ABSTRACT
The potential of two entomopathogenic fungi Metarhizium anisopliae.and Beauveria bassiana for the control of Coelaemenodera elaeidis were evaluated. The objective of this study was to control C. elaeidis using entomopathogenic fungi as biocontrol agents. The pathogenicity of M. anisopliae and B. bassiana isolates were tested using direct contamination bioassay set up of 2.5 g conidia (2.5 g of Metarhizium 2.5 x 1010 or 2.5g of Beauveria 1011) transferred to the centre of plastic Petri dishes. Results from M. anisopliae studies indicated that C. elaeidis changed from yellow to brown and darker brown colour. Death of C. elaeidis was observed on the fourth day post incubation with 100% mortality and the control treatment recorded 14% mortality. C. elaeidis were observed with white mycelia growth on the ninth day, the green conidia appeared around the cadavers. Using B. bassiana, ingestion of spores caused infection from the mandible which spread to the thorax. Infection spread beneath the thorax colonizing the abdomen with white mycelia growth. Death was observed on C. elaeidis with 100% mortality on the sixth day post incubation. The larvae and pupae recorded deaths on the eighth and eleventh days post incubations with 100% each. The control treatment recorded mortalities at 21% for larvae and 7% for pupae. The study results indicated that both M. anisopliae and B. bassiana have a high potential as biocontrol agents for the control of C. elaeidis.
Key words: Biocontrol, mortality, Coelaemenodera elaeidis, M. anisopliae, B. bassiana
Coelaenomenodera elaeidis (Coleoptera: Chrysomelidae: Hispinae) is endemic in almost all oil palm growing areas of West Africa and regarded as the most devastating pest of the oil palm. The larvae are the main cause of damage, especially during swarming when several individuals can be found mining into the epidermis of the leaflets, leading to direct destruction and desiccation of the leaf or reduced photosynthetic surfaces (Aisagbonhi et al., 2004). A number of outbreaks have been reported throughout the West African oil palm belt (Hartley, 1967).
Palms of all ages are attacked, except those under 3 or 4 years old. The leaflets are attacked soon after they are unfurled, the larvae mining under the epidermis. As many as 10 mines per leaflet have been observed, and these mines may cover a considerable area, depending on the number of larvae in each mine. Mines have been observed containing five larvae, which when full-grown have eaten out an area in the parenchyma of the leaflet up to 9 inches long and 1 inch wide. This area turns brown, resembling a large blister. The leaflets are rapidly eaten away and stripped to the mid-rib by the action of wind, a badly attacked palm having the appearance of the after-effects of fire. This naturally causes a falling off in the yield of palm bunches and devitalisation of the tree (Cotterell, 1925).
The adults feed on the lower surface of the leaflets, usually working in an upward direction. The green material between the veins is eaten, leaving brown longitudinal cuts. C. elaeidis larvae mine oil palm leaves, giving them a blistered appearance and causing withering (Bachy, 1963). Damage caused by C. elaeidis comprises drying of the inner leaves, the leaflets being torn, curled or missing except for the midribs, and consequent effect on the metabolism of the tree and reduction of oil yield (Ruer, 1964).
Shaking oil palm leaves in the early morning or evening during an invasion and collecting the adults at the base may be effective, but would require a large amount of labour. Pruning off and burning of the lower leaves at definite periods would also be effective in a small way, as the larger mines would naturally be in the older leaves (Cotterell, 1925). The use of indigenous insect parasitoid species of C. elaeidis had little impact. Mariau and Morin (1971) indicated that the main reason the larval parasitoids were not effective was their limited spectrum of attack.
Metarhizium anisopliae was tested against a range of organisms and found with no particular risks. A few non-target insects can be killed in laboratory when they are stressed and the fungus dose is high enough. Under more realistic conditions using Metarhizium there was no transmission or evidence for any risk of infection in the field (Lomer, 1999). The hyphae of the Metarhizium anisopliae and Beauveria bassiana penetrate the insects and secrete a toxin known as destruxin, which is able to suppress the insect’s immunity (Morae et al., 2003). Since the use of insect parasitoid species on C. elaeidis had little impact because of their limited spectrum of attack, alternatively entomopathogens will be employed to keep C. elaeidis in check for the avoidance of an outbreak with a view of developing an integrated pest management (IPM) strategy against the pest.
