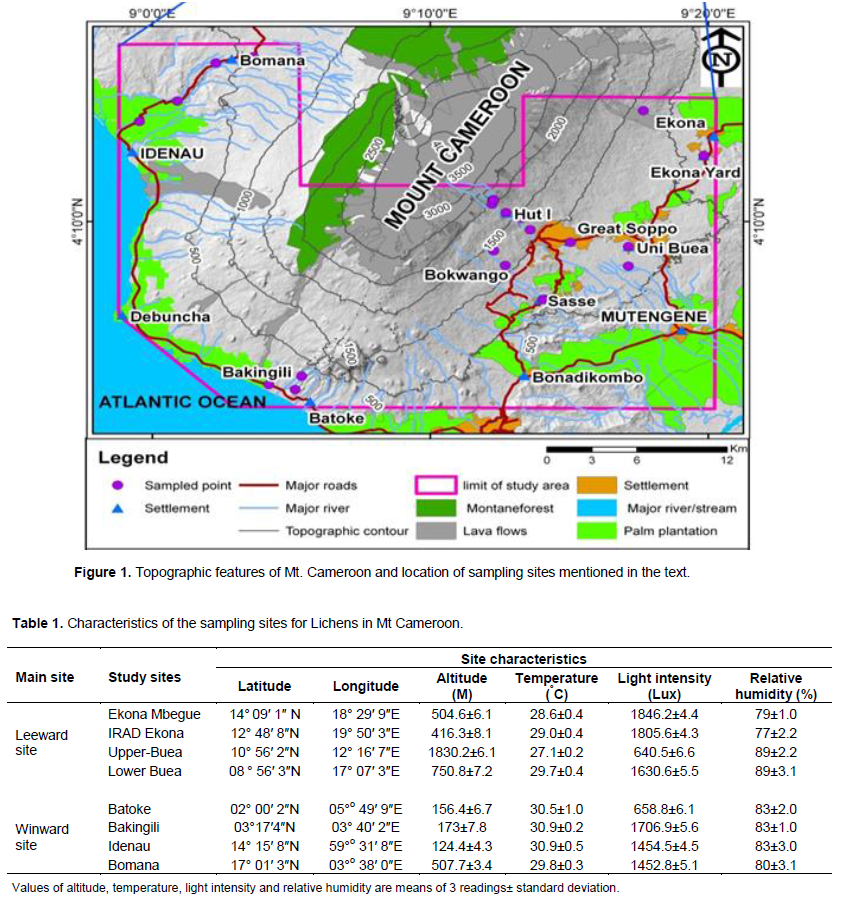
Mount Cameroon is located between 4° North and 6° 20’ North latitude and 8° 50’ East and 10° East longitude in the Southwest region of the Republic of Cameroon, on the coastal belt of the Gulf of Guinea (Figure 1). It covers a land surface area of approximately 24, 900 km2. It is the highest mountain in Central and West Africa, rising up steeply from sea level to 4100 m at the summit (Suh et al., 2003).
This region of the Republic of Cameroon has an average annual rainfall of 4000 mm which declines inland to 1800 mm. Mean temperatures are around 20°C varying with the effects of elevation. Soils are andosols supporting lowland submontane and montane tropical forests, and a microcosm of tropical plantation agriculture. In White’s phytogeographical classification (1983), the area falls within the afromontane eco-region (Ndenecho, 2009). Human-related activities are fragmenting, degrading, and isolating the remaining forest patches despite conservation efforts.
Sampling procedures
This study was carried out in 8 sites, located in ecologically diverse sites of Mt Cameroon, at elevations ranging from 3 to 2178 m above sea level (Figure 1). The study sites and their characteristics are presented in Table 1 and they constitute a general representation of the study area. However, some important habitats remain un-sampled, including sub-alpine and alpine habitats near the mountain summit.
Sampling procedures
The lichen survey consisted of a visual estimate and opportunistic random sampling method described by Jovans (2008) and McClenahen et al. (2007). This sampling method employs the concept of fine focused searches, looking in areas where high diversity is expected. These methods were selected based on their ability to maximize the detection of species diversity. At each sampling point, a circular plot of about 30 m radius with four (04) macro sub circular plots within was created (Figure 2). Each macro subplot was 20 m radius from its centre. In each macro plot, micro plots of 10 m radius from their centre were created. The distance between sub-macro plots centres was 30 m. Sampling was done from each micro subplot to macro subplots from plot 1 to plot 4 systematically.
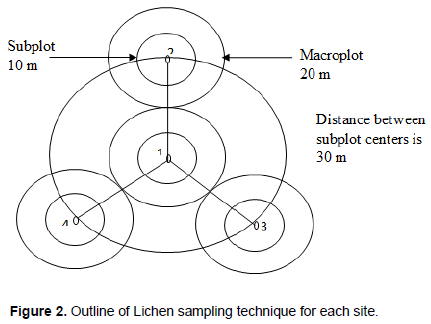
A total of 50 plots were sampled with a minimum of 5 plots per site, except for Lower and Upper Buea where 10 plots each were sampled because of their vast surface area. The chosen plots received a numbered identification plate and the precise positions were marked with a Global Positioning System (GPS). Lichens were observed, and measured in the field. Specimens were collected from live and dead tree barks, fallen branches, twigs, rocks and soil substrates in triplicate. Live trees were sampled from the base to about 2.0 m above the ground. For canopy sampling, some small twigs were cut with a secateurs and falling branches and twigs were sampled. A knife was used to remove lichens from the live and dead branches and twigs of trees. A hammer and chisel were used to remove lichens on rocks, while specimens on the soil were collected with the hand. All the substrates were examined using magnifying lenses of (x10) for morphological identification. Pictures of all specimens collected were taken using a NikonD80/D40 professional digital camera and produced in Buea, Cameroon used for identification. The specimens on wet barks were kept in plant press and tied tightly to avoid curling up. Each specimen was placed in a paper envelope and labelled with appropriate tracking and identification information. A number code such as Sp1, Sp2…. for example, was assigned to the specimens. Quantitative evaluation of lichen species was noted using the abundance rating frequency scale by Jovans (2008). Species were classified as rare (1 -2 individuals in the sampling unit), uncommon (3-5 individuals in the sampling unit), common (6-10 individuals in sampling unit) and abundant (>10 individuals in sampling unit).
