ABSTRACT
Multidrug-resistant tuberculosis (MDR-TB) is a big challenge to the tuberculosis control programmes worldwide. The aim of this work is to determine the rate of MDR-TB in HIV-negative patients with signs and symptoms of pulmonary tuberculosis in Lagos State. 891 adult HIV-negative patients enrolled for the study and tested for acid-fast bacilli by culture; resistance to first-line anti-tuberculosis drugs was performed (isoniazid – 0.2 µg/ml, rifampicin – 40 µg/ml, and ethambutol – 2 µg/ml) by culture method on Lowenstein Jensen (LJ) medium using proportion method. Of the 819 HIV-negative patients, 47.7% were positive for acid-fast bacilli by culture, and 78% of the culture positive were found to be Mycobacterium tuberculosis complex (MTBC). DST analysis on MTBC isolates showed that resistance to only one drug was found in forty-two (13.5%) patients, dual resistance in eighty-seven (28.3%) patients while triple resistance in 45 (14.6%). A total of 117 (38%) MDR-TB cases were recorded in this study. Sex, age, tuberculosis contact, smoking and alcohol consumption were not significantly associated with MDR-TB, P>0.05. However, previous history of tuberculosis was significantly associated with MDR-TB, P = 0.000. This study concluded that there was high MDR-TB rate (38%) among HIV-negative patients and MDR-TB is associated with previous history of tuberculosis. The findings emphasize the importance of continuing the systematic surveillance of drug resistance for prompt diagnosis and iniatiation of treatment.
Key words: Lagos, HIV-negative, Directly Observed Treatment Short-course (DOTS), Multidrug-resistant tuberculosis (MDR-TB).
Abbreviation:
MDR-TB, Multidrug-resistant tuberculosis; TB, Tuberculosis; HIV, Human Immunodeficiency Virus; DOTS, Directly Observed Treatment Short-course; AFB, Acid-fast bacilli; LJM: Lowenstein Jensen Medium; ZN, Ziehl Neelsen; MTB, Mycobacterium tuberculosis; MTBC, Mycobacterium tuberculosis complex; DST, Drug Susceptibility Testing; WHO, World Health Organization; NIMR, Nigerian Institute of Medical Research; IDH, Infectious Disease Hospital; CTBR, Centre for Tuberculosis Research.
Multidrug-resistant tuberculosis (MDR-TB), defined as Mycobacterium tuberculosis that is resistant to isoniazid and rifampicin, has become a great challenge to tuberculosis (TB) control programmes (Gehre et al., 2016). Nearly 1.5 million people are living with MDR-TB worldwide (O’Donnell et al., 2016). In 2016 alone, the World Health Organization (WHO) estimated 490,000 new cases of MDR-TB and the same WHO global report 2016 revealed that about 93,000 cases of MDR-TB occurred in the WHO African region, with Nigeria having 20,000 of these cases (Adebisi et al., 2019). Also, in 2016, the incidence of MDR-TB/RR-TB in Nigeria was 11/100,000 population (Adejumo et al., 2020a).
MDR-TB is associated with human immunodeficiency virus (HIV) infection and clinically HIV worsens TB (O’Donnell et al., 2016). Although HIV patients are at a higher risk of developing MDR-TB than HIV-negative patient, the global TB report in 2016 had revealed that 490,000 new cases of MDR-TB occurred in HIV-negative patients (Ogbo et al., 2018). Furthermore, the WHO Global Tuberculosis Report 2017 and Global Burden of Diseases, Injuries and Risk Factors (GBD) study 2016 reported 6.3 million and 9.0 million new cases of TB among HIV-negative people respectively (Ogbo et al., 2018). These reports highlighted the considerable burden of TB in HIV–negative people.
