ABSTRACT
Elephants in the borderland of Kenya and Tanzania landscape roam freely outside the protected areas. These areas are critical for long term elephant survival and viability. Understanding the ecological conditions in these landscapes and threats to elephants is critical in future elephant management. Using collared elephants, the habitat use and selection was studied. Elephants showed selection for habitats, but selection was independent on season, individual elephant and gender. Bushland and woodland habitats were most preferred by elephants because they represented better habitat patchiness and heterogeneity. This range was also shared by other elephants and wild large mammals particularly zebra, gazelles and giraffes. The presence of livestock in all habitats results in competition for forage and water and leads to conflicts over space and resource. Habitat (and its quality, quantity and risks) may be the most important factor in elephant viability and ranging in the landscape. Further, securing quality and sufficient space and controlling human-elephant conflicts are the most important aspects for elephant management. We therefore recommend focus on space needs and controlling conflicts outside protected areas, and negotiations with land owners for elephant space in this borderland landscape.
Key words: Amboseli ecosystem, African elephants, habitat selection, Kenya, landscape movements.
Elephants interact strongly with the ecosystems they inhabit (Kerley et al., 2008). This is partly because the African elephant is the largest herbivore alive today, with females attaining a maximum body mass of over three tons and males over six tones (Estes, 2012). Coupled with this large size (and hence mega - herbivore status) is a fairly simple digestive system with most digestion taking place in the capacious hindgut, comprising the small intestine and colon. Throughput is relatively rapid, with mean retention time of about 24 h, independent of the daily food intake (Clauss et al., 2007; Davis, 2007). This fast passage (compared with other large herbivores) means that digestive efficiency is quite low, with less than half of the ingested food being assimilated and the remainder passed out as droppings (Estes, 2012).
Elephant are mega-herbivores, consuming vast quantities of food, and are known as ‘wasteful feeders’ (Kerley et al., 2008). They are a savanna keystone species (Western, 1989; Laws, 1970; Penzhorn et al., 1974; Pellew, 1983), meaning that their presence ecolo-gically benefits other wildlife species and due to its ecological role in an ecosystem (Twine et al., 2008), they are important in nutrient cycling and seed dispersal, and elicit plant defense and growth responses (Kerley et al., 2008). Elephants and fire are regarded as drivers of alternate states in ecosystems (Kerley et al., 2008). It is sometimes difficult to disentangle the relative roles of elephant, fire, drought, disease, and other browsers in tree population patterns because they often affect the vegetation in combination (Kerley et al., 2008).
Grainger et al. (2005) investigated the influence of special heterogeneity on use of space and home range in elephants. They hypothesized that heterogeneity may influence ranging behavior of mammals. They related the home range size of elephants living in the Kruger National Park to the number of patches, proportion of each patch, spatial arrangement of patches, patch shape and contrast between neighboring patches. Home range sizes decreased exponentially with an increase in the number of patches per 100 km2 and the home range sizes of bulls were in general more strongly related to measures of heterogeneity. This may reflect differences in perception of heterogeneity between the sexes.
Elephants roam the landscapes utilizing different habitats and its resources that meet their needs and enhance their survival. Within the landscape, habitat patches vary in their composition and spatial arrangement and this complexity represents landscape heterogeneity (O’Neill et al., 1986). So habitat characteristics and resources within them can determine level of preference and use of different habitats (Alldredge and Ratti, 1986, 1992; McClean et al., 1998).
Hierarchy theory predicts that resource selection at smaller scales (e.g. plant resources) will cause an aggregate selection response at larger habitat scale (O’Neill et al., 1986). As bulky feeders, elephants include low-quality plant matter in their diets (Owen-Smith, 1988). However, to maximize their energy intake there should be a trade-off between selection for scarce, high-quality resources and the utilization of lower quality resources that are presumably more abundant (Illius, 2006).
For elephants, nutritional constraints are pronounced as the dry season progresses. In theory, elephants are therefore expected to increase the size of their home ranges during the dry season to include the resources otherwise available during only the wet season. Most often, elephants tend to concentrate their foraging activities in areas close to water during the dry season (Chamaille´-Jammes et al., 2007; de Beer et al., 2006; Gaylard et al., 2003; Leggett, 2006; Osborn and Parker, 2003; Redfern et al., 2003) and they then conceivably depend on lower quality food (Owen-Smith, 1988). The restriction imposed by the distribution of water and possibly away from human infrastructure and presence may therefore coincide with selection for areas with higher food resource availability within the landscape, which may consequently determine the location of elephant home ranges (Damschen et al., 2006). In the Damschen et al. (2006) study, they hypothesized that landscape heterogeneity and water distribution are determinants of the location and size of elephant home ranges in arid savannas. The apparent selection for variables that are encapsulated by land-scape heterogeneity metrics may explain the uneven distribution of elephants across landscapes as an outcome of their preferences for certain habitats. Moreover, by identifying how landscape heterogeneity and water distribution affects the spatial dynamics of elephants, we may be able to predict how elephants will respond to areas in which they do not occur at present. This may facilitate initiatives to improve conservation management plans that incorporate aspects of landscape ecology (Damschen et al., 2006; Potvin et al., 2001).
This study was undertaken to investigate the use of habitats by elephants and the elephant attributes that determine the use and choice of habitat types. The specific objectives of this paper are as follows:
1. To establish the proportion of various habitats found in the landscape used by collared elephants in the Amboseli Ecosystem.
2. To establish the frequency of habitat use by elephants in their home range in the landscape.
3. To establish elephant’s selection of habitats by comparing the habitat proportion available and frequency of use for each habitats by the elephants.
Study area
The Amboseli Ecosystem is located in southern Kenya bordering Tanzania, in the Loitokitok Division of the Kajiado District. It consists of KGR which is 251 km2 of land. Most of the Loitokitok Division is semi-arid and arid rangeland with a bimodal rainfall pattern (Katampoi et al., 1990). Mt. Kilimanjaro casts a rain shadow affect over the region where moisture in the clouds is lost as air masses move up the south side of the mountain and arrive on the north side of the mountain dry (Katampoi et al., 1990).