Processing specimens for identification
All the specimens brought from the field were taken to the University of Buea Life Sciences Laboratory. The specimens were sorted, curated and prepared for drying. The specimens were dried in an oven at 60°C for 6 h. The dried specimens were then placed inside herbarium bags. Crust-like specimens on rock and wood/bark were glued using acid free herbarium glue. Fragile foliose specimens were placed between folded pieces of cotton padding for extra protection inside the envelope. The specimens were placed in the freezer for four days, to kill any insects, before being stored in the herbarium. Three voucher specimen packets were prepared and one packet each was deposited in the herbarium of the Botany laboratory of the University of Buea Cameroon and the Herbarium of the Limbe Botanic garden Cameroon.
Data analysis
Specimens were identified to generic and species level with the aid of “artificial field keys’’ and by extensive matching with correctly identified specimens from herbaria and pictorials from online databases. Microscopic observations of free-hand sections of thallus and fruiting bodies were also made on some of the lichens in order to assess diagnostic characters provided in the keys. Chemical spot tests were carried out to distinguish some species of lichens using freshly prepared calcium hypochlorite, 10% aqueous solution of potassium hydroxide and freshly prepared paraphenylenediamine. Color reactions of the thallus to the above chemicals were observed by applying a small drop to the cortex on the upper surface or to the medulla after scraping off the cortex to expose the medulla. Lichens were preserved in packets by oven drying at 60°C for 6 h. Each specimen was transferred to a new envelope and information such as the name, family, site, altitude, tree species, recorders’ names and date of collection were recorded on the envelope.
The morphological and chemical spot test observations were compared to keys of Coppins (2002), Brodo et al. (2001), May et al. (2000), St. Clair (1999), illustrations of St. Clair (1999), Goward et al. (1994), Goward (1999), and manuals of Gilbert (2004) and St. Clair (1999). The identity of each specimen was confirmed at the Herbarium of Nonvascular Cryptogams at Brigham Young University, Utah, USA.
Descriptive statistics were used to analyze the morphological and chemical identification data. Species diversity, richness and similarity were determined using Shannon-Weaver diversity index (H’), Margalef species richness index (D) and the Sørensen coefficient (Ss), respectively. All analyses were done with Microsoft Excel 2007, Inc, US 19.
RESULTS
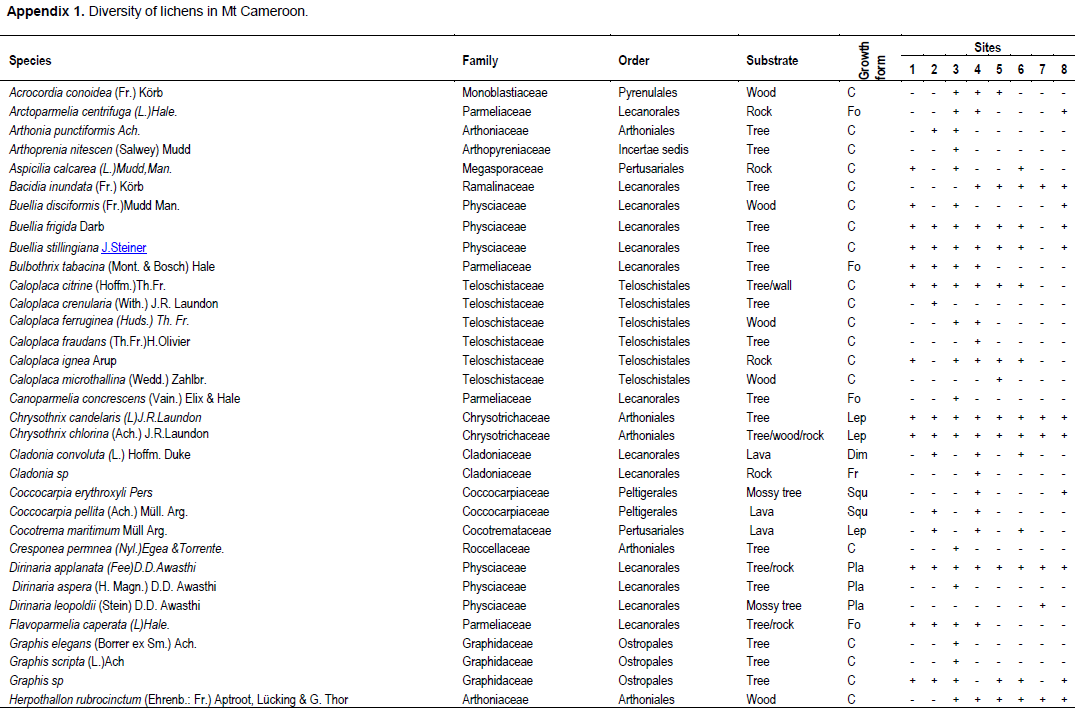
A total of 89 lichen specimens were identified (Appendix 1). Four of the specimens were identified to genus level and eighty-five to species level. The lichens were identified in 27 families (Figure 3). Of these families, Parmeliaceae with 22 species was most represented followed by Physciaceae with 17 species. Twelve families of the lichen samples identified were represented by only one species each.


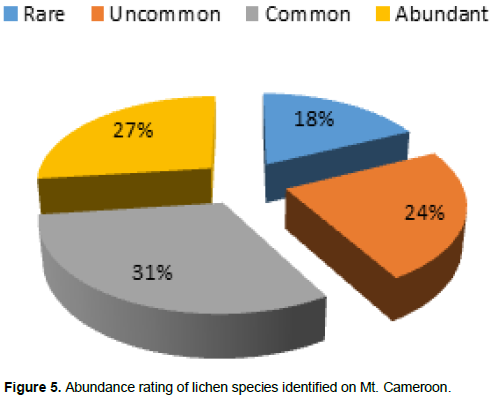
The lichens species identified from Mt. Cameroon belong to 52 genera (Figure 4). The genus Usnea was the most represented with 07 species followed by Heterodermia with 06 species. 31% of the lichen species identified in the study area were rated as common, while 18% were rated as rare (Figure 5).
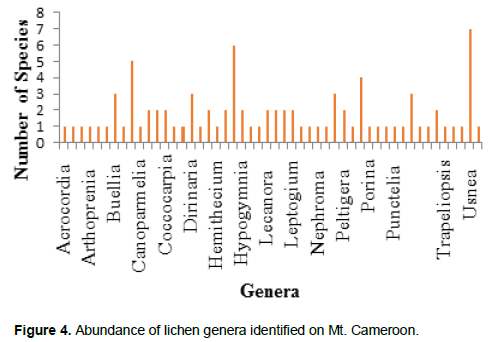
A total of 22 species cut across sites and altitudes (Table 2). The placodioid species Dirinaria applanata with 36 individiuals was the most represented followed by the crustose species Graphis scripta with 24 individuals. Leptogium gelatinosum and Heterodermia obscurata were the most represented foliose species, while the species Caloplaca citrina had the least individuals 10.