An increase of TB and MDR-TB in HIV-negative patients could be attributed to several factors. This could be as a result of poor response to treatment and this draws attention to the need for a prompt response and close monitoring of patient during treatment or the abuse of drugs used for TB in treating other infectious diseases. Furthermore, it could be as a result of periodic visits to clinical settings or from mixing of patients who have MDR-TB. However, previous history of TB therapy is the strongest risk factor for development of MDR-TB and this is partly due to acquired drug resistance. Acquired resistance usually reflects inadequate TB treatment (that is when patients fail to adhere to proper treatment regimens, wrong drugs are prescribed, irregular drug supplies or use of substandard drug) (Fadeyi et al., 2017). Other risk factors include alcohol consumption, smoking, drug addiction, drug side effects, homelessness and treatment failure (Mulu et al., 2015).
Despite the fact that various TB control strategies have been implemented in Nigeria, Nigeria is listed among the 30 high burden countries for MDR-TB with a prevalence of 4.3 and 25% in new and previously treated TB patients respectively in 2016 (WHO Report, 2017). These could be lower than the real number of MDR-TB patients in Nigeria. Although, a systematic review in 2017 on the prevalence of drug-resistant TB in Nigeria reported an increase in the rate of MDR-TB (Onyedum et al., 2017). The case detection rate in the country is one of the lowest worldwide at 16% (Adejumo et al., 2020b). The WHO recommends baseline resistance testing for all HIV positive patients with active TB prior to initiating treatment; thus, more attention is devoted to studying the burden among them while HIV negative population that constitutes the bulk of TB cases was relegated to the background. Ideally, before commencement of treatment in all patients with TB, routine DST should be performed but this is not attainable in most high burden countries because facilities for culture and DST are not readily available. Therefore, in high burden settings like Nigeria, there is need to perform routine baseline resistance testing for all patients with active TB. Thus, this study has been designed to determine the rate of MDR-TB among HIV-negative individual with signs and symptoms of active pulmonary TB as well as possible risk factors of MDR-TB.
Study setting
This study was carried out at two DOTS clinics in Lagos State: The Nigerian Institute of Medical Research (NIMR), Yaba and Infectious Disease Hospital (IDH), Yaba. Lagos State is one of the most populous states in Nigeria occupying about 3577.28 km2 with a projected population of 24 821 418 in 2017 (Adejumo et al., 2020a). Lagos thus has a small landmass with very high population resulting in huge overcrowding in homes, public places and transportation. In 2016, Lagos State contributed nearly 17% of all DR-TB cases diagnosed in the country (Adejumo et al., 2020a). NIMR is a tertiary health institution and serves as a referral site for diagnosis and treatment monitoring of patients with complicated TB cases. IDH which is also called Mainland hospital is a secondary referral hospital for treatment of infectious diseases like TB, MDR-TB and HIV.
Study design and population
This is a prospective study of 819 adult presumptive TB cases with signs and symptoms of pulmonary tuberculosis who were HIV-negative. In the study, only patients that gave written informed consent were enrolled while patients with unknown HIV status who refused to perform HIV testing were excluded from the study.
Definition of terms
(i) New case: Newly diagnosed TB patients that have not received TB treatment or received TB treatment for <1 month.
(ii) Previously treated TB case: TB patients that have received TB treatment for >1 month (returned after default, relapses, or treatment failure) (Sanchez-Padill et al., 2012).
(iii) MDR-TB case: Patients whose Mycobacterium tuberculosis in sputum culture is resistant to both isoniazid and rifampicin.
Sample collection
Two consecutive sputum samples (spot and early morning) were collected from the patients in sterile wide-capped container from July 2015 to April 2016. Prior to sputum collection, patients’ information (such as age, sex, HIV status etc.) and treatment history were obtained using a well-structured questionnaire. After sample collection, all samples were transported to the laboratory at 4 to 8°C within 24 h of collection.
Laboratory procedures
The laboratory procedures were performed at the National TB Reference Laboratory – Centre for Tuberculosis Research (CTBR), Nigerian Institute of Medical Research (NIMR) Lagos.