On the Kenya side, the landscape’s key features include: Amboseli National Park and six (6) Maasai group ranches (Figure 1). On the Tanzanian side, key attributes include Mt. Kilimanjaro and Arusha National Parks, as well as Lake Natron, and the low-lying Savannas of Longido. Some of the main threats facing this landscape include: human-wildlife conflicts, land use changes especially proliferation of agriculture, sedentarization, unsustainable livestock grazing practices, and illegal poaching of wildlife (Ntiati, 2002; Okello and Kiringe, 2004; Okello, 2009).
The critical underlying drivers of these threats are: climate change and variability, human population growth, change subsistence to a commercial lifestyle and increase of poverty levels (Ntiati, 2002; Okello and Kiringe, 2004; Okello, 2009).
The Amboseli eco-system on the Kenyan includes Amboseli National Park and the adjacent six Maasai Group Ranches (GR) and community conservancies. Within the Kilimanjaro Landscape, the group ranches in the Amboseli Ecosystem include former (now subdivided) Kimana, Mbirikani, Kuku, Olgulului/Ololorashi, Eselenkei and Rombo (Figure 1). This is the general area where the elephant subjects in this work ranged on a landscape level. These group ranches comprise many ethnic groups, but are particularly dominated by the Maasai. A diverse ethnic community lives in markets, agricultural clusters and towards the Mt. Kilimanjaro slopes (Ntiati, 2002; Okello and Kiringe, 2004; Okello, 2009)
The long rain season begins from March to early June and the short rains occur in October and November. The average annual rainfall received in KGR is 210 mm with 30% being received during the short rains and 45% received during the long rains (Irigia 1995). The area has a variety of habitats including dense and open shrubland, bushland and woodland. The dominant vegetation in the riverine habitat is Acacia xanthophloea and the drier regions are dominated by Acacia tortillis and Acacia mellifera (Irigia, 1995). Soils in this region are classified as volcanic soils which are generally highly saline and alkaline. In addition, the soils in the KGR area are shallow due to the recent volcanic activity of the region. This volcanic soil is generally unproductive, but near water sources can be extremely fertile (Katampoi et al., 1990). Areas further away from water sources are suitable only for pastoralism and wildlife grazing.
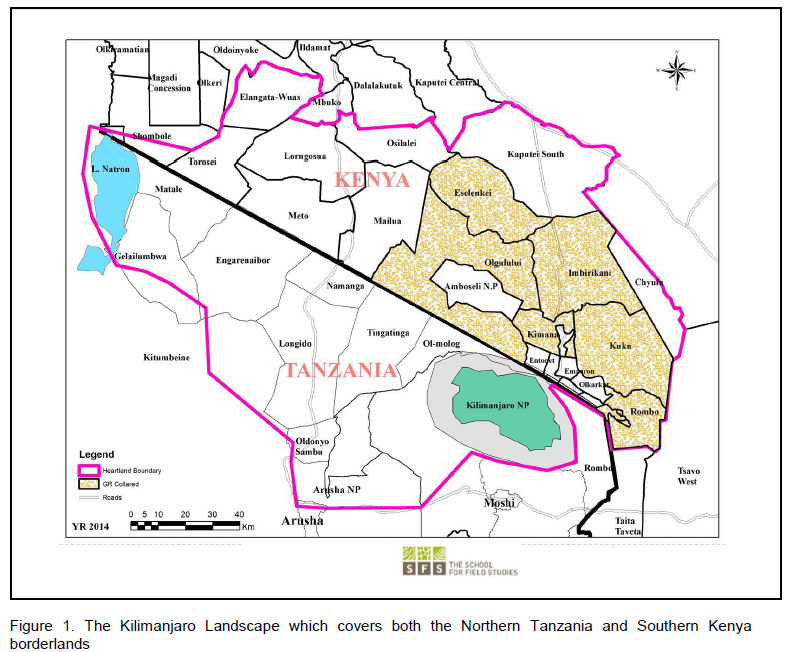
Together, these group ranches create wildlife corridors and dispersal areas that connect the park islands, allowing the parks to support large populations of seasonally migratory mammals (Western, 1975). The group ranches also support large populations of wildlife. In support of this wildlife, Kimana Community Wildlife Sanctuary was established in 1996 (Lichtenfeld, 1998). It provides a concentration area in the group ranch for resident and migrating species between protected areas in the ecosystem.
This socio-economic changes are increasing demand for group ranch subdivision so that people individually feel secure in land ownership. Newer government polices aim to provide a framework for dismantling communal ownership of land and nomadic pastoralism into individual ownership in support for group ranch subdivision (Graham, 1989; Galaty, 1992, 1994; Seno and Shaw, 2002). Kimana is now fully subdivided, and all Maasai group ranches have already begun the process. As subdivision occurs, the Maasai will no longer be able to support their large herds of livestock without depletion of land resources. In response, many Maasai are becoming agro-pastoralists (Okello, 2005) despite the old belief that to till the land is a curse.
Also, land tenure policy promoting subdivision and private ownership increases the opportunity for migrant farmers to lease subdivided land, hence accelerating agriculture expansion in the area (Okello, 2005). This switch to agriculture causing serious problems since cultivation is considered one of the most serious threats to wildlife conservation in this region (Okello and Kiringe, 2004). Almost all agriculture that takes place in KGR requires the use of irrigation except in the areas near Kilimanjaro where rain fed agriculture is possible. The use of irrigation reduces water quantity available to other land uses such as pastoralism and wildlife (Campbell et al., 2000).
