ABSTRACT
The small mammal community at Kogyae Strict Nature Reserve (KSNR) in the Ashanti Region of Ghana were studied in two habitats during the wet and dry seasons to investigate seasonal changes in species richness, abundance, composition and diversity. Ninety-six individuals belonging to nine species were recorded in 720 trap-nights, giving overall trap-success of 13.33%. Species richness (Sr), trap-success (Ts) and relative abundance (Ra) were higher (Sr = 6 species; Ts = 23.1%; Ra = 86.5%) in wooded grassland than forest (Ra = 4 species; Ts = 3.6%; Ra = 13.5%). However, species diversity was higher (Shannon-Wiener index Hʹ = 1.157) in forest than in wooded grassland (Hʹ = 1.089). Mastomys erythroleucus dominated in wooded grassland (68%) and Hylomyscus alleni in forest (53.8%). The species composition was unique for both habitats, with Mus musculoides being the only species common to both habitats. Seasonal changes in community assemblages were evident in both habitats, with species richness, diversity and abundance of the dominant species being highest in the wet seasons. Sex-ratio was unity in both habitats, and remained fairly constant throughout the rainy and dry seasons. Breeding activity was evident all-year-round for most species, but peaked in the rainy season. Our findings are consistent with that of other studies in Ghana and elsewhere in the African subregion, highlighting the importance of rainfall to the ecology of tropical small mammals.
Key words: African rain forest, community dynamics, habitat quality, live-trapping, rodents, tropical biodiversity, wildlife management.
Abbreviation:
Identification of the factors that influence the distribution of species and temporal and spatial abundance, richness and composition of communities are of central importance in ecology, biogeography and biodiversity conservation. Knowledge of how natural environmental changes impact on organisms and how they in turn, respond to these changes can be used to forecast population trends, species turn-over and potential local extinctions (Soule et al., 2003; Manning and Edge, 2008). This in turn, can reveal subtle changes in environments and provide great insights into the threats facing biodiversity (Vos et al., 2000; Zahratka and Shenk, 2008), allowing for effective conser-vation planning and landscape management (Attum et al., 2008).
Small mammals are important contributors of biodi-versity and biomass of most natural and semi-natural ecosystems (Makundi et al., 2009; Habtamu and Bekele, 2012). They are the most diverse group of mammals, with considerable diversity in life-history, morphology and habitat associations. Rodents alone comprise over 40% of all mammalian fauna globally (Wilson and Reeder, 2005). Small mammals have complex effect on the structure, composition and functional diversity of their environment through various ecological interactions. For instance, by feeding on seeds, seedlings, fungal spores and insects, and serving as important sources of food for many medium-sized mammalian predators, raptors and snakes, small mammals maintain many food webs and ecosystem balance (Angelici and Luiselli, 2005). Some small mammals are also sensitive to even small changes in the environment (Malcom and Ray, 2000), as reflected in changes in their abundance, diversity and composition. Changes in the community structure of small rodents can therefore be used as surrogates for, and a quick and cost effective way of, measuring habitat quality or environ-mental disturbance (Avenant, 2011). Some small mammals are pests of agriculture and carriers of zoonotic diseases, causing significant economic losses and serious health implications (Habtamu and Bekele, 2012). Thus, the impacts of environmental change on small mammal populations have been the subject of intense research globally.
The abundance, diversity and community structure of small mammals are affected by several factors, including floristic composition, productivity, resource availability and microhabitat features such as available cover from predators (Nicolas and Colyn, 2003; Garratt et al., 2012). These factors in turn, are affected by climatic variability and disturbance regimes such as fire and habitat clearance (Jackson et al., 2009). In tropical and semi-arid regions, rainfall is often the most important driver of ecosystems’ productivity (Coe et al., 1976). Small mammals therefore experience seasonal and inter-annual changes in abundance, composition and distribution tied to the amount and pattern of rainfall in space and time (Nicolas and Colyn, 2003).
