The commercial use of biotech crops developed using genetic modification (GM) technologies is very limited in sub-Saharan African agriculture. Efforts are on-going to address this. The need to implement best practices in stewardship to support the responsible and safe management of agricultural biotechnology and maintain the highest research standards on product integrity have received less attention. A pioneering initiative on “strengthening capacity for safe biotechnology management in sub-Saharan Africa (SABIMA)” was introduced in six African countries (Ghana, Nigeria, Burkina Faso, Kenya, Uganda and Malawi) during the period 2009 to 2013. This initiative was led by the Forum for Agricultural Research in Africa (FARA) to address the stewardship capacity gap. The train-the-trainer approach was used to train 1515 professionals in the core principles of stewardship. Key stewardship practices like Critical Control Point Analysis (CCPA) and the relevant Standard Operating Procedures (SOPs) were internalised in the public research and development institutions. These institutions also demonstrated their commitment to stewardship by developing and implementing stewardship policies. Evidence of enhanced quality product development was demonstrated by the publication of case studies from project work at beneficiary institutions. These fuelled the demand for further stewardship training in African countries. Training and implementation of the best stewardship principles and practices should be mainstreamed into all research and development programmes creating new varieties using molecular biotechnology to address the needs of farmers and their value chains. Sustainable funding into the post-project period is a challenge to be addressed in future stewardship capacity building projects.
Agricultural productivity in sub-Saharan Africa has been one of the lowest in the world (AFDB, 2016). Cereal production levels averaging 1.45 tonnes/ha are half the global average of 3.9 tonnes/ha (World Bank, 2014). Some factors contributing to these statistics include debilitating pests and diseases, declining soil fertility and climate change phenomena (FARA, 2014; Juma, 2015; FAO, 2017a, b). The low adoption of modern crop varieties and technologies including agricultural biotechnology and genetic modification to tackle these challenges are some of the contributors to continuing low productivity (Walker and Alwang, 2015; Tadele, 2017). Currently, Africa remains the lowest adopter of genetic modification (GM) crops. Only 2.8 million hectares was grown in 2016 in South Africa (2.7 million) and Sudan (0.1 million). This is less than 2% of the global area grown (James, 2016). Hitherto, safety concerns and the need for effective, practical and workable biosafety legal frameworks have been the main focus of national governments in African countries where the deployment of GM technologies in agriculture is under consideration. Development of professional stewardship practices to complement biosafety considerations are less known in Africa. Stewardship is a broad concept, and within the context of plant biotechnology is defined as: “the responsible management of a product from its inception through to its use and discontinuation” (Excellence Through Stewardship, 2017). Stewardship ensures product integrity from the earliest stage of research through to commercialisation and use of new varieties, until they are withdrawn from use (Figure 1). Implementation of a professional stewardship programme provides more confidence in the scientists and the methods they use to develop new products, and the practices by users and stakeholders in the product value chains.