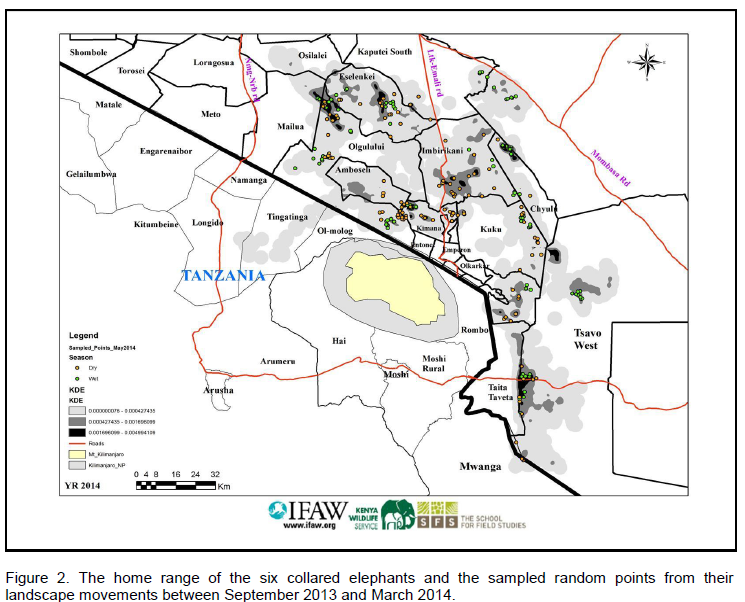
This work was done between September 2013 and March 2014 in the landscape home range of 6 collared elephants. The dry season sampling points were taken from the satellite GPS locations of the elephants between September 2013 and December 2013. The wet season points were randomly sampled from landscape elephant locations in short rainy season (January 2013) and in early rainy season (month of February to March 2014).
A total of 260 landscape GPS elephant locations were selected randomly (rom computer random number generator of point identification numbers) from the 6 general elephant home ranges (determined from the cluster of locations) between September 2013 and March 2014. For the dry season, a total of 129 points were randomly sampled while for the wet season, a total of 131 points were sampled. The home ranges of most elephants overlapped, so an effort was made to restrict sampled points to be those exclusively used by each elephant to reduce compounding factors.
Total composition of habitats
Ten randomly located one kilometer long line transects were established in each elephant home range. The line transects run east to west in each of the general elephant home range. For each line transect, the length covered by each habitat type along the transect was recorded. The average length of each habitat coverage along all line transects was an index of the proportion of availability of that transect.
Total habitat use
For each random point selected from elephant actual locations obtained from the satellite data, the broad habitat type was recorded for both dry and wet season.
The number of random points of elephant actual location selected from each habitat was the frequency of use of that habitat use by the elephants. The proportion of total random locations in each habitat from all the 260 random points sampled gave the proportion of habitat use for that habitat
Animal density
At each sampling point (considered a point transect), alllarge mammal (wildlife and livestock) numbers seen were recorded. Further, the distance from the sampling point to each of the species seen and its number were also recorded. The broad habitat type and season was also indicated. This data was used to summarize large mammals seen by signs, those seen live (wild and livestock) and the density (number of animals per observation point of same radius of one Kilometer, and the number of animals per area of circular sampling point) of each species at each sampling point. The data also gave information for the density per km
2 of live animals by dividing the number seen for each species over the circular area (using

r
2) from the distance of the live seen animals to the sampling point.
Data analysis