Sampling location
The study surveyed oil palm seed garden of the seed production division (SPD) of the Nigerian Institute for Oil Palm Research (NIFOR) main station that were disrupted by C. elaeidis activities at field 42 with geographical position system (GPS) at N 06°33 to 204 latitude, E 005°37 to 348 longitude and 170m altitude. GARMIN trex 10 - Geographical Positioning System (GPS) of coordinates was used.
Sampling period
Sampling was done during the early morning hours till afternoon in the month of May, 2014. This was necessary in order to avoid the rainy season. The various taxonomic group and population dynamics of C. elaeidis were observed. During the field study the mean maximum. temperature was 26ºC and the mean minimum temperature was 22ºC.
Sampling methodology of Coelaemenodera elaeidis
As many as 14 mines per oil palm canopy were observed, and these mines covered a considerable area of the leaflet, depending on the number of larvae in each mine. We collected ninety leaflets showing symptoms of abnormal leaf fall, external feeding, internal feeding and necrotic areas. They were examined in the laboratory. All the collected leaflets were excised. Larvae and pupae were collected. The adults of C. elaeidis were also collected into plastic disposable Petri dishes with aeration holes.
Entomopathogenic fungi
Isolation
The Metarhizium anisopliae and Beauveria bassiana were isolated from soil and soil forest samples collected from different locations at NIFOR using geographic position system (GPS). Veen’s and Fenon (1966) selective medium was used. Micropipette was used to inoculate plates with the solution of soil. 0.3 ml of each solution was used for each plate and three plates per sample were inoculated. The plates were incubated at 25-26oC in the dark.
Insect bait method
The study used Galleria insect bating method (bathing soil with larvae of Helicoverpa armigera was applied as a tool to isolate entomopathogenic fungi) and topical method (isolates from samples were harvested with sterilized water and inoculated on some grasshoppers for pathogenicity)
Monitoring
Cultures were stained with lactophenol cotton blue and examined with x40 magnification using light microscope after 7 days incubation. For each sample, both Metarhizium and Beauveria observed and suspected to be entomopathogens were re-isolated to normal potato dextrose agar (PDA) medium and incubated at 25
26°C in the dark for 2 weeks. The M. anisopliae and B. bassiana from NIFOR’s stock culture (IC30 and Bb S41) and the newly isolated ones were checked for virulence by passing them through C. elaeidis. Only the pathogenic isolates were employed for further study. They were later re-isolated from cadavers onto PDA medium and purified
Spore count
The conidia of each isolate were made by scrapping off the conidia using a sterile glass slide. The conidia were kept in different McCartney bottles of distil-sterilized water. They were shaken vigorously before counted under a light microscope connected to Moticam 2300 digital camera and computer (40X) using a Neubauer haemocytometer
Laboratory trial
The pathogenicity of M. anisopliae and B. bassiana isolates were tested using direct
Contamination bioassay set up conidia (2.5 g of Metarhizium 2.5 x 1010 or 2.5 g of Beauveria 1011) transferred to the centre of plastic Petri dishes with sterilized moisture Whatman No 1 filter paper with humidity of 70%. A blended young oil palm leaf (1 gm) as feed was also introduced to each Petri dishes. A total of 14 each for larvae, pupae and adults C. elaeidis replicated three times were used for the study. The lids of the Petri dishes had four aeration holes (2 mm in diameter) to ensure free flow of air. The control experiment was similar but without treatments. Mortality was recorded daily for twelve days. Light photographs of conidia were taken using Moticam 2300 camera connected to the microscope and computer. Light photographs of C. elaeidis were taken from lower to total magnification using SAMSUNG GALAXY Note 10.1 2014 Edition.