Culture
Laboratory diagnosis of TB was done by solid culture. Sputum samples were decontaminated with NALC-NaOH and concentrated by centrifugation at 3000G force for 15 min. Sediments were re-suspended in 2ml phosphate buffer (pH 6.4) and about 2-3 drops of the sediment was inoculated unto slopes of Lowenstein Jensen medium. The inoculated LJ slopes were incubated at 370C and examined on the third day for contamination and weekly for up to 8 weeks for growth of mycobacteria. All slopes not showing any growth within this period were discarded and recorded as culture negative. For slopes with growths, smear of colonies were made, stained by Ziehl Neelsen (ZN) technique and examined for the presence of Acid-fast bacilli (AFB). Isolates that were acid-fast bacilli were confirmed as positive.
Identification of culture Isolates
Isolates that were positive for AFB were further identified as M. tuberculosis complex (MTBC) using SD Bioline MPT 64 Ag TB rapid identification test kit and para-nitrobenzoic acid (PNB) test. MTBC identification was based on positive TB Ag MPT 64 test and no growth on LJM containing PNB.
Phenotypic drug susceptibility testing (DST)
All isolates showing positive for MTBC were sub-cultured on fresh LJM. DST were performed on LJM by the proportion method on the following first-line anti-TB drugs – isoniazid (0.2ðœ‡g/ml), rifampicin (40 µg/ml), and ethambutol (2 µg/ml) (Kent and Kubica, 1985). Bacterial suspensions were inoculated by concentrations (104 and 102) into drug-free and drug-containing slopes respectively. Susceptibility or resistance was recorded when the proportion of bacteria in drug-containing medium to that of drug-free medium is <1or≥1, respectively.
Statistical analysis
Data generated from the study were entered and analyzed using the Statistical Package for Social Sciences (SPSS) IBM for windows version 26.0. Frequencies were calculated as percentages. Comparison of categorical variables and significance testing was made with Chi-square test. Value of P < 0.05 was considered statistically significant.
Ethical approval
Ethical approval for the study was obtained from Institutional Review Board, Nigerian Institute of Medical Research, Lagos. Permission to carry out the study was granted by the management of the DOTS clinics. Consent was obtained from each participant, and the participants’ confidentiality was maintained throughout the study (Figure 1).
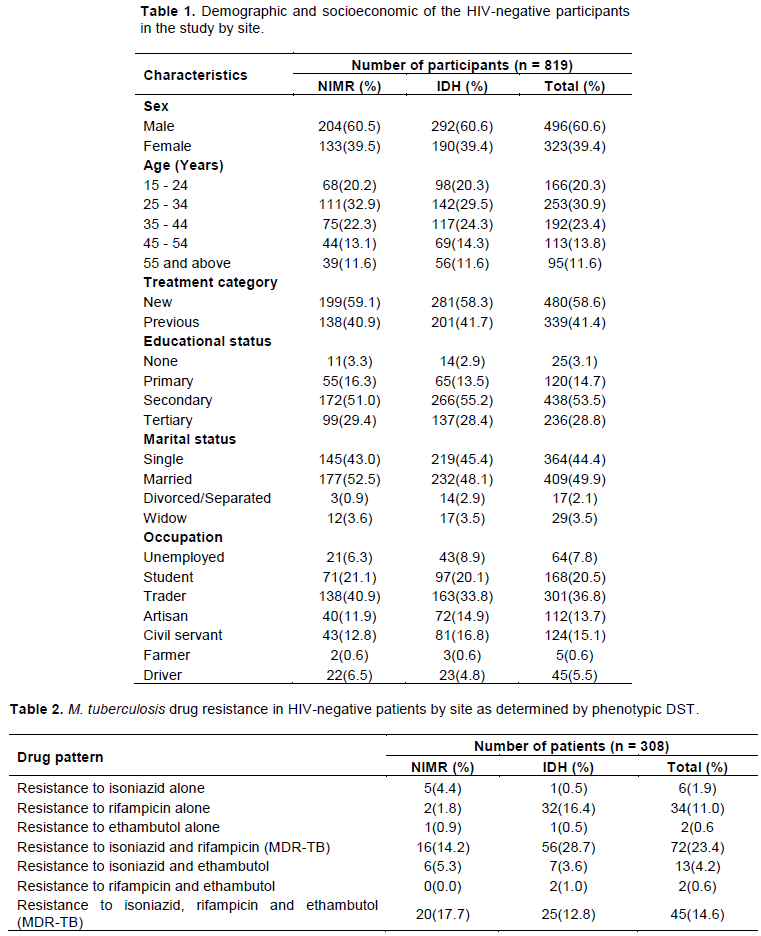
A total of 819 HIV negative subjects were recruited from the two DOTS clinics. More than sixty percent of the cases were males 496 (60.6%) and between the age group of 15 – 55 years with a mean of 34.2± 6.4 years. Majority 480 (58.5%) were new TB cases while most of the participants had received some level of education; 794 (97%) and 409 (49.9%) were married. Among the participants, trading was the highest occupation as shown in Table 1.