Normal mathematical calculations for area and means (with standard error) were applied. For establishing selection, Strauss Linear Index of Food Selection (Strauss 1979) which has which was compared to other complex statistical techniques of resource selection evaluated in the literature (Alldridge and Ratti 1986, 1992; McClean et al., 1998) but giving comparable inferences. Strauss linear index of food selection has advantages of being simple to use, symmetrical for selection and avoidance and being reliable for changes in items available for choice and non-parametric in approach. The Strauss Index was simply calculated as the difference of the portion of use and availability of habitats by elephants based on the equation below:
Strauss Resource Selection, L = (ri – pi), Where L= Strauss measure selection / preference, ri = Proportion or percentage used , pi = Proportion availability of the same prey resource in the environment For the relationship between elephant selection and various elephant attributes (individual, sex) and other independent factors (season and habitat type used by elephants), a chi – square cross tabulations (Zar, 1999) was used to establish dependence of elephant habitat selection and these independent factors. Spatial analysis was done using GIS software to establish general elephant core use areas.
Habitat availability, use and selection by elephants
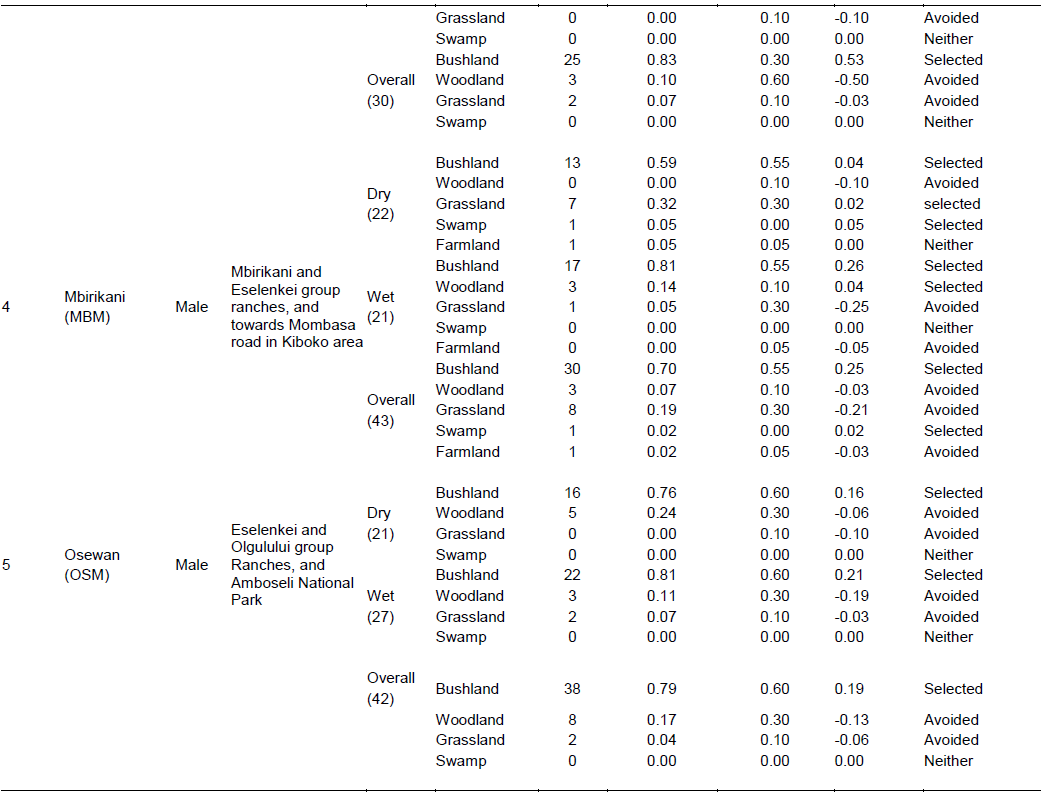
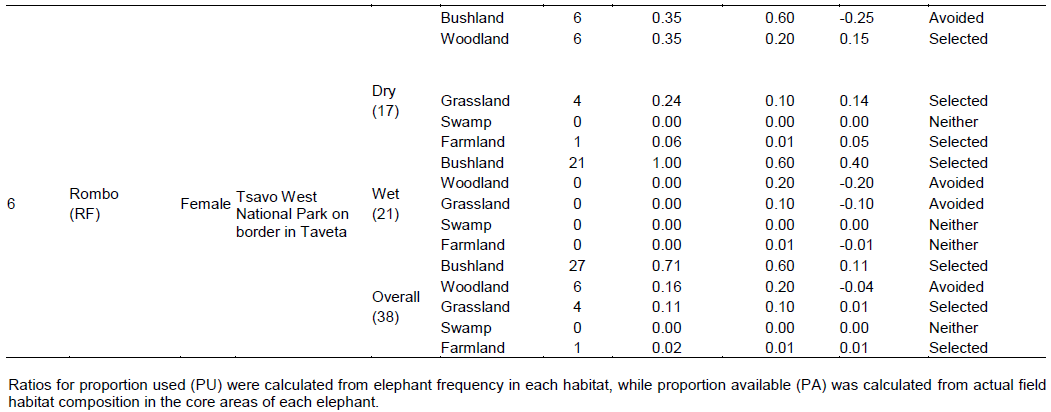
There were four main broad habitat types in elephant home ranges in the Amboseli Ecosystem: bushland, woodland, grassland and swamp (Table 1). In the Eselenkei (ESM) male whose core area is mainly in Eselenkei Group Ranch (Figure 3), the dominant habitat was bushland (70%) followed by woodland (20%). For Kimana (ESM), male elephant core area is in Kimana Group Ranch and Southern Amboseli (Figure 4), the dominant habitat was bushland (60%) followed by woodland (10%). Kuku (KUF) has its core area in Kuku Group Ranch (Figure 5) along the Chyulu Hills, and the dominant habitat in its home range was woodland (60%) followed by bushland (30%). The Osewan (OSM) male has its core use area in Olgulului and Eselenkei group ranches (Figure 6). The dominant habitat in its home range was bushland (60%) followed by woodland (30%). The Rombo (RF) female uses mainly Rombo group ranch and Tsavo West National Park (Figure 7). The dominant habitat in its home range was bushland (60%) followed by woodland (20%).
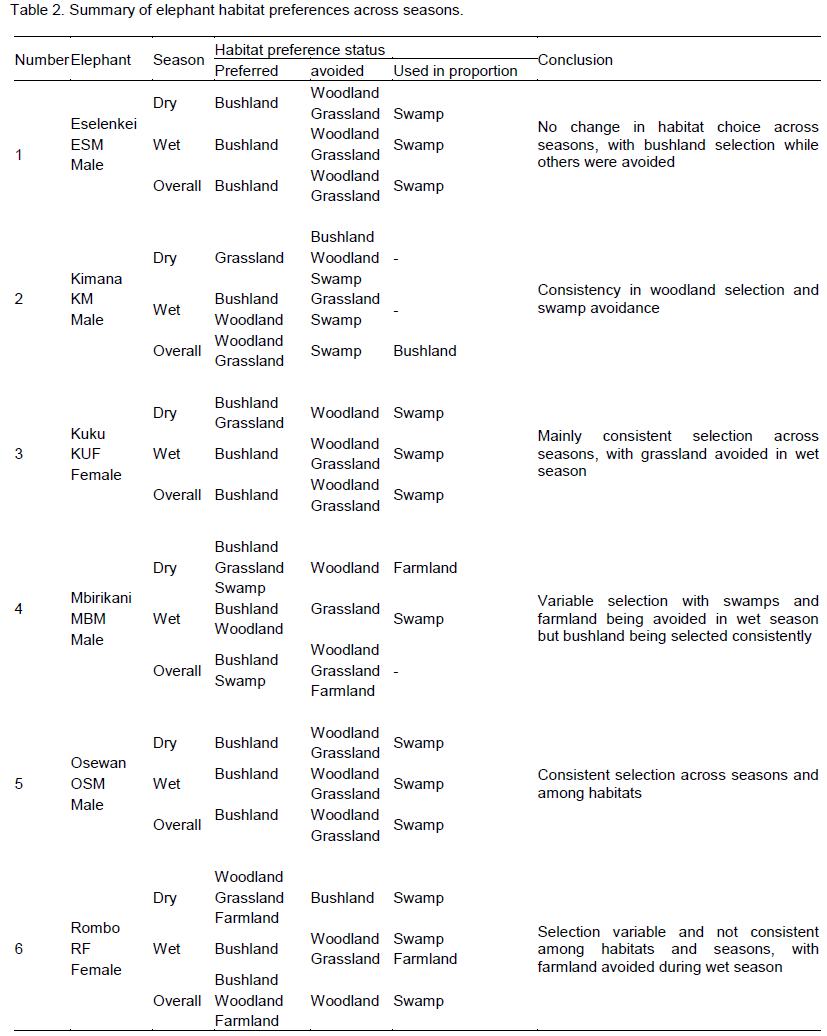
The proportion used for each habitat and for each elephant ranging varied (Table 1). The Eselenkei (ESM) male generally selected bushland and avoided woodland and grassland, often utilizing swamp in proportion to availability (Table 1).