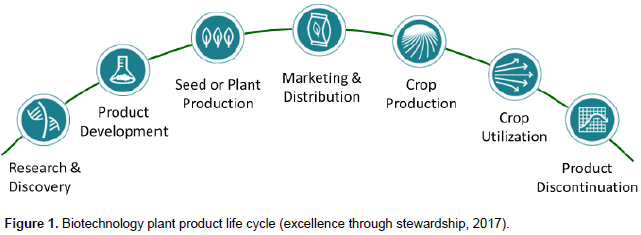
It minimises the potential for the incidence of trade disputes arising from either the product’s genetic composition being compromised during the research and development process, or by the unauthorised adventitious presence of other varieties occurring due to co-mingling during seed production, storage, transport or other stages in the supply chain. Stewardship fully supports regulatory compliance and promotes the sharing of best practices amongst scientists, government officials, farmers and stakeholders in crop production and their value chains. Stewardship requires knowledge of best practices that have been developed within the private sector, and particularly from the experiences and challenges faced by the early developers of agricultural biotechnology. Stewardship is currently not part of the curricula for undergraduate or postgraduate plant science or genetics degree programmes and researchers working in crop improvement require specific professional training. This takes place as part of continual professional development of scientists in the private sector but training has not been generally available to public researchers. In Africa, the focus of public researchers and international donor agencies in agricultural biotechnology has been primarily capacity building in molecular biology and associated modern scientific methods and regulatory compliance of their use. However, the safe and appropriate development and use of agricultural biotechnology and GM products requires wide-scale deployment of excellence in stewardship. This is an ambitious objective, especially given that public institutions operate in a very different organisational environment from the private sector and often use different processes. However, both public and private organisations have a common goal - to enable safe and improved crops to be designed and adopted by farmers to increase crop production and the availability of nutritious food for consumers. Public African research institutions share this objective but also face unique specific challenges. This requires a dedicated approach, starting from basic principles of good stewardship practice and adapting these to the needs and possibilities of the reality of African research and development organisations. During the period 2009 to 2013, the Forum for Agricultural Research in Africa (FARA), with the financial and technical support of the Syngenta Foundation for Sustainable Agriculture (SFSA), introduced the first agricultural biotechnology stewardship programme into sub-Saharan Africa (SFSA, 2011). It was called “Strengthening the capacity for safe biotechnology management in sub-Saharan Africa” and is known as “SABIMA” (FARA, 2015). Its main objective was to introduce and encourage the best stewardship practices into the plant biotechnology research and development activities in public research institutions. The paper is an account of the capacity building process for stewardship application in modern biotechnology research and development and its impact on best stewardship practices in agricultural biotechnology in select Africa countries.
The SABIMA project was introduced into six sub-Saharan African countries (Burkina Faso, Ghana and Nigeria in West Africa; Kenya and Uganda in East Africa and Malawi in Southern Africa). Countries were selected in sub-Saharan Africa based on their progress in development of regulatory frameworks and having operational biotechnology research underway. Only Burkina Faso and Malawi had biosafety laws that could allow commercialisation of GM crops at the start of the project (Table 1). The remaining four countries had legal provisions that allowed the testing of GM crops in confined field trials (CFT). In each country, a focal person was selected to introduce and lead the stewardship programme in their country. They were identified with the assistance of senior management in the key public sector research institutions conducting agricultural biotechnology at the time. Each professional was an experienced and respected plant scientist with expertise in agricultural biotechnology. The SABIMA focal persons together with the Project Coordinator at FARA led the training, implementation and outreach of stewardship best practices within the participating countries (Table 1). For each country, and in addition to the focal persons, a champion was identified to facilitate linkage with the country’s high level policy individuals to promote biotechnology with stewardship implementation. The programme focussed on raising awareness of the need for policy and the best operational practices in stewardship and adapting these to the primary needs of research and development institutions in sub-Saharan Africa. The main elements were based on the stewardship principles and mission advocated by the excellence through stewardship organisation and their stewardship guides on plant product launch (2017) and maintaining plant product integrity (2016). The core best practices include:
(1) Establishing a pan-African biotechnology stewardship policy (FARA), which provides the central elements for all African R&D institutions’ policies, processes and procedures.
(2) Establishing an organisation’s structure and roles and responsibilities to manage stewardship policies, operational practices, set standards and encourage a culture of continuous improvement.
(3) Implementing a quality process to maintain product integrity, manage risks, and strengthen communication.