In most tropical regions, natural habitats are being lost and degraded at alarming rates (FAO, 2007), posing serious threats to wildlife in general, and forest-specialist small mammals in particular, as these species require specific habitat structure and quality. The proliferation and mono-dominance of opportunistic species that are able to tolerate habitat modifications may further impoverish small mammal richness and diversity. It is feared that some tropical small mammal might be exterminated before they are discovered given the current rate of habitat loss in tropical regions. Therefore, it is of utmost necessity to conduct as many surveys as possible, particularly in unsurveyed areas that have experienced no or relatively less human modifications.
The composition of small mammal communities in many habitats and regions in Ghana is incompletely known. Knowledge of the effects of changing environments on this group of animals also is limited, despite recent efforts to bridge this knowledge gap (Yeboah, 1998; Decher and Bahian, 1999; Ryan and Attuquayefio, 2000; Decher and Abedi-Lartey, 2002; Attuquayefio and Wuver, 2003; Attuquayefio and Ryan, 2006; Barriere et al., 2009; Ofori et al., 2013a). A recent review of the status and challenge for conservation of small mammal assemblages of Ghana showed that 34 species of rodent (excluding squirrels, grasscutter), 14 species of shrews and one species of hedgehog have been recorded in the country between 1975 and 2014 (Ofori et al., unpublished). Most studies have been conducted in the southern part of the country. As yet, no small mammal study has been published from the forest-savanna transition zone and the two upper regions of Ghana (Ofori et al., unpublished).
In this study, we conducted small mammal trapping in two habitats: (i) forest and (ii) wooded grassland during the dry season and the minor and major rainy seasons to investigate seasonal changes in species richness, abundance, diversity and composition at the Kogyae Strict Nature Reserve in the Ashanti Region of Ghana.
Study area
Kogyae Strict Nature Reserve (07° 12’N 01° 11’W), with an area of 386 km², is located in the forest/savanna transition zone in the Sekyere Central District of the Ashanti region. The climate is typical Transitional Woodland, with annual rainfall ranging between 1,200-1,300 mm (mean: 1,254 mm) and elevation of 120-130 m (WCMC, 2006). The area is bordered by the Afram River and riparian forest along its south-western boundary, as well as small pockets of dry forest and small rocky hills in the west. Much of the reserve has lost its status of "strict nature reserve", with an increasing number of farms encroaching from the south and east, as well as logging and hunting activities (Kyerematen et al., 2014). Common plant species included Anogeissus leiocarpus, Ceiba pentandra, Cola gigantea, Khaya senegalensis, Milicia excelsa, Triplochiton scleroxylon. Daniellia oliveri, Ekebergia senegalensis and Manilkara multinervis. Much of the grassland was replaced by the invasive Chromolaena odorata. Other tree species include Afzelia africana, Cussonia arborea, Detarium microcarpum, Lannea barteri, Pterocarpus erinaceus, Terminalia laxiflora. Lophira lanceolata, Parkia biglobosa, palms like Borassus aethiopum and figs (Ficus platyphylla).
Methods
Small mammals were live-trapped in forest and wooded grassland, which form the major habitats in the study area. The wooded grassland is characterized by grasses and sedges, notably
Sporobolus pyramidalis, Vertiveria fulvibarbis, Panicum maximum, Andropogon gayanus and
Heteropogon contortus, with scattered trees. Trees in the wooded grassland included
Daniellia oliveri, Ekebergia senegalensis, and
M. multinervis,
T. laxiflora.
L. lanceolata, P. biglobosa and the palms like
B. aethiopum. In each habitat, two permanent transects, each about 210 m long were established. Twenty standard Sherman collapsible live-traps (23 x 9 x 7.5 cm; H.B. Sherman Traps Inc., Florida, USA) were placed at about 10 m intervals along the length of each transect. Traps were baited with a mixture of corn meal and peanut butter, and were set during the day at about 16:30 GMT and checked the following morning from 07:30 to 10:00 GMT. Traps were set for
three
consecutive nights during the minor rainy season (September 2011), the dry season (January 2012), and the major rainy seasons (June 2012). There was therefore a total trapping effort of 360 trap-nights per habitat and an overall effort of 720 trap-nights.