The highest lichen diversity (4.70) by Shannon-Weaver index was recorded from Upper Buea in the Leeward Flank and the lowest (2.41) from Idenau in the Windward Flank (Table 3).
Distribution of lichen growth forms on Mt. Cameroon
Lichens from this study belong to six growth forms (crustose, foliose, fruticose, leprose, placodioid and squamulose). Foliose was the most represented with 35 species identified, while squamulose with 02 species was the least represented (Figure 6).
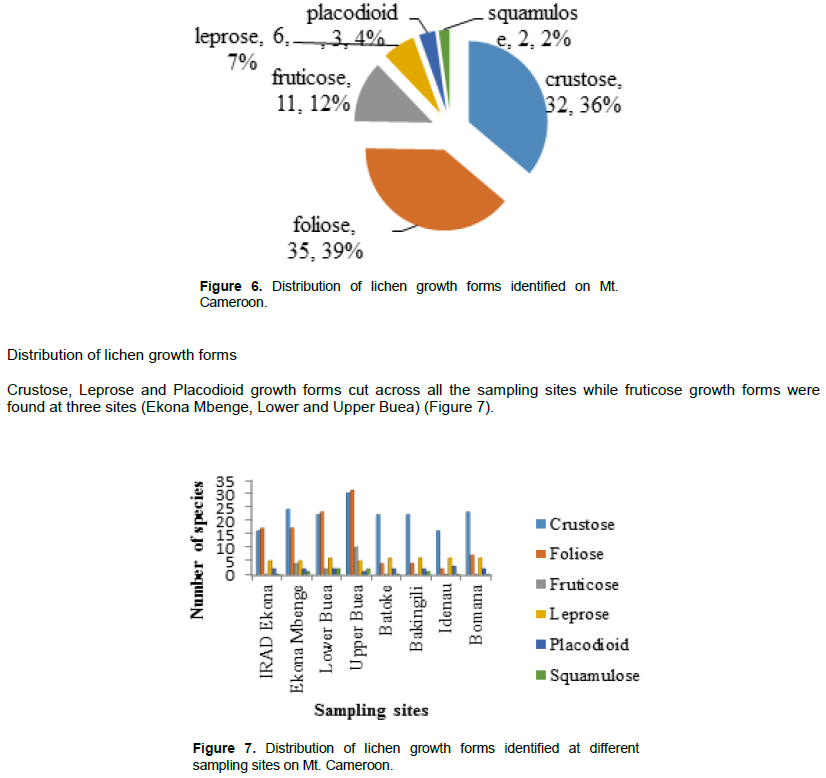
Distribution of growth forms in different flanks
Out of the six growth forms identified in the study, no fruticose types were identified from the windward flank (Figure 8).
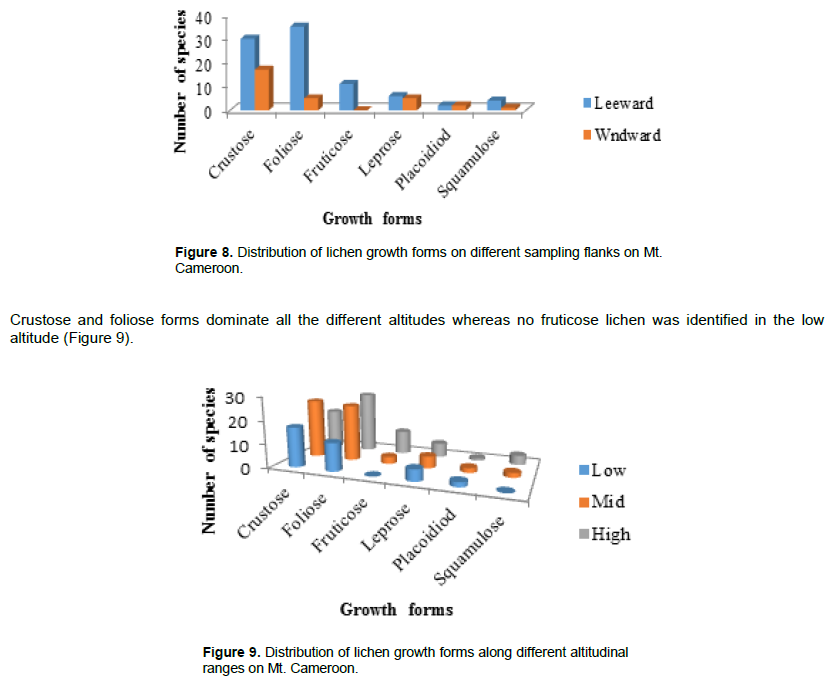
Distribution of lichen substrates
Lichens were identified from nine substrate types (Figure 10). Most species were corticolous lichens, while tree/rock/soil and tree/rock/wood species were the least represented.
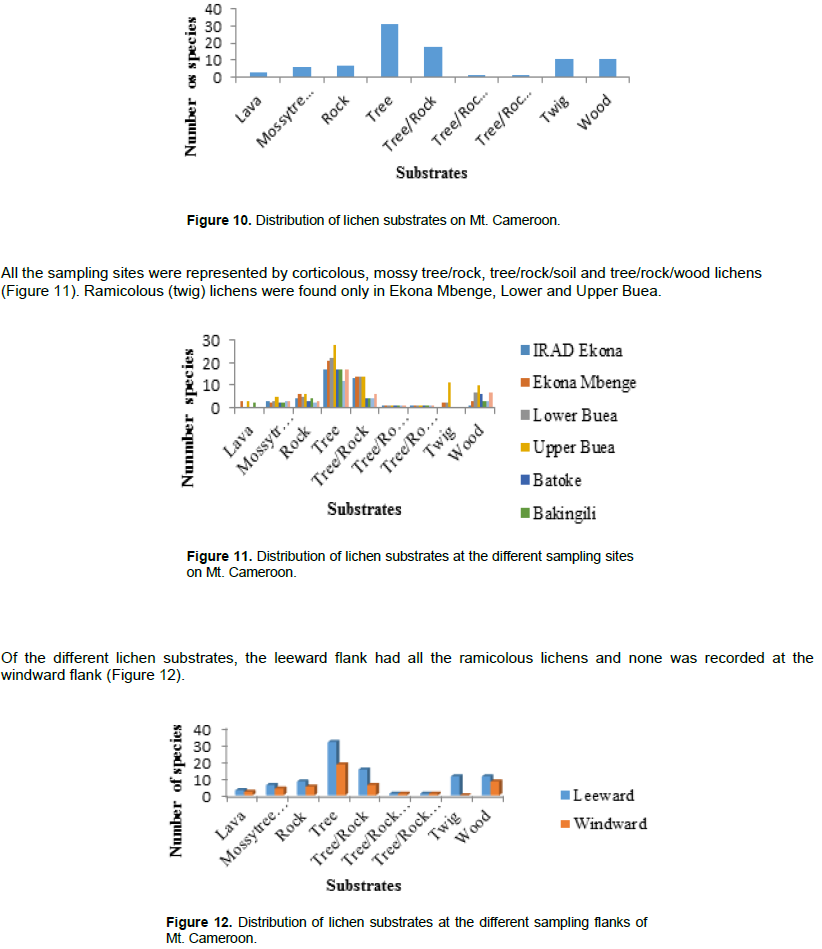
Distribution of substrates along different altitudes
Eight of the nine different substrates were represented in the three altitudes (Figure 13). Ramicolous lichens were absent in the low altitude.