There was no change in habitat choice across dry and wet season (Table 2). The Kimana (KM) male generally selected woodland and grassland, but avoided swamp, often utilizing woodland in proportion to availability (Table 1). There was no change in habitat choice dry and wet season (Table 3). For the Kuku (KUF) female, it generally selected the bushland habitat, but avoided woodland and grassland, only utilizing swamp in proportion to availability (Table 1). There was consistency in habitat choice in dry and wet season (Table 2). The Mbirikani (MBM) male generally selected Bushland and Swamp, but avoided woodland and grassland. It also avoided cultivated areas (Table 1). The elephant seemed to change its habitat choice based on season (Table 2). For the Osewan (OSM) male, it generally selected the bushland habitat, but avoided woodland and grassland, only utilizing swamp in proportion to availability (Table 1). There was consistency in habitat choice in dry and wet season (Table 2). The Rombo (RF) female generally selected bushland and grassland. This elephant also seemed to prefer using farmlands (Table 1). The elephant seemed to change its habitat choice based on season (Table 2). We established that habitat selection was independent on the individual elephant characteristics (χ2 = 5.30, df = 10, p = 0.87), implying that selection of habitat by elephants is consistent with any elephant (Table 4). In terms of gender, habitat selection was also independent on gender (χ2 = 0.23, df = 2, p = 0.88), implying that habitat selection is consistent irrespective of the elephant gender. Further, in terms of seasons, habitat selection by elephants was independent on the season (χ2 = 5.90, df = 2, p = 0.74), implying that habitat selection was consistent irrespective of season. However, despite habitat selection by elephants being independent of individual elephants, gender and season, selection was dependent on habitat type (χ2 = 4652, df = 8, p < 0.001). Habitat type influenced habitat use and selection by the elephants (Table 3).
Animal density across habitats in elephant home range
Overall in terms of habitats, density of animals seen (per 1 km sampling radius point) was highest (Table 4) in the swamp (107.80 ± 76.36 animals per sampling point) followed by woodland (13.95 ± 4.71 animals per point), bushland (11.51 ± 2.13 animals per point) and grassland (11.39 ± 4.09 animals per point). In terms of livestock, the highest livestock density was found in swamp habitat (500.00 ± 100.00 per point) followed by bushland (129.95 ± 10.36 animals per point), grassland (113.18 ± 29.71 animals per point), farmland (101.75 ± 98.85 animals per point) and woodland (96.72 ± 16.98 animals per point).
In the Eselenkei (ESM) male home range, the highest density of wild large mammals occurred in bushland (4.48 ± 0.86 animals per point). Further, the highest density of livestock was also in the bushland habitat (77.00 ± 8.14 animals per point). In the Kimana (KM) male home range (Table 4), the highest density of wild large mammals occurred in bushland (26.63 ± 8.34 animals per point) followed by woodland (17.53 ± 7.93 animals per point). Further, the highest density of livestock was also in the bushland habitat (114.25 ± 27.33 animals per point) followed by woodland (100.30 ± 25.89 animals per point). In the Kuku (KUF) female home range, more common large mammal (wild and livestock) signs were in woodland (Table 4), but live wild large mammals occurred only in woodland (17.53 ± 7.93 animals per point) followed

by bushland (1.33 ± 0.33 animals per point). However, the highest density of livestock was also in the grassland (500.00 ± 0.00 animals per point) followed by woodland (100.30 ± 25.89 animals per point).
In the Mbirikani (MBM) male home range, although it was more common to see large mammal (wild and livestock) signs in woodland and bush land habitats, (Table 4), the highest density of live wild large mammals occurred in bushland (4.48 ± 0.86 animals per point) followed by woodland habitat (26.63 ± 8.34 animals per point). Further, the highest density of livestock was also in the swamp habitat (500.00 ± 100.00 animals per point) followed by bushland habitat (114.25 ± 27.33 animals per point). In the Osewan (OSM) male home range, more common large mammal (wild and livestock) signs were in woodland and bushland (Table 4), but the highest density of live wild large mammals occurred in bushland (6.11 ± 1.70 animals per point) followed by woodland (5.00 ± 1.53 animals per point). However, the highest density of livestock was found in the bushland habitat (115.80 ± 19.57 animals per point) followed by woodland (57.00 ± 29.94 animals per point). In the Rombo (RF) female home range, more common large mammal (wild and livestock) signs were in bushland and grassland (Table 4), but live wild large mammals occurred in grassland habitat (80.00 ± 0.00 animals per point) followed by woodland (12.20 ± 6.80 animals per point).
However, the highest density of livestock was also in the bushland (155.75 ± 24.18 animals per point) followed by woodland (127.50 ± 47.77 animals per point).
In terms of species numbers across habitats, giraffe, Grants gazelle, Thomson’s gazelle, elephants, Kirk’s dik dik and impala were commonly seen in the bushland habitat, which had 20 large mammal species seen in the habitat (Table 5). In terms of density, the most abundant was Thomson gazelle followed by Kirk’s dik dik, Olive baboons, impala, lesser kudu and Oryx (Table 5). Livestock was dominated by mixed herd of goats and sheep (acronym shoats) and cattle. In the woodland habitat, there were 6 large mammal wild species and ostrich. The most common were giraffe followed by Grant’s gazelle, impala, zebra, Thomson’s gazelle, common waterbuck and elephants. The most abundant in terms of density was Thomson’s gazelle followed by zebra, waterbuck, giraffe, common eland and Kirk’s dikdik. The shoats and cattle also dominated the habitat but were relatively fewer than the bushland.
The grassland had a total of 14 species with the most commonly seen being Grant’s gazelle followed by ostrich, elephants, giraffes and zebra.