Statistical analysis
Mortality data were corrected for the corresponding control mortality by the formula:
CM (%) = (T(%) – C(%) / 100 - C(%) x 100 (Abbott, 1925). Where CM is corrected mortality, T is mortality in treated insects and C is mortality in untreated insects and graphs were plotted using Star version 2.0.1 (2014).
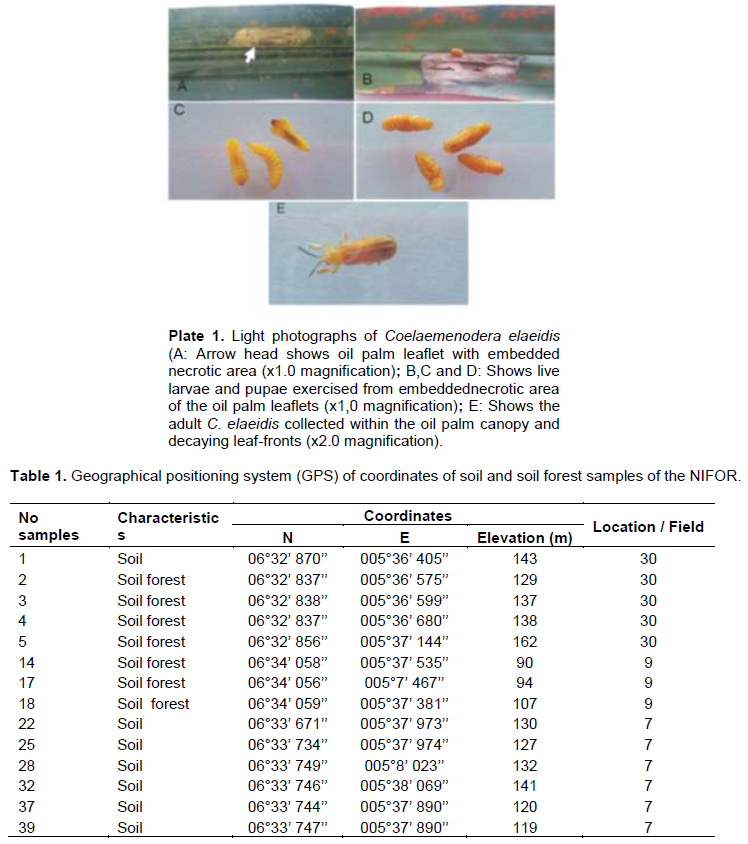
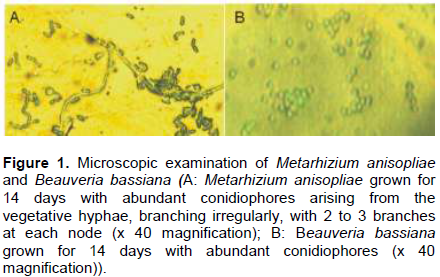
The study collected leaflets with the appearance of embedded necrotic area (Plate 1A). Mines contained from one to three larvae or pupae, which ate out an area in the parenchyma tissues of the leaflet up to 3 cm long and 1 cm wide. This area turned brown. The leaflets were rapidly eaten away to the mid-rib with the appearance of necrotic area or brown color (Plate 1B). Live larvae and pupae were collected from exercised leaflets. (Plate 1 B, C and D). The adults C. elaeidis were also collected within the oil palm canopy and decaying leaf-fronts (Plate 1E). Geographical positioning system (GPS) of coordinates of soil and soil forest samples of NIFOR for the isolation of M. anisopliae and B. bassiana are presented in Table 1. Metarhizium anisopliae grown for 14 days had spores produced in columnar stands with abundant conidiophores arising from the vegetative hyphae, branching irregularly, with 2 to 3 branches at each node, (Figure 1A). Beauveria bassiana also had conidiophores abundant, arising from the vegetative hyphae, 1-2µM wide, bearing group of clustered conidiogenous cells measuring 3-6 x 3-5 µM (Figure 1B).