Small mammal trapping and handling protocols followed standard methods (Wilson et al
., 1996; Martin et al., 2001) and complied with the animal care and use guidelines of the American Society of Mammalogists (Gannon et al., 2007). Captured individuals were identified on the spot (when possible), weighed to the nearest gram, sexed using the anal-genital distance (which is shorter in females) (Attuquayefio and Ryan, 2006) and checked for reproductive condition (scrotal testes in males and perforate vagina, enlarged nipples and pregnancy in females). Standard morphometric data, including head and body length (HB), tail length (TL), hind foot length (HF) and ear length (EL) were recorded. Each individual captured was marked by toe-clipping, before being released at the point of capture.
Voucher
specimens of species that could not be identified on site were sent to the University of Ghana zoological museum for identification.
Analysis of data
Trapping success (Ts)
This was estimated as the number of rodents captured per 100 trap-nights (a trap-night = 1 trap set for 1 night) (Nicolas and Colyn, 2003). Thus,

Relative abundance (Ra)
This was estimated as the number of individuals of the ith species caught per 100 individuals. Thus,
Diversity
For diversity of rodents, the Shannon-Wiener index (H?) (Pianka, 1966) was estimated as follows:
H? = - Σ pi ln pi (3)
Where Nt is number of rodents captured, Ni is the number of individuals of the ith species, Tn is the total number of trap-nights and Pi is the proportion of the ith species in the total sample.
Species composition
The similarity of small mammal composition between wooded grassland and forest was computed using Sorenson’s index (CN) (Krebs, 2001) as follows:
CN = 2c/(a+b) (4)
Where
a and
b are the number of species at the first and second habitats, respectively, and
c is the number of species common to the
two
habitats. The value of
CN may range from 0 to 1, with a value of 0 (zero) indicating that the species composition of the
two
sites are distinct with no common species shared between them, whereas, a value of 1 means the species composition of both habitats are identical.
Overall trap-success, species richness, relative abundance, composition and diversity
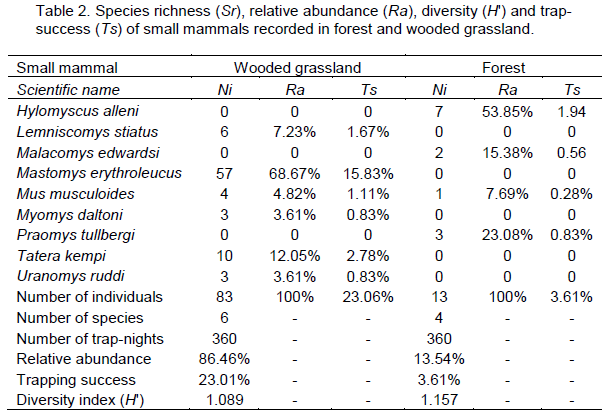
Ninety-six individuals belonging to nine species were recorded over the entire trapping session, giving an overall trap-success of 12.63% and species diversity of 1.16 for the study area (Table 1). Eighty-three individuals (86.5%) of six species were recorded in wooded grassland, while 13 individuals of four species were recorded in the forest. The total trap-success was therefore higher (23.1%) in the wooded grassland than in the forest (3.6%). The total species diversity was however, slightly higher in forest (H? = 1.157) than in wooded grassland (H? = 1.089) (Table 2).
M. erythroleucus recorded the highest captures (68.7%) in wooded grassland, and together with T. kempi, comprised 80.7% of the total number of individuals recorded in this habitat. H. alleni was the dominant (53.9%) species in forest (Table 2). Species composition was unique for both habitats (Sorenson’s index CN = 0.2), with M. musculoides being the only species common to both habitats.

Seasonal changes in species richness, abundance, diversity and composition
Wooded grassland
Species richness was higher in the rainy seasons, with both the major and minor rainy season recording five species each (Table 3). The total number of individuals (abundance), relative abundance and trap-success were highest in the minor rainy season (
Ni = 34,
Ra = 35.41%,
Ts = 28.3%) and lowest in the major rainy season (
Ni = 22,
Ra = 22.91%,
Ts = 18.3%). Species diversity was however, highest (
H? = 1.184) in the major rainy season and lowest in the dry season (
H? = 0.754) (Table 3).