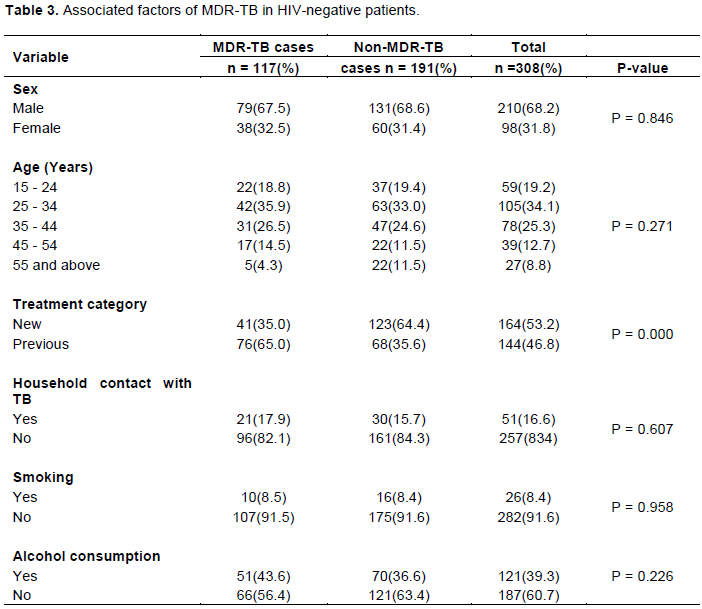
The phenotypic DST was performed on three hundred and eight patients (308) as shown in Table 2. Mono-resistance (resistance to one drug only) was found in 42 (13.6%) patients; Mycobacterium tuberculosis (MTB) culture consists of 6 (1.9%) in isoniazid, 34 (11%) in rifampicin and 2 (0.6%) in ethambutol. Dual drug resistance to isoniazid and rifampicin (MDR-TB), isoniazid and ethambutol, and rifampicin and ethambutol were found in 72 (23.4%), 13 (4.3%) and 2 (0.6%) patients samples, respectively. Forty-five (14.6%) patients MTB cultures were found to be resistant to the three drugs of isoniazid, rifampicin and ethambutol tested (MDR-TB). A greater number of MDR-TB samples were observed at Infectious Disease Hospital (81) than at Nigerian Institute of Medical Research (36), P = 0.092.
A total of 117 (38%) MDR-TB cases were recorded in the study consisting of 72 (23.4%) of dual resistance of isoniazid and rifampicin, and 45 (14.6%) of triple resistance of isoniazid, rifampicin and ethambutol. Although the proportion of males among the MDR-TB cases 79 (67.5%) was lower than the proportion of non-MDR-TB cases 131 (68.6%), the difference was not statistically significant (P = 0.846) (Table 3). The number of cases less than 35 years with MDR-TB diagnosis was less than the number among those without MDR-TB. However, the difference was not statistically significant, P = 0.271. The proportion of previous tuberculosis cases among the MDR-TB cases 76 (65.0%) was significantly higher than the proportion of previous tuberculosis cases 68 (35.6%) among the non-MDR-TB cases (P = 0.000). The percentage of MDR-TB cases that had tuberculosis contact was lower than in non-MDR-TB (17.9%) (P = 0.607). Smoking and alcohol intake were also lower among MDR-TB cases compared to non-MDR-TB cases (8.5 and 43.6%, respectively) (Table 3).