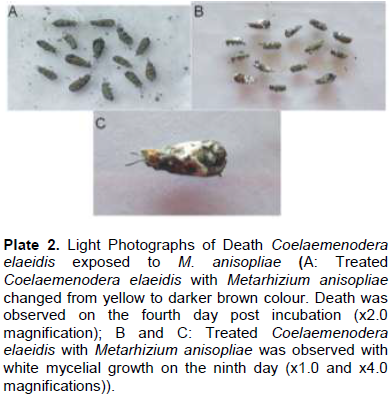
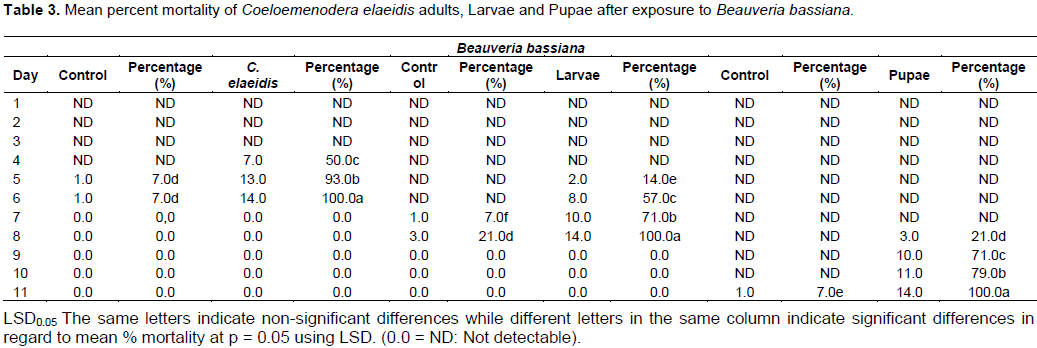
After comparing the pathogenicity of all the isolates, M. anisopliae IC30 and B. bassiana Bb S41 were employed for further studies. Pathogenicity test using M. anisopliae show the diseased C. elaeidis changed from yellow to brown and darker brown color (Plate 2A). Death of C. elaeidis was observed on the fourth day post incubation (Plate 2A) with 100% mortality. Based on the number of days, there was a significant difference between day 3 and day 4, and when compared with the control treatment on percentage mortality for the adult C. elaeidis (Table 2), the control treatment recorded 14% mortality. C. elaeidis were observed with white mycelia growth on the ninth day (Plate 2A, B, C,). Using B. bassiana, ingestion of the spores caused infection from the mandible which spread to the thorax. Infection spread beneath the thorax colonizing the abdomen with white mycelia growth (Plate 3A, B, C). Death was observed on C. elaeidis with100%mortalityonthesixthdaypostincubation(Table3).The larvae and pupae recorded deaths on the eighth and eleventh days post incubation with 100% each. The control treatment recorded mortalities at 21% for larvae and 7% for pupae (Table 3). The graphs of percent mortalities from Figures 2 and 3 confirmed the results obtained from Tables 2 and 3.