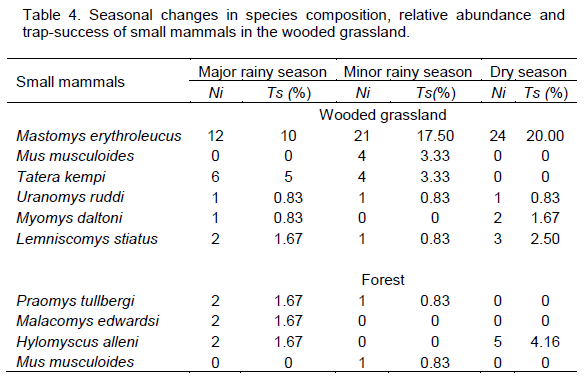
Three
species,
M. erythroleucus,
U. ruddi and
L. striatus were recorded in the major and minor rainy season and in the dry season.
M. erythroleucus, the dominant species in the wooded grassland, was most abundant in the dry season with 24 individuals and least abundant (12 individuals) in the major rainy season.
T. kempi and
M. daltoni were recorded in at least
two
seasons, while
M. musculoides was recorded in the minor rainy season only,
T. kempi recorded the highest trapping success (5%) and relative abundance (60%) during the major rainy season (Table 3).
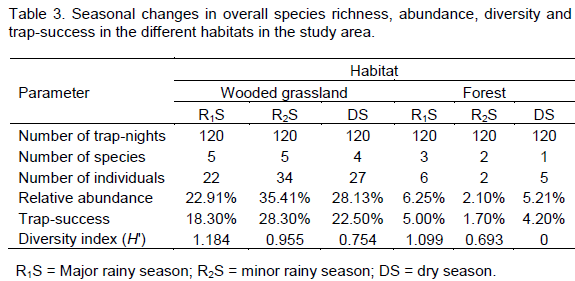
Forest
In the forest habitat, species richness and diversity were highest (3 species, H? = 1.099) in the major rainy season and lowest (1 species, H? = 0) in the dry season. Relative abundance and trap-success were highest (Ra = 5.0%, Ts = 46.1%) in the major rainy season and lowest (Ts = 1.7%, Ra = 23.4%) in the minor rainy season (Table 3). Praomys tullbergi was recorded during the major and minor rainy seasons only, while H. alleni was recorded during the major rainy and dry seasons, but not in the minor rainy season. H. alleni was also the only species recorded during the dry season. Malacomys edwardsi was recorded in the major rainy season only, whereas M. musculoides was recorded in the minor rainy season only (Table 4). P. tullbergi, M. edwardsi and H. alleni were equally abundant (33.33% each) during the major rainy season. M. musculoides and P. tullbergi also recorded similar relative abundance (50% each) and trap success (0.83% each) during the minor rainy season (Table 4).
Seasonal changes in sex-ratio
For individual species, sample sizes were too small to indicate any seasonal trends in sex ratio. Sex-ratio of M. erythroleucus, the most abundant species in the study area, was female-biased (?1:?2) in the major rainy season, male-biased (?1.6:?1) in the dry season and unity (?1:?1) in the minor rainy season.
Seasonal changes in breeding activity Small mammals showed signs of breeding activity during the major and minor rainy season, with 93.3% of males having scrotal testes and 77% of females with perforate vaginas during the rainy seasons. All the captured males of M. erythroleucus had scrotal testes in the major and minor rainy seasons, but only 46% had scrotal testes in the dry season. The highest percentage (75%) of M. erythroleucus females with perforate vaginas was recorded in the dry season, and the lowest (18.2%) in the minor rainy season. All the individuals recorded in forest showed signs of breeding activity during the wet season.