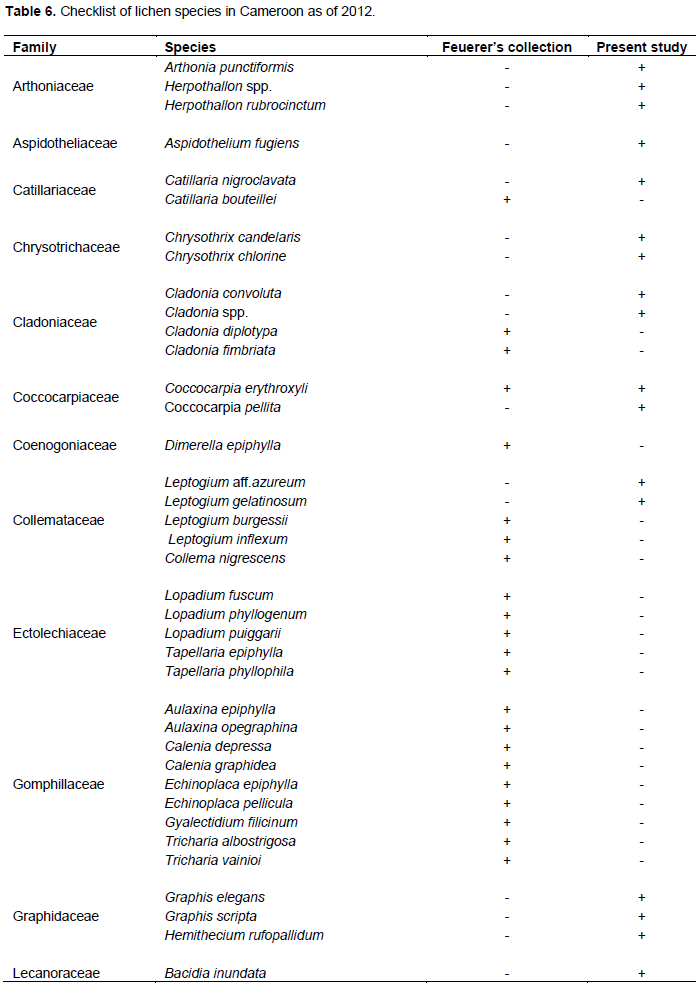
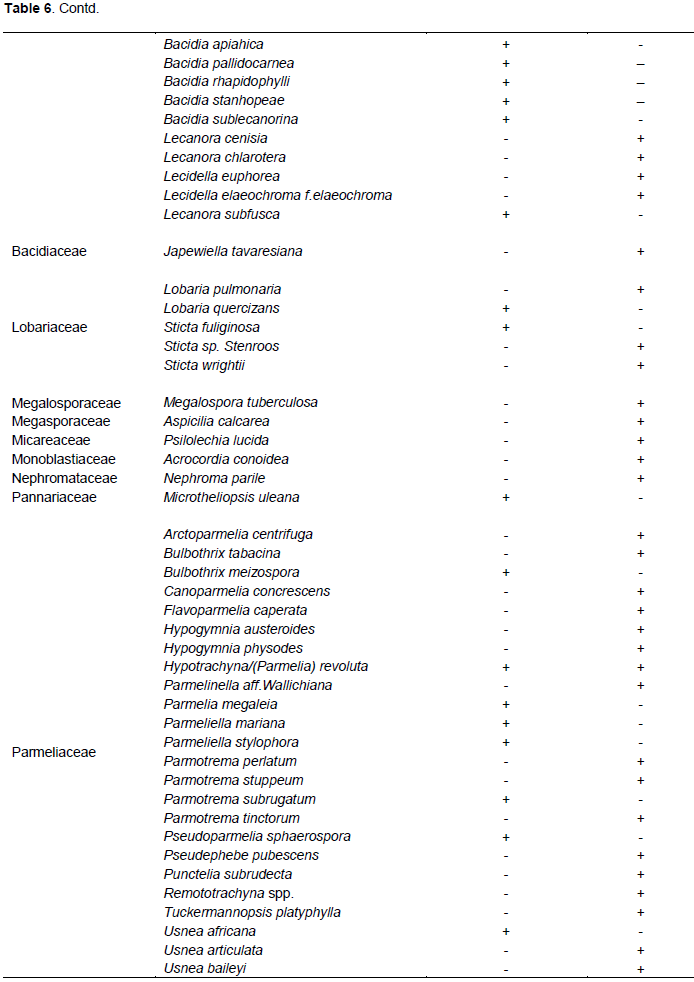
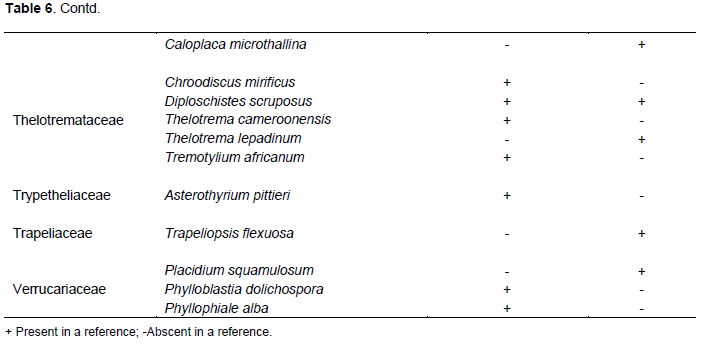
According to this inventory, out of the 89 lichen species identified, 07 were common with that of Feuerer (2011), while 82 were not found and are therefore added as new species records for Cameroon (Table 6).