Specifically, emphasis was placed in the training programme on not just understanding stewardship policy and concepts, but equipping research managers and scientists to fully implement and operate a stewardship management system. This management system involves: analysing processes (ex-ante) to identify steps which should be controlled using Critical Control Point Analysis (ILSI, 2004), then establishing infrastructure, equipment and Standard Operating Procedures (SOPs) to exert such controls, together with verification of the identified measures and record keeping. The training was an innovative approach that took the stance that capacity building would be best achieved through leadership and education in Africa by Africans. Therefore, the methodology used to maximise efficient and effective outreach to scientists and stakeholders in the use of new products across the six countries was based on a training-of-trainers (ToT) approach. Firstly, the country focal persons and their deputies received comprehensive training by an independent, experienced private sector expert in stewardship and biotechnology regulatory affairs. On completion of their implementation assignments in their home institutions these focal persons became fully certified as “Stewardship Trainers“ by FARA and formed the core educators in the programme. Rather than providing the training as a single event, the education programme was structured in three modules spread over several months in order to allow the trainees an opportunity to apply the training content in their home institutions and research programmes. Module 1 covered core stewardship principles and their implementation in core Research and Development organisations, while Module 2 addressed issues of policy development and implementation and stewardship communication and incident management. Module 3 addressed verification and audit procedures. These stewardship trainers educated others in their countries along the product value chain, beginning with research scientists on laboratory and field research. In each country the target audience for participation in the training programme was based on where research materials and biotechnology projects were in their lifecycle. For example, in Burkina Faso Bt cotton was being commercialised and so training focussed on professionals working in the seed arena and on cotton farms, whereas in Kenya trainees were mainly early stage researchers working in laboratories or were conducting confined field trials. As part of the training, the stewardship trainers were encouraged to write case studies and share their own experiences and challenges of applying stewardship best principles and processes in their own institutions (Johnson et al., 2011). Peer audit visits were undertaken by stewardship trainers to each other’s institutions to verify and critique stewardship practices being implemented.
By the time the SABIMA project was completed in 2013, a total of 1,515 Africans had received agricultural biotechnology stewardship training and were applying these principles. The majority of these professionals were introduced and trained by the certified African Stewardship trainers. They trained 1,412 persons across the project countries, i.e. a reach of over 10:1. The impact of the stewardship training by the SABIMA project has been positive. As countries understood and implemented stewardship principles acquired during the training, there was the call to expand the project to more African countries. The First Pan-African Conference on Stewardship in Agricultural Biotechnology was held in Accra, Ghana on November 29 to 30, 2011 (FARA, 2012). Highlights of the conference included the presentation of country case studies on stewardship. Stewardship principles learnt have been successfully applied to non-GM (conventional crops-certified seeds) and to animal research. In 2016 (three years after the project closure) the status of biotechnology with stewardship in project beneficiary countries was evaluated. In general, progress is noted from the standpoint of putting in place enabling legislation and approvals for confined field trials (CFTs) and environmental release. These actions set the stage for mainstreaming stewardship activities. As an example, in Burkina Faso, there is a project funded by the Islamic Development Bank for quality seed production. Stewardship forms a major component of this project (Oumar Traore, Personal Communication, 2014). Burkina Faso faces a current challenge of Bt cotton cultivation rejection by farmers due to a reduction in the staple length of the current Bt cotton variety preferred by farmers. The problem has been traced to an inadequate number of backcrossing or introgression steps of the Bt genes into the recurrent Bt cotton lines (Anon, 2017a). This poses a stewardship challenge to be addressed. Sofitex, the major Bt cotton company in Burkina Faso, is introducing strict stewardship principles into cotton seed production to pre-empt any quality challenges that could arise. This is a cautionary step following the current negative experience with commercial Bt cotton production. Ghana has completed multi-location trials on Bt/herbicide tolerant cotton but is not able to proceed with evaluation for commercial release until approval of biosafety regulations is given for commercial release of GM crops. (Dr Emmanuel Chamba, Personal Communication, 2016).