Relative abundance, diversity and composition
The small mammal species richness at the KSNR (9 species) compared well with that obtained in the Accra Plains of Ghana (Decher and Bahien, 1999) and the Muni-Pomadzi Ramsar site in the Central region of Ghana (Attuquayefio and Ryan, 2006). However, it fell short of the 11 species reported from the Amansie West District of the Ashanti Region (Ofori et al., 2013) and the 14 species in the Western Region (Yeboah, 1998). The low abundance and diversity of small mammals recorded in this study could be, in part, due to the low trapping effort. It normally takes considerable trapping effort to capture naturally rare species that have low population sizes and small distributional ranges (Fichet-Calvet et al., 2009a). Placing the traps on the ground only, might also have resulted in a bias of trapping effort towards forest-floor dwelling species.
Species composition at the study area was typical of West African small mammal assemblage (Yeboah, 1998; Decher and Bahian, 1999; Attuquayefio and Ryan, 2006; Fichet-Calvet et al., 2009a; Nicolas et al., 2010). The distinctiveness of species composition in the two habitats corresponded well with the habitat type. P. tullbergi, H. alleni and M. edwardsi, which are known forest-associated species, were recorded in forest only, whereas M. erythroleucus, L. striatus, T. kempi and U. ruddi are typical open grassland species with some preference for farmbush and forest clearings (Happold, 1987; Decher and Abedi-Lartey, 2002; Ofori et al., 2013a). Mus musculoides and M. daltoni are widespread habitat generalists that occur in a wide range of habitats and seem to depend on leaf litter (Decher and Abedi-Lartey, 2002). The high trapping success and relative abundance of M. erythroleucus in wooded grassland was not unexpected. The species is well-documented as very dominant in open grasslands and thickets, as well as in more arid areas (Decher and Bahian, 1999; Attuquayefio and Ryan, 2006; Makundi et al., 2009). The local and regional abundance of M. erythroleucus could be explained by its opportunistic feeding on grasses, leaves, seeds, seedlings, and insects, and its ability to adapt to modified habitats from open woodland to perennial grassy habitats (Decher and Bahian, 1999).
West African forests are usually dominated by either P. tullbergi or Hylomyscus sp. (Decher and Bahian, 1999). Most small mammal studies in forests in Ghana have reported P. tullbergi as the dominant species (Cole, 1975; Jeffrey, 1977; Garshong et al., 2013; Ofori et al., 2013b). In this study however, H. alleni was the most abundant forest-specialist species, even though the numerical difference in individual numbers between these species was not as large as their relative abundance scores might suggest.
Seasonal changes in species richness, abundance, diversity and composition
Seasonal variations in rodent communities were evident in the study area, supporting previous studies (Fichet-Calvet et al., 2009b; Makundi et al., 2009; Ofori et al., 2013a). The high species richness and abundance in the rainy season could be attributed to abundance of food and vegetation cover for small mammals during the wet season (Habtamu and Bekele, 2012). The high trap-success and relative abundance in the dry season could be attributed to the mono-dominance of M. erythrleucus, an opportunistic feeder, in the dry season (Decher and Bahian, 1999).
Seasonal changes in sex-ratio and breeding activity
Even though the male : female ratio of small mammals in this study did not deviate very much from unity, males were generally more abundant than females. This observation is supported by several other studies (Nicolas and Colyn, 2003; Garshong and Attuquayefio, 2013; Ofori et al., 2013ab). This may be because dispersal in small mammals is male-biased, increasing the chance of males encountering traps and getting captured (Garshong and Attuquayefio, 2013; Ofori et al., 2013b).
Overall breeding pattern of rodents in the study area was seasonal and related to rainfall. It is probably because the abundance of protein-rich diets like foliage, seedlings and insects, and lush vegetation cover during the wet season provide adequate security for lactating females and their offspring (Attuquayefio and Wuver, 2003; Nicolas and Colyn, 2003; Makundi et al., 2005, 2009; Fichet-Calvet et al., 2009b; Habtamu and Bekele, 2012). The findings of this study also showed that breeding activity in M. erythroleucus continued during the dry season indicating perhaps, a year-round breeding activity in this species (Fichet-Calvet et al., 2009b). Further studies will however be needed to confirm this, even though opportunistic feeding behaviour of the species coupled with a year-long breeding activity could account for its local dominance.