DISCUSSION
Among the sites surveyed at Mt Cameroon, Upper Buea situated on the leeward flank at high altitude >1000 m, recorded the highest diversity, similar to studies on Mt. Uludag (Öztürk and Güvenç, 2010) with maximum diversities recorded at 1400 m and poor below 600 m. The highest lichen diversity and richness on Mt, Cameroon from the mid-high altitude (>500 m Lower Buea, Ekona Mbenge Bomana and Upper Buea) and lowest diversity at low altitude (<200 m IRAD Ekona, Batoke, Bakingili and Idenau) may be attributed to a number of factors influencing the abundance and richness of lichens such as altitude, humidity temperature, forest type and land use changes from human activities (Sevgi et al., 2019). Notably, Shyam et al. (2010) reported altitude as one of the main factor controlling the diversity and distribution of epiphytic lichens in Kollihills. Furthermore, Bassler et al. (2016) and Chitra et al. (2009), found decrease in lichen diversity along increasing altitude and their preference of corticolous habitat can be attributed to the abiotic factors such as humidity, light, temperature and substrate. All the sampling sites above 500 m in this study with high diversities, recorded higher humidity levels (>80 mm) than the sites below this elevation with lower humidity levels (<80 mm). Matteucci et al. (2012) in their studies observed that one of the most lichen-rich forest types with maximum species diversity and abundance are humid and cooler montane forest located in the cloud zones like the Upper Buea and Ekona Mbenge sampling sites. Most researchers (Nascimbene et al., 2010) have shown that, the composition of lichen communities depends on factors that operate at multiple spatial and temporal scales. At the local scale, lichen composition is mainly related to microclimatic and substrate factors associated with forest structure and continuity, while at broader scales climate and dispersal limitations are further important drivers.
The richness of species, growth forms and substrate types in this sites may be attributed to the well-developed preferences for habitats and microhabitats by lichens which are influenced by small differences in chemical (mineral contents) and physical factors (light, temperature, humidity, wind, altitude and unpolluted air, substrate) playing an important role in shaping lichen species richness patterns. A relationship between the concentration of certain lichen secondary compounds and the degree of exposure to environmental variables such as light, moisture, altitude, climate change and pollution have demonstrated (Brodo et al., 2001). A lichen’s ability to protect itself from high levels of visible light and ultraviolet radiation is determined by the production of secondary compounds which influenced the habitats it is able to occupy (Hess et al., 2008; Paolo, 2019). Also, as lichens possess a wide range of optima with regards to light, moisture, temperature, substrate quality and stability, and nutrient inputs, various life-history traits (biomolecules accumulation from different photobiont in association) are expected to modify the lichen response to various environmental factor and land use (Aptroot and van Herk, 2007; Chuquimarca et al., 2019).
The abundant families (Physciaceae and Parmeliaceae) in this study correspond to the studies of Divakar et al. (2010), who found these as the most common families in tropical regions, particularly abundant in humid climates at mid-high elevations. The family Physciaceae with 17 species, harbours thermophilic lichens like H. obscurata and Physcia americana (Moberg, 2004) which response to distributional changes are attributed to global warming (Hickling et al., 2005) and are among the most common and particularly abundant in humid climates at low and mid elevations (Ellis and Coppins, 2017).
The most represented genus Usnea with 07 different species in tree canopies at high elevations is similar to study performed in West Greenland (Bjerke and Dahl, 2002) which showed that, lichen species with high concentrations of usnic acid inhabit more light-exposed areas than species with lower levels of this compound. Presumably, this may be the reason for the highest diversity and richness of canopy ramicolous fruticose and foliose usnic acid producing species at Upper Buea and Ekona Mbenge sampling sites. Most of these species and others were positive red and yellow with spot test than the species of the low altitude. This is findings are consistent with a survey of lichen distribution in Thailand by Wolseley and Aguirre-Hudson (1997) who found that, lichen which showed red and yellow colours with spot test were found at high-altitude montane oak forest and lacking in low-altitude tropical mixed deciduous forest. They reported that, lichen communities must experience a threshold level of light intensity before it becomes adaptive to begin producing expensive secondary compounds for protection from intense irradiance. This adaptation may be attributed to the absence of usnic acid producing species in the low altitude sampling sites of this work. Although these lower altitude sampling sites recorded higher mean light intensities (1706.9±5.6 lux) than high altitude (640.5±6.6 lux), the lichen species may not have experienced the threshold to produce irradiance-protecting substances because of land use changes.
At Mt. Cameroon, macro lichens (foliose, fruticose and squamulose) growth forms recorded highest diversities in the mid and high altitudes, while micro lichens (crustose, leprose and placodioid) growth forms were highest in the low altitude sites might have been influenced by land use activities. According to Ahti (2000), lichens show preferences to either sexual or asexual reproductive methods due to differences in morphological tolerance to trampling. For example, macro-lichens are more susceptible to trampling and so rarely reproduce sexually, while micro-lichens are more tolerant to trampling therefore reproduce both sexually and asexually. Therefore, foliose growth forms with the most representative probably relates to the fact that the presence of foliose lichens is independent of landscape condition and relies on a small amount of fragmentation to disperse to new sites (Cáceres et al., 2007). Managed forests like those in the low altitude (Batoke, Bakingili, Idenau and Ekona IRAD) are normally young and fragmented for wood fuel, timber and plantation agriculture in which young tree stems are repeatedly cut down to near ground level with short rotation cycles (Kaufmann et al., 2017). Management activities such as forestry (Aragon et al., 2010) agriculture (Styres et al., 2010) livestock grazing and quarrying (Boudreault et al., 2008) have been recognized as the most important driving forces for lichen species richness and composition. Habitat fragmentation (Kubiak et al., 2016) has led to the isolation of many lichen communities by creating limitations and hampering their dispersal and reproduction (Lorenzo et al., 2011). The generation time, from spore to spore determined in some lichens takes so many years (Ranius et al., 2008). Red-list species Cliostomum corrugatum and Lobaria pulmonaria for example, have reach an average age of 30 and 35 years respectively before they are able to produce and disperse their spores (Edman et al., 2008).