MDR-TB has reached alarming levels worldwide with the emergence of M. tuberculosis strains that are nearly untreatable with the existing anti-TB drugs. MDR-TB, which is caused by M. tuberculosis strains that do not respond to standard therapies, not only poses problems for individuals but also for the control of TB in populations as it represents lapses in public health. This study also observed high rates of MDR-TB (38%) among HIV-negative patients with tuberculosis studied. This is much higher than rate previous reported in Nigeria (Otokunefor et al., 2018; Onyedum et al., 2017; Dinic et al., 2012) and elsewhere (Saldanha et al., 2019; Yeboah-Manu et al., 2012; Fenner et al., 2012). However, the rate of MDR-TB among HIV-negative patients observed in this study was lower than the rate reported by Okoro et al. (2019) in Eastern part of Nigeria. The high rate reported in this study may be poor adherence as a result of tuberculosis associated stigma in our environment, challenges in practicing classic DOTS and high population in Lagos that favours transmission of tuberculosis. The relative high burden of HIV in Lagos despite low prevalence may also contribute to the high rate.
Mono-resistance to isoniazid and rifampicin of 1.9 and 11% respectively was found in our study population. This is similar to previous reports in Nigeria (Dinic et al., 2012) and Togo (Dagora et al., 2015). However, worrisome is the rifampicin mono-resistance of 11% as resistance to rifampicin is the surrogate marker for MDR-TB (Mohammad et al., 2017). The observed high rate of mono drug resistance may be connected to the uncontrolled sale of antibiotics including tuberculosis drugs in open market in Nigeria. In Nigeria, antimicrobials including antibiotics and anti-tuberculosis drugs can be purchased in the open market and drug stores. The quality of counselling at such places is doubtful, impacting adherence and implication for the high resistance rate recorded especially among previous tuberculosis cases. This study was conducted in Lagos with its transportation challenge which makes daily DOTS difficult. In centres that insist on daily DOTS, adherence may be a challenge. This study was conducted in two centres with one practicing modified DOTS and the other daily DOTS.
With regard to gender and MDR-TB in this study, majority of the MDR-TB patients were males (67.5%), P = 0.401, an observation which is in agreement with the fact that male gender has been identified as a risk factor for TB (Pokami et al., 2013). This could be attributed to increased male activities that increase the risk of contacts with infected persons in work place, during visits to overcrowded recreation centers or males engaging in more risk activities, hence more prone to tuberculosis disease. It could also be that women do not have time or they do not have the final decision when to seek help. This may be attributed to the stigma associated with TB and also to the fact that women prefer to visit traditional healers and seek alternative medicine rather than mainstream medical health professionals (Ehsanul Huq et al., 2018). This finding is in contrast to what is reported by Olowe et al. (2017) where TB was higher in females than males. The non- statistically significant association between gender and MDR-TB in this study may have account for the variation in distribution of sex among different studies.
We also found higher MDR-TB rate in the patients aged 15 - 44 years than the other age group. Worldwide, TB affects the most productive age group. Ogbo et al. (2018) reported high burden of tuberculosis among HIV-negative people within the age group 15 – 49 years. Also, this finding is in concordance with the reports by Abdulazeez et al. (2019), Fadeyi et al. (2017) and Pokami et al. (2013) and further confirms that these age groups are more vulnerable to tuberculosis infection.
Although majority of the patients were new cases, previous TB cases had the highest rate of MDR-TB in this study (P = 0.000). This shows that level of acquired resistance to anti- TB drugs among TB patients is high. Previous studies have highlighted a higher proportion of MDR-TB in previous cases than new cases (Saldanha et al., 2019; Onyedum et al., 2017; Yeboah-Manu et al., 2012) and this preponderance may be attributed to poor patient compliance to treatment, irregular drug supplies, inadequate treatment regimens or poor quality of drugs. In addition, the MDR-TB rate among new cases observed in this study is above the current WHO estimates of 4.3% for Nigeria (WHO Report, 2017; Onyedum et al., 2017). This shows that among new cases, the burden of MDR-TB could be under reported given that the facilities for culture and DST are not readily available in the country (Onyedum et al., 2017; Pokam et al., 2013). Also, the presence of MDR-TB among new cases could be attributed to active transmission of resistant strains in the community from the infectious DR-TB patients who are not on treatment and this calls for close monitoring of DR-TB patients (Onyedum et al., 2017).