Malawi, in April 2016, granted a permit for the commercial release of Bt cotton in the country. (Boniface Mkoko, Personal Communication, 2016). This positive sign will, hopefully, stimulate the application of stewardship criteria in the Bt cotton production to ensure the product’s success. In Nigeria, not only is there a biosafety Law (enacted in 2015), but a permit has been granted for the environmental release of Bt cotton and confined field trials for Bt maize with herbicide tolerance. Permits for both events were issued on 1st May, 2016. Both situations create opportunities for the engagement of stewardship principles in product development and cultivation. In Uganda, late blight in potatoes is a major problem for potato growers. Uganda conducted a very successful confined field trial on GM potatoes resistant to late blight, during the period October 2015 to January 2016. The relatively short time span taken for development of this highly efficacious product makes this product development unique. The original imported GM research variety containing the resistance trait was introgressed into the local more acceptable varieties and tested for resistance to late blight. Varieties were identified with high resistance to potato blight that could benefit smallholder farmers (ISAAA, 2016). Other successful development products in the pipeline (post-2013) and waiting for multi-locational trials in farmers’ fields in Uganda include genetically engineered Xanthomonas bacterial wilt (BXW) resistant banana. Development lines are ready for multi-locational testing on farmers’ fields as soon as the Uganda biosafety bill is passed into law (Anon, 2017b). Stewardship capacity built in Uganda during the SABIMA project contributed to the success of these landmark trials. This positive contribution of stewardship to GM product development stems from the fact that its application enhances product integrity thereby ensuring quality and consumer confidence. Resistance or refugia management, a specific case of stewardship application, prevents resistance build-up in a target pest.
Challenges
Many challenges face major capacity building programmes in research and development in sub-Saharan Africa. The three major ones that have influenced stewardship implementation were:
(1) Greater institutional recognition and support by senior management was needed for the project country focal persons, to empower them to lead the mainstreaming of stewardship practices and create change in their home institutions.
(2) High work loads of focal persons and time pressures limited optimum inter-country communications, sharing of best practices and project reporting.
(3) Financial support has not been forthcoming to deepen the know-how amongst already trained professionals by African governments or international donor agencies, and to scale-out stewardship best practices to public research institutions in more African countries undertaking GM trait research.
Lessons learnt
A range of key lessons were learnt during the start-up, training and implementation phase of the stewardship programme. These stretched from strategic insights to practical operational conclusions that can be drawn upon to benefit future stewardship capacity building projects. These are outlined below:
(1) Scientific excellence: It was discovered that implementing stewardship principles can bring scientific rigour, excellence and enhanced risk management, not just for GM programmes but for all research and development programmes involved with agricultural biotechnology for crop and animal improvement. The primary reason is that using the CCP/SOP approach requires a very thorough review of existing processes and discussions between all staff members: glasshouse staff to junior technicians and research scientists to senior managers involved with the research. For instance, in all countries, improper labelling of research material was identified as a Critical Control Point (CCP) that needed to be addressed by appropriate Standard Operating Procedures (SOPs).
(2) Training methodology and scale-up: The lead trainer coached a total of 103 persons including the 12 African focal persons within the first year. The African focal persons then trained 1412 further persons within the remainder two-year period of the project. Peer-review visits for focal persons organized as part of the training were highly beneficial to consolidate learning and reinforce principles on verification and auditing. The train-the-trainer approach enabled the scale-up to be managed in Africa by Africans and was well received because of this fact and that training and examples were tailored for their own situations and specific needs.
(3) Effective implementation in research and development institutions:
(a) The change management required to introduce and implement stewardship principles in research and development institutions requires ownership by both the heads of institutions and the lead scientists
(b) To encourage the upkeep and preservation of improved practices, stewardship processes need to be mainstreamed into each institution’s research activities and not treated as a stand-alone initiative
(c) Stewardship implementation is best achieved by involving all staff that contribute to specific research processes, no matter what level of seniority, from research project leaders to non-technical staff
(d) Scientists need to be encouraged and recognised by their management for mainstreaming stewardship principles into their research and product development activities and encouraged to publish their experiences in local and international journals.
(4) Project management and outreach:
(a) A key success factor for the SABIMA stewardship project concerned the presence of highly motivated committed and influential project coordinators from FARA, together with highly professional and respected country focal persons. It was this leadership, know-how and commitment that enabled the scale of training and implementation of best practices that ensued
(b) Support and harmonisation of activities by the sub-regional organisations (SROs) exemplified by the West African Council for Agricultural Research and Development (CORAF/WECARD) is crucial in the pursuit of a regional initiative such as the SABIMA project.