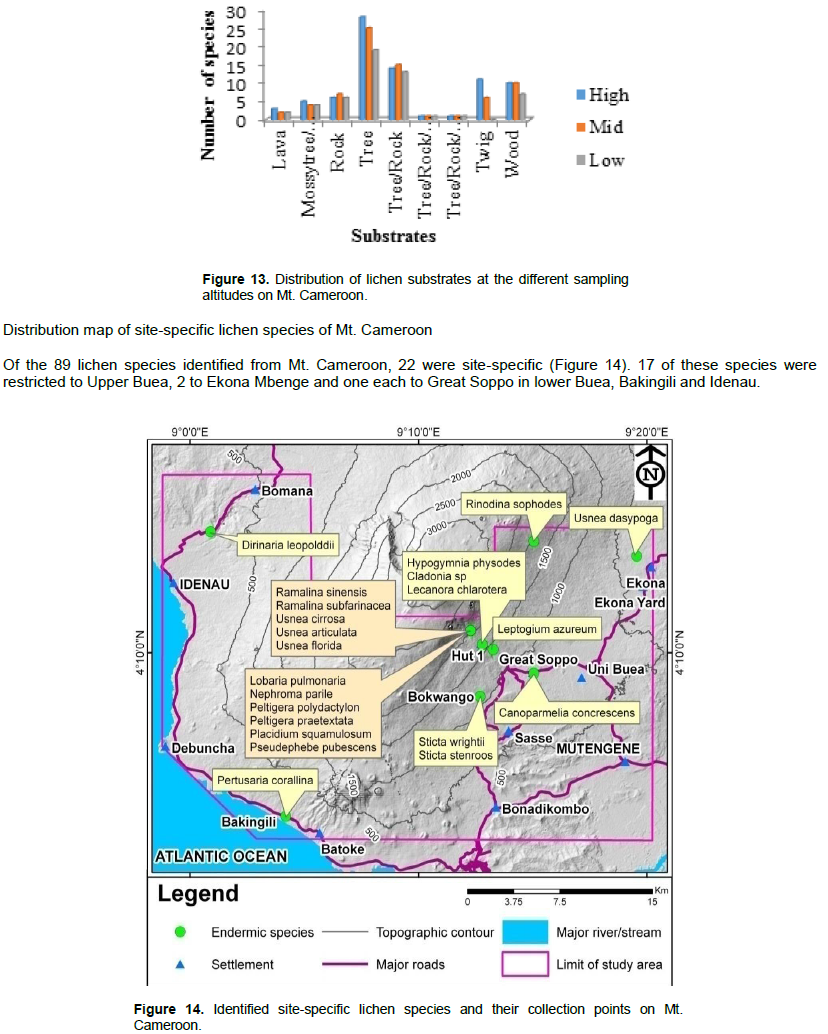
Mature forests like Upper Buea sampling site with high conservation value are forest where regeneration is usually of seedling origin, either natural or arti?cial (or a combination of both) and the rotation cycles are generally long to harbour red-list threaten species (Hofmeister et al., 2015). Owing to the evidence of their low dispersal ability and long generation the red-list has been widely used as an indicator species of undisturbed forest ecosystems and forest areas of a high ecological continuity and conservation (Nascimbene et al., 2014; Vondrak et al., 2015). This dispersal limitation may be one of the reasons for more (17) site-specific species including L. pulmonaria at Upper Buea in the high. Furthermore, the IUCN (2000) red-list criteria emphasizes the conservation of lichen species in areas were rating is not abundant, like case with Mt. Cameroon where rating is mostly common (31%).
Most lichens occurring in Mt. Cameroon are corticolous (tree) species with the highest diversity of perennial substrates in Upper Buea sampling site. Ioana (2011) revealed some lichens, especially the endangered red-listed species are restricted to specific perennial substrates and habitats in montane regions. The vegetation in Upper Buea still covers relatively large and continuous areas with perennial substrates for colonisation and stability of the genera Lobaria, Nephroma, Ramalina, Cladonia and Usea. These red-list species are found on branches and twigs with dense canopies of mature trees with rough barks. According to Ellis and Coppins (2007), these lichens have some degree of substrate specificity and stability of the habitats over a long period giving an impact of lichen status. Therefore, the probable reason for the high diversity of quality perennial substrates in Upper Buea site may be attributed to forest maturity and reduced human activity unlike IRAD Ekona, Batoke, and Idenau which are highly disturbed by land use changes from anthropogenic activities like agriculture. Since many species are restricted to specific microclimatic pockets, any change in the original structure of the forest will decrease species diversity (Ben?tez et al., 2018).
Deforestation of old forest for instance leads to the removal of decaying logs and stumps and conversion to secondary forest with different substrate quality in the low altitude resulting in a decrease in species diversity like the case of this study. Pinho et al. (2008) reported that the nature of the substrate (tree age, size and bark nature, that is, rough or smooth) has considerable influence on the diversity and abundance of lichen species. This may be reflected by smooth bark Graphis scripta, Arthonia punctiformis, Lecidella elaeochroma species for example, recorded in the young tree with smooth barks found in the lower altitude sampling sites.
The Mt. Cameroon windward flank (Batoke, Bakingili, Idenau and Bomana) of this study, recorded fewer lichen species than the leeward flank (Upper and lower Buea, IRAD and Ekona Mbenge) which can be attributed to air quality. Bjerke (2011) reported that wind often favours lichen growth, distribution and abundance positively if the wind circulation is nutrient-rich or negatively if the wind circulation is pollutant-rich (Paolo, 2019). This may be the effect of the poor lichen diversity and richness in the windward flank because, studies by Suh et al. (2008) reveal the dominant prevailing winds direction of Mt. Cameroon is from the NE-SW direction, which tends to drive volcanic ash plumes and degassing over the south-western (windward) flank. Upper Buea sampling site situated in the leeward flank recorded all the fruticose air quality sensitive species and none of this species was recorded in any of the sampling sites in the windward flank. Perlmutter (2010) recognised air quality as one of the main factors influencing development of lichen species. They found that shrubby lichens (fruticose) thrive in clean air while only crusty lichen thrives in polluted air. Using the Rose and Harkworth (1970) scale documented in Brodo et al. (2001), the assemblage of lichen species in Upper Buea in the high altitude has the best air quality. This was indicated by the occurrence of all the fruticose and foliose lichen species in the genera (Lobaria, Nephroma, Ramalina, Cladonia, Usea and Flavoparmelia) associated with good air quality.