History of contact with TB patient has not been found to be associated with MDR-TB in this study (P = 0.607). This is in agreement with the study by Mulu et al. (2015). The association between history of close contact with TB patients would be attributed to acquiring of primary drug resistant bacteria from the patient (Mulu et al., 2015). Tuberculosis is transmitted through close contact with an individual who is infected with active TB that spreads the bacteria through coughing (Demile et al., 2018). As soon as the bacilli is inhaled, the infection is established with or without a visible primary lung lesion; lymphatic and hematogeous spread usually within 3 weeks of infection (Demile et al., 2018).
Previous studies have reported that smoking is associated with an increased risk of tuberculosis infection and tuberculosis disease (Wang et al., 2018). Tuberculosis patients who had a history of smoking exposure are 1.57 times more likely to develop drug resistance tuberculosis compared with non-smoker tuberculosis cases (Wang et al., 2018). The 8.5% rate of MDR-TB in this study among HIV-negative persons that smoke has shown that there is association between MDR-TB and smoking, though not statistically significant.
We also observed that one of the risk factors for MDR-TB is alcohol use. Samuels et al. (2018) reported alcohol consumption as one of the risk factors for MDR-TB. In addition, Ogbo et al. (2018) documented that alcohol use was essential contributor to the burden of TB in Nigeria. This could be due to impairment of the host immune system (innate and adaptive response), which increases vulnerability to TB infection, or reactivation of latent TB infection (Ogbo et al., 2018).
This study had some limitations. The study population only represents the population of patients diagnosed through the two DOTS clinics. Hence, the rate of MDR-TB in HIV-negative patients obtained in this study may not reflect the true rate of MDR-TB in Lagos State. Furthermore, our study was limited by the design of the study which did not give us the opportunity to explore all other risk factors such as diabetes, malnutrition, history of prison drug addiction, drug side effects, homelessness and others associated with MDR-TB. However, despite these limitations, the study provides information about the rate of MDR-TB and associated risk factors in HIV-negative patients in Lagos State where there are limited data.
Our study concluded that there was high MDR-TB rate (38%) among HIV-negative patients and MDR-TB is associated with previous history of tuberculosis. The findings emphasize the importance of continuing the systematic surveillance of drug resistance for prompt diagnosis and iniatiation of treatment. There is therefore the need for the National Tuberculosis Control Program to increase the use of commercial rapid DNA-based kits, the Genotype MTBDRplus (Hain Lifescience, Germany) for routine testing of sputum samples from all patients with symptoms suggestive of TB.
The authors have not declared any conflict of interests.
The authors are grateful to the patients who participated in the study and also, thank the clinical and laboratory staff of NIMR and IDH. We especially thank Mr. Aje T., Ms. Nureni AR and Mr. Igwubor FO.
MDR-TB, Multidrug-resistant tuberculosis; TB, Tuberculosis; HIV, Human Immunodeficiency Virus; DOTS, Directly Observed Treatment Short-course; AFB, Acid-fast bacilli; LJM: Lowenstein Jensen Medium; ZN, Ziehl Neelsen; MTB, Mycobacterium tuberculosis; MTBC, Mycobacterium tuberculosis complex; DST, Drug Susceptibility Testing; WHO, World Health Organization; NIMR, Nigerian Institute of Medical Research; IDH, Infectious Disease Hospital; CTBR, Centre for Tuberculosis Research.