(c) The participation of regulatory officials in stewardship training and outreach activities facilitated the understanding of the stewardship role in the assurance of quality and integrity of GM crops.
(5) Community of practice: A strong network between implementing countries is required. Such community of practice encourages sharing of experiences and contributes to project sustainability. Case studies are an excellent way for groups to share experiences and help others to practise good stewardship and apply one of the key principles of continuous improvement. Ideally, an annual or biennial stewardship conference is organised to ensure community of practice develops and strengthens over time.
(6) Knowledge sharing with the private sector: Special linkage with biotech companies proficient in stewardship that are willing to share their expertise in project management has been richly rewarding. Nevertheless, it must also be acknowledged that public sector research and agriculture in Africa present a very different reality to the situations in industrialized countries and private sector organisations. Stewardship practices need to be thoroughly tailored to fit the needs of African organisations and agriculture in Africa.
(7) Excellence through Stewardship (ETS): Membership of this professional association will allow continued access to information on current developments in stewardship globally, thereby enhancing capacity in the field in a cost-effective manner. Apart from the project coordinating agency, FARA, due to financial considerations none of the participating countries have so far subscribed to ETS.
(8) Donors and trait research: Donors need to include stewardship in their project requirements when funding trait research. This is to encourage standard setting and good practices by recipient institutions. This will also enhance the sustainability of the stewardship concept in the research and development processes of Africa.
(9) Sustainability of best practices: As with many capacity-building projects supported by international funding, sustainability of activities after cessation of the project funding has been problematic. Greater attention on achieving sustainability of know-how and continued use of new skills and processes should be a prerequisite for any training programme.
All the lessons learnt and listed will need to be considered in the design and implementation of similar capacity-building initiatives in the future. Stewardship application involves a continuous learning process. Frequent updating will be required through the peer review mechanisms and the association with international organisations promoting stewardship ideals in agricultural research, development and technology transfer. The current absence of special funding support for stewardship activities in many African countries poses a threat to the continued building in capacity for the engagement of stewardship principles in research, product development and use throughout value chains. The situation needs to be addressed by African governments and the donor community so that stewardship is seen as an integral part of conducting professional crop research programmes and is embedded in daily activities. An important outcome from this capacity-building project has been that rigorous stewardship processes not only maintain product integrity and reduce potential risks of non-regulatory compliance for genetically modified traits, but also enhance and encourage excellent and well-documented scientific practices. These are applicable to all research and development activities using molecular biology and advanced crop improvement techniques. Since this stewardship training was mainstreamed in Africa, scientific discoveries have accelerated within the gene-editing arena and targeted mutagenesis (Ricroch and Hénard-Damave, 2016; Songstad et al., 2017; Flavell, 2017). Attention is shifting into research areas where genetic modification can be achieved without incorporating foreign DNA into crop plant genomes. Government authorities and scientists alike around the world are reviewing the implications of these new molecular technologies and how they, and products made using them, should be regulated (Ledford, 2016; Wolt and Bing, 2016; Sprink et al., 2016; Van de Wiel et al., 2017). Consequently, as modern molecular methods develop further into the 2020s there is an even greater imperative to mainstream agricultural biotechnology stewardship across Africa. In this way, stewardship will not only encourage excellent, reputable science but will also ensure that risk management approaches are fully operational. Thus, ensuring the identity and integrity of research materials and new varieties being developed are maintained throughout their product life-cycles.
The authors have not declared any conflict of interests.
The authors are grateful to both the Forum for Agricultural Research in Africa (FARA) and the Syngenta Foundation for Sustainable Agriculture (SFSA) for the hosting and provision of financial and technical support, respectively, to the project on “Strengthening capacity for safe biotechnology management in sub-Saharan Africa (SABIMA)”. The content of which provided the material for this publication. Thanks to the six project countries and the respective focal persons who showed great enthusiasm and dedication in project implementation and post-project information sharing. Without this commitment, the sharing of knowledge from this publication would not have been possible.