Feuerer (2011), listed only 101 lichenized fungal for the Republic of Cameroon, but according to these findings, out of the 89 lichen species identified, 82 species were not found on Feuerer list, therefore are added as new species records for Cameroon lichen check list. According to Feuerer and Hawksworth (2007), the lichen mycobiota in most regions, especially in the tropics, is still largely unknown and surveys will always reveal new species. The numerous new species in Cameroon indicates that Mt. Cameroon lichens are still unexplored, and new inventories are required to understand the potential of its diversity. This research generates new data on lichen of MC and contributes to a better understanding of the existing lichen flora.
CONCLUSION
Despite harbouring rich lichen diversity, the Mt. Cameroon region in particular and Cameroon in general is grossly under explored. Mt Cameroon has a thick moist forest vegetation which provide a suitable habitat for the colonization of different lichen species on different substrates. The high altitudes and the Leeward sites show less anthropogenic pressure and less habitat alteration. It is no surprise that the habitats with the highest lichen species diversity are the remnants of ancient forests and undisturbed ecosystems like Upper Buea sampling site.
Current study reveals region-specific distribution of lichens, which is very different in the sampling sites of Mt. Cameroon region. This disparity can be primarily attributed to the different climatic factors and anthropogenic pressures in the form of land use patterns influencing the lichen communities of the area.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Ahti T (2000). Cladoniaceae. Flora Neotropica Monograph 78:1-362. |
|
|
Aragon GI, Izquierdo MP, Belinchon R, Escuderon A (2010). Effects of forest management on epiphytic lichen diversity in Mediterranean forests. Applied Vegetation Science 13:183-194. |
|
|
Aptroot A, van Herk CM (2007). Further evidence of the effects of global warming on lichens, particularly those with Trentepohlia phycobionts. Environmental Pollution 146:293-298. |
|
|
Bassler C, Cadotte MW, Beudert B (2016). Contrasting patterns of lichen functional diversity and species richness across an elevation gradient. Ecography 39:689-698. |
|
|
Ben?tez A, Aragon G, Gonzalez Y, Prieto M (2018). Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richness and diversity. Ecological Indicators 86:18-26. |
|
|
Bjerke JW, Dahl T (2002). Distribution patterns of usnic acid-producing lichens along local radiation gradients in West Greenland. Nova Hedwigia 75:487-506. |
|
|
Bjerke JW (2011). Winter climate change: Ice encapsulation at mild subfreezing temperatures kills freeze-tolerant lichens. Environmental and Experimental Botany 72:404-408. |
|
|
Boudreault C, Coxson D S, Vincent E, Bergeron Y, Marsh J (2008). Variation in epiphytic lichen and bryophyte composition and diversity along a gradient of productivity in Populus tremuloides stands of northeastern British Columbia, Canada. Ecoscience 15:101-112. |
|
|
Brodo IM, Sharnoff SD, Sharnoff S (2001). Lichens of North America. Yale University Press New Haven. |
|
|
Cáceres MES, Lücking R, Rambold G (2007). Phorophyte specificity and stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycological Progress, Tübingen 6:117-136. |
|
|
Chitra BB, Torstein S, Yngvar G, Michael WP (2009). The elevation gradient of lichen species richness in Nepal. The Lichenologist 42(01):83. |
|
|
Chuquimarca L, Gaona FP, Iñiguez-Armijos C, Benítez Á (2019). Lichen Responses to Disturbance: Clues for Biomonitoring Land-use Effects on Riparian Andean Ecosystems. Diversity 11:73. |
|
|
Coppins BJ (2002). Checklist of lichens of Great Britain and Ireland. British Lichen Society, London. |
|
|
Divakar P, Figueras G, Hladun N, Crespo A (2010). Morphological versus phylogenetic species: an example from Melanelixia glabra (Parmeliaceae, Ascomycota). Fungal Diversity 42:47-55. |
|
|
Edman M, Eriksson A, Villard S (2008). Effects of selection cutting on the abundance and fertility of indicator lichens Lobaria pulmonaria and Lobaria quercizans. Journal of Applied Ecology 45:26-33 |
|
|
Ellis C, Coppins BJ (2007). Reproductive strategy and the compositional dynamics of crustose lichen communities on aspen (Populus tremula L) in Scotland. The Lichenologist 39:377-391. |
|
|
Ellis CJ, Coppins BJ (2017). Taxonomic survey compared to ecological sampling: are the results consistent for woodland epiphytes? Lichenologist 49:141-155. |
|
|
Ellis CJ (2019). Climate Change, Bioclimatic Models and the Risk to Lichen Diversity. Diversity 11:54. |
|
|
Feuerer T, Hawksworth DL (2007). Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan's floristic regions. Biodiversity and Conservation 16:85-98. |
|
|
Feuerer T (2011). Checklists of lichens and lichenicolous fungi, |
|
|
Gilbert OL (2004). The Lichen Hunters Hardback, 208p. |
|
|
Giordani P, Brunialti G (2015). Sampling and Interpreting Lichen Diversity Data for Biomonitoring Purposes. In Recent Advances in Lichenology; Upreti DK, Divakar PK, Shukla V, Bajpai R. Eds Springer New Delhi, India, pp. 19-46. ISBN 978-81-322-2180-7. |
|
|
Goward TB, McCune D, Meidinger V (1994). The Lichens of British Columbia: Illustrated Keys. Part 1: Foliose and Squamulose Species; MoF, Research Program, Special Report No. 8. 181pp. |
|
|
Goward T (1999). Lichens of British Columbia, Illustrated Keys. Part 2: Fruticose Species. MoF Forestry Division Service Branch, Special Report No. 9. 319 pp. |
|
|
Hofmeister J, Hos?ek J, Brabec M, Dvo?a'k D, Beran M, Deckerova' H (2015). Value of old forest attributes related to cryptogam species richness in temperate forests: A quantitative assessment. Ecological Indicators 57:497-504. |
|
|
Hess D, Coker DJ, Loutsch JM, Russ J (2008). Production of oxalates In Vitro by Microbes Isolated from Rock Surfaces with prehistoric paints in the Lower Pecos Region, Texas. Geoarchaeology 23(1):1-175. |
|
|
Hickling R, Roy DB, Hill JK, Thomas CD (2005). A northward shift of range margins in British Odonata. Global Change Biology 11:502-506. |