.png)
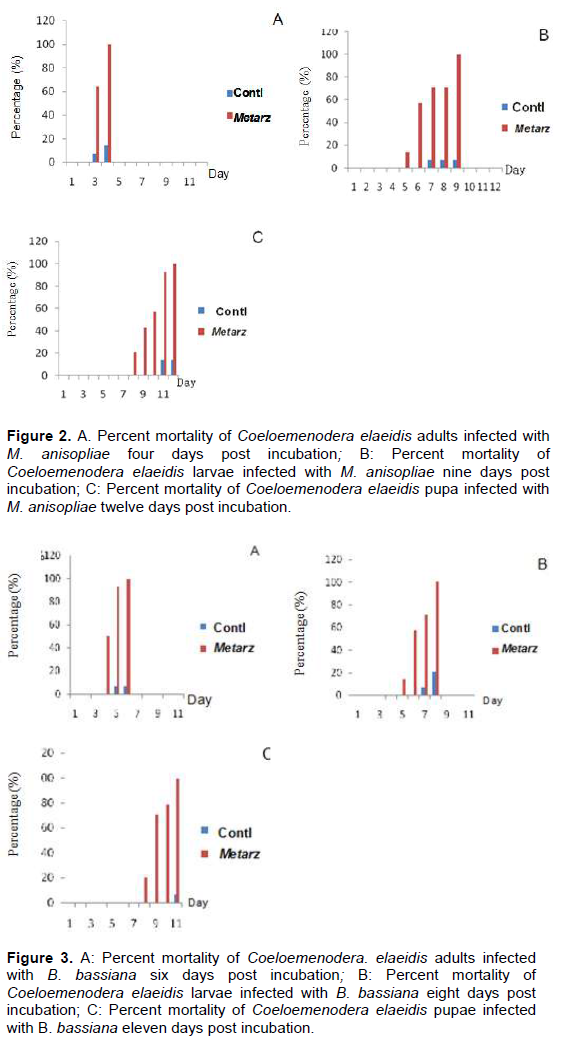
The present study showed that M. anisopliae and B. bassiana conidia (2.5 g of Metarhizium 2.5 x 1010 or 2.5g of Beauveria 1011) concentration caused 100% mortality on adults C. elaeidis, on the sixth day. Similar study by Addisu et al. (2013), stated that 1 x 109 M. anisolpliae caused more than 95% mortality for seven days on Macrotermes. The ability of M. anisopliae and B. bassiana to cause 100% mortalities with the emergence of fungi mycelia growths and sporulation of conidiophores from the cadavers of C. elaeidis clearly indicated that the entomopathogen strains were pathogenic. This agrees with Reddy et al. (2014) and Schapovaloff et al. (2014) on the susceptibility of Cerambycid beetle Hedypathes betulimus to B. bassiana and M. anisopliae. Maoye et al., (2014), reported that B. bassiana isolate had strong pathogenic and exhibited great potential for sustainable control of brown planthopper Nilaparavata lugeens.
The mycelia proliferated by feeding on the host. The process of host invasion caused depletion of nutrient, dehydration and death of C. elaeidis. The mortality on C. elaeidis depends on virulent isolates of M. anisopliae and B. bassiana. Similar studies have attributed the high mortality caused by the entomopathogens to some enzymatic and toxin activities (Dong et al., 2009). Brogden et al. (2005), reported that mortality increased because specific dextruxin penetrated into insect hemocoel. Barra et al. (2015) also reported that entomopathogen fungus Purpureocillium lilacinum had extracellular enzymes that degraded cuticle components, mainly hydrocarbons used as energy source. According to some authors, entomo-pathogenic fungi infect insects to produce a large amount of secondary metabolites, including toxins attributed to pathogenicity, among which are low molecular weight compounds and other peptidic nature as well as enzymes involved in the attacks on the host (Franco et al., 2011; Rohlfs and Churchhill, 2011).
The flying C. elaeidis spread the conidia through contact there-by increasing the rate of mortality. Ingestion of B. bassiana comidia caused infection from the mandible, this spread further to colonize the abdomen with white mycelia growth. This agrees with other similar study on transmission of conidia (Grace and Zoberi, 1992). Direct contamination bioassay with appropriate concentration was the key to better control of C. elaedis. The bait method would be tested in field trials for more isolation of entomopathogenic fungi. This phenomenon was found in a study by Wang and Powell (2004).
The study results demonstrate pathogenic effect of M anisopliae and B. bassiana against C. elaeidis under laboratory condition. Further study is necessary to determine the effectiveness of these entomopathogens under field condition and to examine its impact on non-target insects.
The authors have not declared any conflict of interest.
REFERENCES
|
Abbott WS (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol., 18:265-267.
Crossref
|
|
|
|
Addisu S. Waktole S, Mohamed D (2013). Laboratory Evaluation of Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana against Termite Macrotermes (Isoptera: Termidae). Asian J. Plant Sci. 12:1-10.