The present study is the first detailed survey of small mammals at the Kogyae Strict Nature Reserve, and the forest-savanna transition of Ghana. The findings of this study therefore will serve as baseline information for future monitoring programmes, which are necessary to evaluate impacts of environmental changes on small mammals.
The authors did not declare any conflict of interest.
This study was undertaken under the building capacity to meet the climate change challenge (B4C), Ghana project funded by the Open Society Foundations. The authors wish to thank the two anonymous reviewers whose comments helped in improving this paper.
REFERENCES
|
Angelici FM, Luiselli L (2005). Patterns of specificdiversity and population size in small mammals from arboreal and ground-dwelling guilds of forest area in southern Nigeria. J. Zool. Lond. 265: 9-16.
Crossref
|
|
|
|
Attum O, Rabea B, Osman S, Habinan S, Baha EL, Din SM, and Kingsbury B. (2008). Conserving and studying tortoise: A local community visual-tracking or radio-tracking approach? J. Arid Environ. 72: 671-676.
Crossref
|
|
|
|
|
Attuquayefio DK, Ryan, JM. (2006). Taxonomic report on small mammals from two coastal wetland (Ramsar) sites in Ghana. W. Afri. J. Appl. Ecol. 10: 1-11.
|
|
|
|
|
Attuquayefio DK, Wuver AM (2003). A study of bushfire in a Ghanaian coastal wetland. I. Impact on small mammals. W. Afri. J. Appl. Ecol. 4: 1-11.
|
|
|
|
|
Avenant N (2011). The potential utility of rodents and other small mammals of indicators of ecosystem integrity of South African grasslands. S. Afri. J. Wildl. Res. 38: 626-639.
Crossref
|
|
|
|
|
Barriere P, Nicolas V, Oduro LK (2009) A rapid biodiversity assessment of the Ajenjua Bepo and Mamang River Forest Reserves, Ghana. In: McCullough J, Hoke P, Naskrecki P, Osei-Owusu Y (Eds.). A rapid biological assessment of the Ajenjua Bepo and Mamang River Forest Reserves, Ghana. RAP Bulletin of Biological Assessment 50. Conservation International, Arlington, VA, USA, Pp 54-57.
|
|
|
|
|
Coe MJ, Cumming DH, Phillipsi J (1976). Biomass and productivity of large African herbivores in relation to rainfall and primary production. Oecol. 22:341-354.
Crossref
|
|
|
|
|
Cole LR (1975). Foods and foraging places of rats (Rodentia:Muridae) in the lowland evergreen forest of Ghana. J. Zool. Lond. 175: 453-471.
Crossref
|
|
|
|
|
Decher J, Abedi-Lartey M (2002). Small mammal zoogeography and diversity in West African forest remnants. Final Report. Ghana Wildlife Division, National Geographic Committee for Research and Exploration, University of Vermont, 32 pp.
|
|
|
|
|
Decher J, and Bahian LK. (1999). Diversity and structure of terrestrial small mammal communities in different vegetation types on the Accra Plains of Ghana. J. Zool. Lond. 247: 395-408.
Crossref
|
|
|
|
|
Fichet-Calvet E, Audenaert L, Barriere P, Verheyen E (2009a). Diversity, dynamics and reproduction in a community of small mammals in Upper Guinea, with emphasis on pygmy mice ecology. Afri. J. Ecol. 48: 600-614.
Crossref
|
|
|
|
|
Fichet-Calvet E, Lecompte E, Veyrunes F, Barriere P, Nicolas V, Koulemou K (2009a). Diversity and dynamics in a community of small mammals in coastal Guinea, West Africa. Belg. J. Zool. 139 (2): 93-102.
|
|
|
|
|
Food and Agricultural Organization (FAO) (2007). Forest outlook study for Africa, FAO Forestry Paper 141, Rome, Italy.
|
|
|
|
|
Gannon WL, Sikes RS (2007). The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mamm. 88: 809-823.
Crossref
|
|
|
|
|
Garrat CG, Minderman J, Whittingham MJ (2012). Should we stay or should we go now? What happens to small mammals when grass is mown, and the implications for birds of prey? Ann. Zool. Fenn. 49: 113-122.