|
|
Ioana V (2011). Preliminary study using lichen species diversity as an Indicator of local environmental quality within two nature reserves from Romania. Analele Universit??ii din Oradea. Fascicula Biologie Tom. XVIII 1:53-58. |
|
|
IUCN (International Union for Conservation of Nature and Natural Resources) (2000). |
|
|
Jovans S (2008). Lichen bioindication of biodiversity, air quality and climate: baseline results from monitoring in Washington, Oregon and California. General Technical Report. PNW- GTR-737. Portland, OR: U.S. Department of Agriculture, Forest Service. |
|
|
Kelly LJ, Hollingsworth PM, Coppins BJ, Ellis CJ, Harrold P, Tosh J and Yahr R (2011). DNA barcoding of lichenized fungi demonstrates high identification success in a floristic context. New Phytologist 191:288-300. |
|
|
Kubiak D, Osyczka P, Rola K (2016). Spontaneous restoration of epiphytic lichen biota in managed forests planted on habitats typical for temperate deciduous forest. Biodiversity and Conservation 25:1937-1954. |
|
|
Kaufmann S, Hauck M, Leuschner C (2017). Comparing the plant diversity of paired beech primeval and production forests: Management reduces cryptogam, but not vascular plant species richness. Biodiversity and Conservation 400:58-67. |
|
|
Lorenzo M, Nascimbene J, Nimis PL (2011). Large-scale patterns of epiphytic lichen species richness: Photobiont-dependent response to climate and forest structure. Science of the Total Environment 127:60-66. |
|
|
Lumbsch HT, Ahti T, Altermann S, Amo de Paz G, Aptroot A, Arup U, Barcenas Peña A (2011). One hundred new species of lichenized fungi: a signature of undiscovered global diversity. Phytotaxa 18:1-127. |
|
|
Matteucci E, Renato B, Paolo G, Rosanna P, Deborah I (2012). Epiphytic lichen communities in chestnut stands in Central-North Italy. Biologia 67(1):61-70. |
|
|
May PF, Brodo IM, Esslinger TL (2000). Identifying North American Lichens: A Guide to the Literature. Farlow Herbarium, Harvard University, Cambridge, Massachusetts. Available, |
|
|
Moberg R (2004). The lichen genus Heterodermia in Europe and the Macaronesian Islands. Bibliotheca Lichenologica 88:453-463. |
|
|
McClenahen JR, Davis DD, Hutnik RJ (2007). Macrolichens as bio-monitors of air-quality change in western Pennsylvania. North-eastern Naturalist 14:15-26. |
|
|
Nascimbene J, Marini L, Nimis PL (2010). Epiphytic lichen diversity in old-growth and managed Picea abies stands in Alpine spruce forests. Forest Ecology and Management 260:603-609. |
|
|
Nascimbene J, Nimis PL, Dainese M (2014). Epiphytic lichen conservation in the Italian Alps: the role of forest type. Fungal Ecology 11:164-172. |
|
|
Ndenecho EN (2009). Herbalism and resources for the development of ethnopharmacology in Mount Cameroon region. African Journal of Pharmacy and Pharmacology 3:078-086. |
|
|
Orock EA, Leavitt SD, Fonge BA, St. Clair LL, Lumbsch HT (2012). DNA-based identification of lichen-forming fungi: Can publicly available sequence databases aid in lichen diversity inventories of Mount Cameroon (West Africa)? The Lichenologist 44(6):1-8. |
|
|
Öztürk P, Güvenç P (2010). The distribution of epiphytic lichens on Uludag fir (Abies nordmanniana (Steven) Spach subsp. bornmuelleriana (Mattf.) Coode and Cullen) forests along an altitudinal gradient (Mt. Uludag, Bursa, Turkey). Ekoloji 19(74):131-138. |
|
|
Perlmutter GB (2010). Bioassessing air pollution effects with epiphytic lichens in Raleigh, North Carolina, U.S.A. The Bryologist 113(1):39-50. |
|
|
Pinho P, Augusto S, Maguas C, Pereira MJ, Soares A, Branquinho C (2008). Impact of neighbourhood land-cover in epiphytic lichen diversity: Analysis of multiple factors working at different spatial scales. Environmental Pollution 151:414-422. |
|
|
Paolo G (2019). Lichen Diversity and Biomonitoring: A Special Issue. Diversity 11:171. |
|
|
Ranius T, Johansson P, Berg N, Niklasson M (2008). The influence of tree age and microhabitat quality on the occurrence of crustose lichens associated with old oaks. Journal of Vegetation Science 19:653-662. |
|
|
Rocha B, Pinho P, Vieira J, Branquinho C, Matos P (2019). Testing the Poleotolerance Lichen Response Trait as an Indicator of Anthropic Disturbance in an Urban Environment. Diversity 11:55. |
|
|
Shyam KR, Thajuddin N, Upreti DK (2010). Diversity of lichens in Kollihills of Tamil Nadu, India. International Journal of Biodiversity and Conservation 3(2):36-39. |
|
|
St. Clair LL (1999). A Color Guidebook to Common Rocky Mountain Lichens. Brigham Young University Press, Provo, Utah. 243 pp. |
|
|
Styres DM, Chappelka AH, Marzen LJ, Somers LG (2010). Developing a land-cover classification to select indicators of forest ecosystem health in a rapidly urbanizing landscape. Landscape and Urban Planning 94:158-165. |
|
|
Suh CE, Luhr JF, Njome MS (2008). Olivine-hosted glass inclusions from Scoriae erupted in 1954-2000 at Mount Cameroon volcano, West Africa. Journal of Volcanology and Geothermal Research 169:1-33. |
|
|
Suh CE, Sparks RSJ, Fitton J G, Ayonghe SN, Annen C, Nana R and Luckman A (2003). The 1999 and 2000 eruptions of Mount Cameroon: eruption behaviour and petrochemistry of lava. Bulletin of Volcanology 65:267-281. |
|
|
Sevgi E, Y?lmaz OY, Çobanoglu Özyigitoglu, G, Tecimen HB, Sevgi O (2019). Factors Influencing Epiphytic Lichen Species Distribution in a Managed Mediterranean Pinus nigra Arnold Forest. Diversity 11:59. |
|
|
Vondrak J, Mal??ek J, Soun J, Pouska V (2015). Epiphytic lichens of Stuzica (E Slovakia) in the context of Central European old-growth forests. Herzogia 28:104-126. |
|
|
Will-Wolf S, Geiser LH, Neitlich P, Reis AH (2006). Forest lichen communities and environment - How consistent are relationships across scales? Journal of Vegetation Science 17:171-184. |
|
|
Wolseley PA, Aguirre-Hudson B (1997). The ecology and distribution of lichens in tropical deciduous and evergreen forests of northern Thailand. Journal of Biogeography 24:327-343. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0