Crossref
|
|
|
|
Aisagbonhi CI, Airede CE, Appiah FO, Kolade KO (2004). Key pests and diseases of oil palm in Africa –Their biology, epidemiology and method of control. Proc. of the Int. Conf. on Pests and Diseases of Importance to the Oil Palm Industry, Namibia. pp. 287-323.
|
|
|
|
Bachy A (1963). Insectes et animaux nuisibles au palmier à huile. Oléagineux, 18:173-176.
|
|
|
|
Barra P, Etcheverry M, Nesci A (2015). Improvement of the Insecticidal Capacity of Two Purpureocillium lilacinum strains against Tribolium confusum. Insects 6:206-223.
Crossref
|
|
|
|
Brogden KA, Guthmiller JM, Salzet M, Zasloff M (2005). The Nervous System and Innate Immunity: The Neuropeptide Connection. Nat. Immunol. 6:558-564
|
|
|
|
Cotterell GS (1925). The hispid leafminer (Coelaenomenodera elaeidis Maul.) of oil Palms (Elaeis guineensis Jacq.) on the Gold Coast. Bull. Entomol. Res. 16:77-83.
Crossref
|
|
|
|
Dong C, Zhang J, Huang H, Chen W, Hu Y (2009). Pathogenicity of new China variety of Metarhizium anisopliae (M. anisopliae var. Dcjhyium) to subterranean termite Odontotermes formosanus. Microbiol. Res. 164:27-35.
Crossref
|
|
|
|
Franco CKG, Rodriguez NS, Cervantes MJF, Barranco FJE (2011). Soc. Rural Prod. Medio Ambiente. 11:143-160.
|
|
|
|
Grace JK, Yates JR (1992). Behavioural effect of neem insecticide on Coptotermes formosanus (Isoptera: Rhinotermitidae). Tropical Pest Mgt. 38:176-180.
Crossref
|
|
|
|
Hartley CWS (1988). The Oil Palm Elaeis guineensis Jacq. Longman Scientific and Technical, New York, 3rd Edn., 761 p.
|
|
|
|
Lomer C (1999). Lubilosa phase 3 final report. pp. 7-15.
|
|
|
|
Maoye L, Shiguang L, Amei X, Huafeng L, Dexin C, Hui W (2014). Selection of Beauveria Isolates Pathogenic to Adults of Nilaparvata lugens. J. Insect Sci. 14:1-12.
|
|
|
|
Morae CK, Schrank A, Vainstein MH (2003). Regulation of Extracellular Chitinases and Proteases in the Entomopathogen and Acaricide Metarhizium anisopliae. J. Curr. Microbiol. 46:205-210.
Crossref
|
|
|
|
Morin JP, Mariau D (1971). The study of the Biology of Coelaenomenodera elaiedis MlK. III. Reproduction. Oleagineux, 26:373-378.
|
|
|
|
Reddy GVP, Tangtrakulwanich K, Wu S, Miller JH, Ophus VL, Prewett J, Jaronski ST (2014). Evaluation of the effectiveness of entomopathogens for the management of wireworms (Coleoptera: Elateridae) on spring wheat. J. Invertebrate Pathol. 120:43-49.
Crossref
|
|
|
|
Rohlfs M, Churchhill ACL (2011). Fungal secondary metabolites as modulator of interactions with insects and other arthropods, Fungal Genet. Biol. 48:23-34.
Crossref
|
|
|
|
Ruer P (1964). Les conditions de lutte contre un prédateur du palmier à huile, Coelaenomenodera elaeidis. Oléagineux, 19:387-390.
|
|
|
|
Schapovaloff ME, Aives LFA, Fanti AL, Alzogaray RA, Lopez LCC (2014). Susceptibility of adults of the Cerambycid beetle Hedypathes betulimus to the entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae and Purpureocillium lilacinum. J. Insect Sci. 14(1):127.
Crossref
|
|
|
|
Veen KH, Ferron P (1966). A selective medium for isolation of Beauveria tenella and of Metarrhizium anisopliae. J. Invertebrate Pathol. 8:268-269.
Crossref
|
|
|
|
Wang C, Powell JE (2004).Cellulose bait improves the effectiveness of Metarhizium anisopliae as a microbial control of termites (Isoptera: Rhinotermitidae). Biol. Control 30:523-529.
Crossref
|