Crossref
|
|
|
|
|
Garshong R, Attuquayefio D (2013). Aspects of the ecology of rodents in the Owabi Wildlife Sanctuary, Ghana: Sex ratio, age structure and reproductive characteristics. A. J. Appl. Sci. 1: 134-140.
|
|
|
|
|
Garshong RA, Attuquayefio DK, Holbech LH, Adomako JK (2013). Distribution and abundance of small mammals in different habitat types in the Owabi Wildlife Sanctuary, Ghana. J. Ecol. Nat. Environ. 5(5): 83-87.
Crossref
|
|
|
|
|
Habtamu T, Bekele A (2012). Species composition, relative abundance and habitat association of small mammals along the altitudinal gradient of Jiren Mountain, Jimma, Ethiopia. Afri. J. Ecol. 51: 37-46.
Crossref
|
|
|
|
|
Happold DCD (1987). Reproduction, growth and development of a West African forest mouse, Pryomys tullbergi (Thomas). Mammalia. 42: 74-95.
|
|
|
|
|
Jackson ST, Betancourt JL, Booth RK, Gray ST (2009). Ecology and the ratchet of events: climate variability, niche dimensions, and species distributions. Proc. Natl. Acad. Sci, 106:19685-19692.
Crossref
|
|
|
|
|
Jeffrey SM (1977). Rodent ecology and land use in Western Ghana. Gh. J. Appl. Ecol. 14:741-755.
Crossref
|
|
|
|
|
Krebs CJ (2001). Ecology: the Experimental Analysis of Distribution and Abundance (5th Ed). Benjamin Cummings, San Francisco.
|
|
|
|
|
Kyerematen R, Owusu EH, Acquah-Lamptey D, Anderson RS, Ntiamoa-BaidunY. (2014). Species composition and diversity of insects of the Kogyae Strict Nature reserve in Ghana. Open J. Ecol. 4:1061-1079.
Crossref
|
|
|
|
|
Makundi RH, Massawe, AW, Mulungu LS (2005). Rodent population fluctuations in three ecologically distinct locations in north-east, central and south-west Tanzania. Belg. J. Zool. 135: 159-165.
|
|
|
|
|
Makundi RH, Massawe AW, Mulungu LS, Katakweba A. (2009). Species diversity and population dynamics of rodents in a farm-fallow field mosaic system in Central Tanzania. Afr. J. Ecol. 48: 313-320.
Crossref
|
|
|
|
|
Malcom JR, Ray JC (2000). The influence of timber extraction routes on central African small mammal communities, forest structure and tree diversity. Conserv. Biol. 14: 1623-1638.
Crossref
|
|
|
|
|
Manning JA, Edge WD. (2008). Small mammal responses to fine woody debris and forest fuel reduction in southwest Oregon. J. Wildl. Manage. 72: 625-632.
Crossref
|
|
|
|
|
Martin RE, Pine RH, DeBlase AF (2001). A manual of mammalogy: With Keys to Families of the World (3rd Ed). Wm. C. Brown Co., Dubuque, Iowa, pp. xii+436.
|
|
|
|
|
Nicolas V, Colyn M (2003). Seasonal variations in population and community structure of small rodents in a tropical forest of Gabon. Can. J. Zool. 81: 1034-1046.
Crossref
|
|
|
|
|
Nicolas V, Bryja J, Akpatou B, Wendelen W, Peterhans K, Olayemi A, Decher J, Missoup A, Denys C, Barriere P, Cruaud C, Colyn M. (2010). Molecular and morphometric variation in two sibling species of the genus Praomys (Rodentia: Muridae): implications for biogeography. Zool. J. Lin. Soc. 160: 397-419.