REFERENCES
|
Abdulazeez AA, Margaret AA, Sulaiman NA, Abdulazeez AI (2019). Multiple-drug resistant (MDR) tuberculosis among HIV sero-positive and sero-negative populations in Ilorin, North-Central Nigeria. South Sudan Medical Journal 12(3):93-96.
|
|
|
|
Adebisi YA, Agumage I, Sylvanus TD, Nawaila IJ, Ekwere WA, Nasiru M, Okon EE, Ekpenyong AM, Lucero-Prisno DE (2019). Burden of Tuberculosis and Challenges Facing Its Eradication inWest Africa. International Journal of Infection 6(3):e92250.
Crossref
|
|
|
|
|
Adejumo OA, Olusola-Faleye B, Adepoju VA, Gidado M, Onoh MO, Adegboye O, Razzaq HA, Moronfolu O, Shogbamimu Y (2020a). The pattern of comorbidity and its prevalence among drug-resistant tuberculosis patients at treatment initiation in Lagos, Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene 00:1-9.
Crossref
|
|
|
|
|
Adejumo OA, Daniel OJ, Adepoju VA, Femi-Adebayo T, Adebayo BI, Airauhi AO (2020b). Challenges of tuberculosis control in Lagos State, Nigeria: A qualitative study of health-care providers' perspectives. Nigerian Medical Journal 61(1):37-41.
Crossref
|
|
|
|
|
Dagora AY, Miaga KD, Adjoh K, Kadanga E, Disse K, Adekambi T (2015). Prevalence of multidrug-resistant tuberculosis cases among HIV-positive and HIV-negative patients eligible for retreatment regimen in Togo using GeneXpert MTB/RIF. New Microbe and New Infections 8:24-27.
Crossref
|
|
|
|
|
Demile B, Zenebu A, Shewaye H, Xia S, Guadie A (2018). Risk factors associated with multidrug resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infectious Diseases 18:249.
Crossref
|
|
|
|
|
Dinic L, Akande P, Idigbe EO, Ani A, Onwujekwe D, Agbaji O, Akanbi M, Nwosu R, Adeniyi B, Wahab M, Lekuk C, Kunle-Ope CN, Nwokoye N, Kanki P (2012). Genetic Determinant of Drug-Resistant Tuberculosis among HIV-Infected Patients in Nigeria. Journal of Clinical Microbiology 50(9):2905-2909.
Crossref
|
|
|
|
|
Ehsanul Huq KATM, Moriyama M, Zaman K, Chisti MJ, Long J, Islam A, Hossain S, Shiri H, Raiha MJ, Chowdhury S, Rahman MM (2018). Health seeking behaviour and delayed management of tuberculosis patients in rural Bangladesh. BMC Infectious Diseases 18:515-523.
Crossref
|
|
|
|
|
Fadeyi A, Desalu OO, Ugwuoke C, Opanwa OA, Nwabuisi C, Salami AK (2017). Prevalence of rifampicin-resistant tuberculosis among patients previously treated for pulmonary tuberculosis in North-Western, Nigeria. Nigerian Medical Journal 58:161-166.
Crossref
|
|
|
|
|
Fenner L, Gagneux S, Janssens J, Fehr J, Cavassini M, Hoffman M, Bernasconi E, Schrenzel J, Bodmer T, Bottger EC, Helbling P, Egger M (2012). Tuberculosis in HIV-negative and HIV-infected patients in a low-incidence country: Clinical characterisitics and treatment outcomes. PLoS ONE 7(3):e34186.
Crossref
|
|
|
|
|
Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S, Kehinde OA, Adebiyi O, Salako K, Baldeh I, Jallow A, Jallow M, Dagnra A, Dissé K, Kadanga EA, Idigbe EO, Onubogu C, Onyejepu N, Gaye-Diallo A, Ba-Diallo A, Rabna P, Mane M, Sanogo M, Diarra B, Dezemon Z, Sanou A, Senghore M, Kwambana-Adams BA, Demba E, Faal-Jawara T, Kumar S, Tientcheu LD, Jallow A, Ceesay S, Adetifa I, Jaye A, Pallen MJ, D'Alessandro U, Kampmann B, Adegbola RA, Mboup S, Corrah T, de Jong BC, Antonio M (2016).
|
|
|
|
|
The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Medicine 14:160.
|
|
|
|
|
Kent PT, Kubica GP (1985). A Guide for the Level III Laboratory.
|
|
|
|
|
Mohammad AB, Iliyasu G, Habib AG (2017). Prevalence and genetic determinant of drug-resistant tuberculosis among patients completing intensive phase of treatment in a Tertiary Referral Center in Nigeria. International Journal Mycobacteriology 6:47-51.