In terms of density, the most abundant was elephant followed by gerenuk, giraffe, zebra, Grant’s gazelle and ostrich (Table 5). The shoats and cattle also dominated but they were less abundant as compared to the bushland and woodland. In the swamp habitat, there were 7 most commonly seen large mammals species and ostrich. The most abundant was elephant followed by Thomson’s gazelle and wildebeest. This habitat has also shoats and cattle, but the numbers being less than those in bushland, woodland and grassland (Table 5).
The home range of elephants in the Amboseli Ecosystem has a diversity of habitats, dominated mainly by bushland, woodland and grassland. Within these broad habitats is different vegetation structure with a varying amount of openness (with grassland) and density of woody plants. These vegetation structure and composition is critical for elephant use of the range because it provides heterogeneity which is a precursor for a diverse resource types needed for elephant survival. Elephants seem to prefer landscapes with vegetation and plant heterogeneity and such areas become their core use home ranges (De Beer and Van Arde, 2008). However, the habitats in all the elephant range in Amboseli are heavily shared with livestock from the Maasai pastoralists and also used by the local people for plant resources (for housing, fuel wood, fencing and medicinal purposes) and so the vegetation structure and composition is constantly being modified by people, livestock and wildlife. When there is over-utilization of plant resources by people, livestock and wildlife (especially elephants), habitat degradation will occur which may reduce the appeal of the range for elephant use.
Elephant use of habitats available to it varied because they actively select for it and also a combination of it will maximize vegetation heterogeneity. So, it was clear from the results that the most important aspect influencing elephant selection of habitat for use was the type of habitat. This implies that habitat composition in the landscape will influence the location and even size of elephant home range. Any factor that influences habitat quantity (plant harvesting and usage) and quality (range condition and risks to elephants through threats such as poaching and snaring) will determine elephant use. From the results, the bushland seemed the most common habitat, but also the most selected for across elephants and across the seasons. This may be due to the preference of elephants on browsing on woody plants especially during the dry season when they switch from grass to branches and bark of trees. The bushland also provides different woody vegetation that may be providing different level of nutrients and palatability that elephants will specifically select for. Further, bushland may be more heterogeneous with varying amount of grass (open or dense) that provides the aspect of heterogeneity in the landscape that is critical for elephant use of the landscape.
In terms of factors influencing selection, it was noteworthy that neither season nor individual elephants influenced habitat selection as much as habitat type. This implies that selection pattern do not vary from an elephant to another and generalization to this effect is valid (Young et al., 2009). However, to be completely sure, more data duration as well as more elephant will provide more power of the test to make this inference. But going by the results, it then implies that any colored elephant will give information on habitat selection. Further, habitat selection was independent on gender. This implies that elephants sometimes generally will make decisions on habitat selection irrespective of gender. Again since only two female elephants collared gave data, more equal and or increased number of female elephants may improve the power of this test and inference. Results also indicated that season was not a factor in elephant habitat selection. This may be due to the fact that the area is mostly dry and many years can go by before rainy season occur or that rainy season is brief and patchy enough to influence habitat selection by elephants. So, the most prevailing circumstances are dry season circumstances. Since dry season is often hot and dry, it represents the limiting factor to elephant use of range and this is why selection of habitats and resources in the dry season will often represent the elephant survival strategy in the landscape. So given the results, the critical influence on habitat selection is habitat types (and their characteristics) and not individual elephant, gender or season. Therefore, to enhance elephant well-being and survival, focus should be on habitats (quantity, heterogeneity and quality) in the landscape.
Elephants shared their range with other wild large mammals and therefore securing of the elephant home ranges will also secure space for other large mammals that use the same space and possibly utilizing resources in same way and time with some species, and also in different ways and different times with other species ((Valeix et al., 2007). There were 16 species most of them using the bushland, woodland and grassland habitats. These Included giraffes, Grants’ gazelle, Thomson’s gazelle, zebra, wildebeest, impala and other elephant herds. These large mammals are engaged in various activities, with feeding and drinking being the most critical for their survival. The highest frequency of sharing habitats with wild large mammals was in the bushland, woodland and grassland, but highest density of wild large mammals was in swamp and woodland. This means that potential competition between elephant and other wild large mammals’ may occur often in bushland but the intensity of competition was more severe in the swampland. Elephants will compete with other large mammals for space, water and plant resources (Owen-Smith, 1988; Illius, 2006). The competition may not be that intense because elephants switch their diet to browsing on woody vegetation in dry season and therefore can easily associate with other wild species. But for water and grass, the swamps can be areas of intense competition. Since elephants are keystone species, they will often co-exist with other wild animals and it was not at all surprising that they share their home range with many other large mammals, especially in the bushland and woodland habitats. Given that bushland habitat is more common, and both bushland and woodland may have a varying degree of openness, that heterogeneity in patches will provide for more niches and feeding opportunities for more large herbivores and allow for more coexistence.
Scarcity of resources may result in high levels of animal aggregation; interference competition can occur in such a scenario and play a role in resource acquisition (Valeix et al., 2007). In Hwange National Park, Zimbabwe, waterholes were monitored in order to study agonistic interactions between elephants and other herbivore species. Results showed that in drier years, waterholes are crowded with elephants early in the afternoon. In general, the species most affected by interference competition with elephants shift their temporal niches at the waterholes, thus maintaining a constant temporal overlaps with elephants. The species less affected by interference competition with elephants showed no temporal niche shifts and increased their temporal overlap with elephants at waterholes, as predicted from a noncompetition hypothesis. This provided evidence that interference competition with a behaviorally dominant large species influences the temporal niches of smaller species, and suggests that the potential costs associated with interference between elephants and other herbivores at waterholes are linked to shifts in diurnal activities rather than interactions and water acquisition itself (Valeix et al., 2007).