Crossref
|
|
|
|
|
Ofori BY, Attuquayefio DK, Gbogbo F (2013a) Terrestrial small mammal community structure in an anthropogenically-altered moist semi-deciduous forest zone in Ghana. Int. J. Dev. Sust. 2(2): 1156-1168
|
|
|
|
|
Ofori BY, Attuquayefio DK, Owusu EH. (2013b). Aspects of the ecology of the Tullberg's soft-furred mouse, Praomys tullbergi Thomas, 1894) in Mount Afadjato, Ghana. J. Exp. Biol. Agr. Sci. 1(5): 398-404.
|
|
|
|
|
Pianka ER (1966). Latitudinal gradients in species diversity: a review of concepts. The A. Natl. 1100(910): 33-46.
Crossref
|
|
|
|
|
Ryan JM, Attuquayefio DK (2000). Mammal fauna of the Muni-Pomadze Ramsar site, Ghana. Biodivers. & Conserv. 9:541-560.
Crossref
|
|
|
|
|
Soule ME, Estes JA, Berger J, Del Rio CM (2003). Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 17: 1238-1250.
Crossref
|
|
|
|
|
United Nations Environment Programme (UNEP)-World Conservation Monitoring Centre (WCMC) (2006). WDPA Consortium 2006 World Database on Protected Areas.
|
|
|
|
|
Vos P, Meelis E, Ter Keurs WJ (2000). A framework for the ecological monitoring programs as a tool for environmental and nature management. Env. Mon. & Assess. 61: 317-344.
Crossref
|
|
|
|
|
Wilson DE, Cole FR, Nichols DJ, Rudran R, Foster MS (1996). Measuring and Monitoring Biological Diversity: Standard methods for mammals. Smithsonian Institution Press, Washington, DC, pp. xvii+409.
|
|
|
|
|
Wilson DE, Reeder DM (2005). Mammal Species of the World: A taxonomic and geographic reference. JHU Press, Baltimore, MD, USA. Pp2142.
|
|
|
|
|
Yeboah S (1998). Small mammal diversity in the Kakum National Park in Ghana. Gh. J. Sci. 38:25-32.
|
|
|
|
|
Zahratka JL, Shenk TM (2008). Population estimates of snowshoe hares in the southern Rocky Mountains. J. Wildl. Manage. 72:906-912.
Crossref
|
|
 and trapping protocol
and trapping protocol consecutive nights during the minor rainy season (September 2011), the dry season (January 2012), and the major rainy seasons (June 2012). There was therefore a total trapping effort of 360 trap-nights per habitat and an overall effort of 720 trap-nights.
consecutive nights during the minor rainy season (September 2011), the dry season (January 2012), and the major rainy seasons (June 2012). There was therefore a total trapping effort of 360 trap-nights per habitat and an overall effort of 720 trap-nights. specimens of species that could not be identified on site were sent to the University of Ghana zoological museum for identification.
specimens of species that could not be identified on site were sent to the University of Ghana zoological museum for identification. habitats. The value of CN may range from 0 to 1, with a value of 0 (zero) indicating that the species composition of the two
habitats. The value of CN may range from 0 to 1, with a value of 0 (zero) indicating that the species composition of the two sites are distinct with no common species shared between them, whereas, a value of 1 means the species composition of both habitats are identical.
sites are distinct with no common species shared between them, whereas, a value of 1 means the species composition of both habitats are identical. species, M. erythroleucus, U. ruddi and L. striatus were recorded in the major and minor rainy season and in the dry season. M. erythroleucus, the dominant species in the wooded grassland, was most abundant in the dry season with 24 individuals and least abundant (12 individuals) in the major rainy season. T. kempi and M. daltoni were recorded in at least two
species, M. erythroleucus, U. ruddi and L. striatus were recorded in the major and minor rainy season and in the dry season. M. erythroleucus, the dominant species in the wooded grassland, was most abundant in the dry season with 24 individuals and least abundant (12 individuals) in the major rainy season. T. kempi and M. daltoni were recorded in at least two seasons, while M. musculoides was recorded in the minor rainy season only, T. kempi recorded the highest trapping success (5%) and relative abundance (60%) during the major rainy season (Table 3).
seasons, while M. musculoides was recorded in the minor rainy season only, T. kempi recorded the highest trapping success (5%) and relative abundance (60%) during the major rainy season (Table 3).