Elephants also shared their home range with livestock. Most common type as well as in abundance was shoat (sheep and goats together) and cattle. The highest number of livestock was in the swamp and bushland in elephant home range. This means that competition for space, water and forage is most intense in swamps and woodlands. Water is a scarce and important determinant of elephant use of the landscape so potential conflicts and competition with other wild species and livestock may be around water resources as compared to either space or forage (Valeix et al., 2007). While elephants can co-exist with other wild large mammals, the same is not true for livestock. Frequent interaction and increased number of livestock in core elephant use and around critical resources such as water and salt licks can lead to conflicts (Valeix et al., 2007) in which elephants will attack if threatened by man or livestock (Ochola et al., 2013). This becomes a source of elephant-human conflicts and can elicit retaliation from Maasai herds and increase general negative attitude towards elephant presence in the Maasai pastoralists.
Livestock also have a more severe degradation effect on habitats (especially over grazing when the habitats are overgrazed or not given enough time to recover from grazing). This leads to general decline of habitat quality (due to overgrazing, decreased plant productivity and declining range condition). This will eventually affect elephant use of range. It is very important to balance the grazing pressure of both wild and domestic large mammals’ elephant home ranges to contain the conflicts and also safeguard habitat integrity. To do this, there is need for negotiations and awareness with local Maasai since most of the elephant ranges were on their land and outside the network of protected areas. Diversifying and properly locating more water sources and protecting existing ones will help alleviate conflicts especially around the water.
In conclusion, the diverse habitats were critical for elephant use of the landscape in Amboseli Ecosystem. The bushland and woodland habitats seemed most critical for elephants because they represented better habitat patchiness and heterogeneity (because of varying degree of openness) that promote elephant use of the landscape. This range was shared by other elephants and other large mammals particularly the zebra, gazelles and giraffes. These animals co-existed with elephant in the elephant range, with common activities being feeding and drinking especially in the swamps. Elephant habitat selection was not influenced by individual elephants, their gender and seasons. It was the habitat type (and may be its quality, quantity and risks to elephants) that was most critical in determining elephant selection of range. Human presence was common, mostly homesteads, roads and other infrastructure and this presence would increase competition for space and plant resources (Kiringe and Okello, 2005) and rate of encounter and therefore conflicts with elephants. The habitat destruction (through cutting of trees for firewood and other uses and for making charcoal) was the frequent habitat destruction activities of people. Further, clear risks directly to elephants occurred particularly in Kuku, Rombo and Tsavo West National Park as evidenced by presence of snares, elephant carcass and carcass of other large mammals.
The presence of livestock and competition for forage and water especially in bushland, woodlands and swamps may also likely lead to direct conflicts when elephant kill livestock, or increased habitat degradation due to overgrazing in critical elephant habitats. Since most elephants range outside the parks and the land belongs to the Maasai, unexpected encounter with elephants and competition for space and resources will likely increase. We recommend focus on the critical habitats needed by elephants outside of national parks, and negotiations with land owners so that the area can be made into wildlife sanctuaries and tourism investment brings direct benefits to the land owners, in addition to government support for such land owners in elephant management and appropriate compensation opportunity costs (Western, 1982). This should complement awareness and joint management of elephant forums between the government and local land owners who support elephants on their lands.
Authors did not declare any conflict of interest.
REFERENCES
|
Alldredge JR, Ratti JT (1986). Comparison of some statistical techniques for analysis of resource selection. J. Wildlife Mgt. 50(1):157-165
Crossref
|
|
|
|
Alldredge JR, Ratti JT (1992). Further comparison of some statistical techniques for analysis of resource selection. J. Wildlife Mgt. 56(1):1-9
Crossref
|
|
|
|
|
Campbell DJ, Gichohi H, Mwangi A, Chege L (2000). Land use conflict in Kajiado District, Kenya. Land Use Policy 17: 337-348.
Crossref
|
|
|
|
|
Chamaille´-Jammes S, Valeix M, Fritz H (2007). Managing heterogeneity in elephant distribution: interactions between elephant population density and surface-water availability. J. Appl. Ecol. 44: 625-633.
Crossref
|
|
|
|
|
Clauss M, Streich WJ, Schwarm A., Ortmann S, Hummel J (2007). The relationship of food intake and ingesta passage predicts feeding ecology in two different mega - herbivore groups. Oikos 116: 209–216.
Crossref
|
|
|
|
|
Damschen EI, Haddad NM, Orrock JL, Tewksbury JJ, Levey DJ (2006). Corridors increase plant species richness at large scales. Sci. 313: 1284-1286.
Crossref
|
|
|
|
|
De Beer Y, Killian W, Versfeld W, van Aarde RJ (2006). Elephants and low rainfall alter woody vegetation in Etosha National park, Namibia. J. Arid Environments 6: 412–421.
Crossref
|
|
|
|
|
De Beer Y, Van Aarde RJ (2008). Do landscape heterogeneity and water distribution explain aspects of elephant home range in southern Africa's arid savannas? J. Arid Enviro. 72: 2017-2025.
Crossref
|
|
|
|
|
Davis S (2007). Endozoochory in Subtropical Thicket: comparing effects of species with different digestive systems on seed fate. MSc thesis, Nelson Mandela Metropolitan University, South Africa.
|
|
|
|
|
Galaty J (1992). The land is ours: social and economic factors in the privatization, sub – division and sale of Maasai ranches. Nomadic peoples 30: 26-40.
|
|
|
|
|
Galaty J (1994). Rangeland tenure and pastoralism in Africa. In Fraktin, E., Galvin, K.A., and Roth, E.A. (Eds.). African Pastoral Systems: An Integrated Approach. Boulder, Colorado, USA. pp. 212-219
|
|
|
|
|
Gaylard A, Owen-Smith N, Redfern J (2003). Surface water availability: implications for heterogeneity and ecosystem processes. Oryx 42, 66-75.
|
|
|
|
|
Grainger M, Van Aarde RJ, Whyte I (2005). Landscape Heterogeneity And The Use Of Space By Elephants In The Kruger National Park, South Africa. Afri. J. Ecol. 43: 369-375.
Crossref
|
|
|
|
|
Illius AW (2006). Linking functional responses and foraging behavior to population dynamics. In: Danell, K., Bergstro¨m, R., Duncan, P., Pastor, J. (Eds.), Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge University Press, UK.
Crossref
|
|
|
|
|
Irigia BK (1995). Kenya Wildlife Service environmental impact assessment of the proposed Kimana Wildlife Sanctuary. Community Wildlife Service, KWS, Nairobi, Kenya.
|
|
|
|
|
Katampoi K, Genga G, Mwangi M, Kipkan J, Seitah J, Van Klinken M, Mwangi SM (1990) Kajiado District Atlas. ASAL Programme Kajiado, Kajiado, Kenya.
|
|
|
|
|
Kerley GI, Landman M, Kruger L, Owen–Smith N, Balfour D, Boer WF, Gaylard A, Lindsay K, Slotow R (2008). Effects of elephants on ecosystems and biodiversity. In Scholes, RJ, Mennell KG (Eds.). Elephant Management: A scientific Assessment of South Africa. Pp. 146 – 157. Wits University Press, Johannesburg, South Africa. ISBN 9781868144792;
|
|
|
|
|
Kiringe JW, Okello MM (2005). Use and availability of Tree and Shrub resources on Maasai Communal Rangelands near Amboseli, Kenya. African J. Range Forage Sci., 22(1): 37-46
Crossref
|
|
|
|
|
Laws RM (1970). Elephants as agents of habitat and landscape change in East Africa. Oikos 21: 1–15.
Crossref
|
|
|
|
|
Leggett K (2006). Effects of artificial water points on the movement and behavior of desert-dwelling elephants in north-western Namibia. Pachyderm 40, 40-51.
|
|
|
|
|
Lichtenfeld LL (1998). Local Participation and Conservation in Kenya: A Case Study if the Kimana Community Wildlife Sanctuary. Yale University, School for Forestry and Environmental Studies, New Haven, Connecticut, USA. The 12th Annual Meeting of the Society of Conservation Biology. July 13-16.
|
|
|
|
|
McClean SA, Rumble MA, King RM, Baker WL (1998). Evaluation of resource selection methods with different definitions of availability. J. Wildlife Mgt. 62(2):793-801.
Crossref
|
|
|
|
|
Ntiati P (2002). Ranches subdivision study in Loitokitok division of Kajiado district, Kenya. Land Use Change Impacts and Dynamics (LUCID) Working Paper 7 International Livestock Research Institute. Nairobi, Kenya.
|
|
|
|
|
Okello MM (2009). Contraction of Wildlife Dispersal Area and Displacement by Human Activities in Kimana Group Ranch near Amboseli National Park, Kenya. The Open Conserv. Biol. J. 3:49-56.
|
|
|
|
|
Okello MM (2005). Land Use Changes and Human - Wildlife Conflicts in the Amboseli Area, Kenya. Human Dimensions of Wildlife 10(1):19-28
Crossref
|
|
|
|
|
Okello MM, Kiringe JW (2004). Threats to Biodiversity and the Implications in Protected and adjacent dispersal areas of Kenya. J. Sustainable Tourism 12(1): 55-69.
Crossref
|
|
|
|
|
O'Neill RV, DeAngelis DL, Waide JB, Allen TFH (1986). A Hierarchical Concept of Ecosystems. Princeton University Press, Princeton.
|
|
|
|
|
Osborn FV, Parker GE (2003). Linking two elephant refuges with a corridor in the communal lands of Zimbabwe. Afri. J. Ecol. 41: 68-74.
Crossref
|
|
|
|
|
Owen-Smith N (1988). Megaherbivores. The influence of very large body size on ecology. Cambridge University Press, Cambridge.
Crossref
|
|
|
|
|
Pellew RAP (1983). The impacts of elephant, giraffe and fire upon the Acacia tortilis woodlands in the Serengeti. Afri. J. Ecol. 21:41-74.
Crossref
|
|
|
|
|
Penzhorn BL, Robbertse PJ, Olivier MC (1974). The influence of the African elephant on the vegetation of the Addo Elephant National Park. Koedoe 17: 137-158.
Crossref
|
|
|
|
|
Potvin F, Lowell K, Fortin MB, langer L (2001). How to test habitat selection at the home range scale: a resampling random windows technique. Ecoscience 8: 399–406.
|
|
|
|
|
Redfern JV, Grant R, Biggs H, Getz WM (2003). Surface-water constraints on herbivore foraging in the Kruger National Park. South Afri. J. Ecol. 84: 2092–2107.
|
|
|
|
|
Seno SK, Shaw WW (2002). Land tenure policies, Maasai traditions, and wildlife conservation in Kenya. Society and Natural Resources 15: 79-88.
Crossref
|
|
|
|
|
Strauss RE (1979). Reliability index for Ivlevs electivity index, the forage ratio, and a proposed linear index of food selection. Transactions of American Fisheries Society 108: 344-352
Crossref
|
|
|
|
|
Twine W, Mogome H (2008). Interactions between elephants and people . In Scholes, R.J. and Mennell, K.G. (eds). Elephant Management: A scientific Assessment of South Africa. 4:206-256. Wits University Press, Johannesburg, South Africa.
|
|
|
|
|
Valeix M, Chamaillé-Jammes S, Fritz H (2007). Interference competition and temporal niche shifts: elephants and herbivore communities at waterholes. Oecologia 153(3):739-48.
Crossref
|
|
|
|
|
Western D (1975). Water availability and its influence on the structure and dynamics of a savannah large mammal community. East Afri. Wildlife J. 13: 265-286.
|
|
|
|
|
Western D (1982). Amboseli National Park: enlisting landowners to conserve migratory wildlife. Ambio 11(5): 302-308.
|
|
|
|
|
Western D (1989). The ecological role of elephants in Africa. Pachyderm 12:43-46.
|
|
|
|
|
Young KD, Ferreira SM, Van Aarde RJ (2009). Elephant spatial use in wet and dry savannas of Southern Africa. J. Zool. 278: 189-205.
Crossref
|
|
|
|
|
Zar JH (1999). Biostatistical Analysis. 4th ed. Upper Saddle River, NJ: Prentice Hall Publishers.
|
|