Crossref
|
|
|
|
|
Mulu W, Mikonnen D, Yimer M, Admassu A, Abera B (2015). Risk factors for multidrug resistant tuberculosis patients in Amhara National Region State. African Health Sciences 15(2):368-377.
Crossref
|
|
|
|
|
O'Donnell MRO, Daftary A, Frick M, Hirsch-Moverman Y, Amico KR, Senthilingam M, Wolf A, Metcalfe JZ, Isaakidis P, Davis JL, Zelnick JR, Brust JCM, Naidu N, Garretson M, Bangsberg DR, Padayatchi N, Friedland G (2016). Re-inventing Adherece: towards a patient-centered model of care for drug-resistant tuberculosis and HIV. International Journal Tuberculosis Lung Disease 20(4):430-434.
|
|
|
|
|
Ogbo FA, Ogeleka P, Okoro A, Olusanya BO, Olusanya J, Ifegwu IK, Awosemo AO, Eastwood J, Page A (2018). Tuberculosis disease burden and attributable risk factors in Nigeria, 1990 - 2016. Tropical Medicine and Health 46:34.
Crossref
|
|
|
|
|
Okoro CE, Ibhawaegbele SO, Ezema CI, Ezeugwu UA, Igweagu CP, Dozie Nwakile OC (2019). Prevalence of multi-drug-resistant tuberculosis among human immunodeficiency virus and nonhuman immunodeficiency virus-positive pulmonary tuberculosis patients of two referral hospitals in Southeast Nigeria. Ibnosina Journal of Medicine and Biomedical Sciences 11:111-115.
|
|
|
|
|
Olowe OA, Makanjuola O, Adekanmi AS, Adefioye OJ, Olowe RA (2017). Epidemiological characteristics and clinical outcome of HIV-related tuberculosis in a population of TB patients in South-Western Nigeria. European Journal of Microbiology and Immunology 7(2):127-132.
Crossref
|
|
|
|
|
Onyedum CC, Alobu I, Ukwaja KN (2017). Prevalence of drug-resistant tuberculosis in Nigeria: A systematic review and meta-analysis. PLoS ONE 12(7):e0180996.
Crossref
|
|
|
|
|
Otokunefor K, Otokunefor TV, Omakwele G (2018). Multi-drug resistant Mycobacterium tuberculosis in Port Harcourt, Nigeria. African Journal of Laboratory Medicine 7(2):805.
|
|
|
|
|
Pokam BT, Asuquo AE, Abia-Bassey LN, Idasa MB, Umoh NO, Eko FO, Rastogi N (2013). Multidrug resistance and demography of newly diagnosed tuberculosis patients in Cross River State, Nigeria. International Journal of Mycobacteriology 2:89-93.
Crossref
|
|
|
|
|
Saldanha N, Runwal K, Ghanekar C, Gaikwad S, Sane S, Pujari S (2019). High prevalence of multi drug resistant tuberculosis in people living with HIV in Western India. BMC Infectious Diseases 19:391-396.
Crossref
|
|
|
|
|
Samuels JP, Soods A, Campbell JR, Khan FA, James, Johnston C (2018). Comorbidities and treatment outcomes in multidrug resistant tuberculosis: a systematic review and meta-analysis. Scientific Reports 8:4980.
Crossref
|
|
|
|
|
Sanchez-Padilla E, Diamini T, Ascorra A, Rush-Gerdes S, Tefera ZD, Calain P, de la Tour R, Jochims F, Richter E, Bonnet M (2012). High Prevalence of Multidrug-Resistant Tuberculosis, Swaiziland. Emerging Infectious Diseases 18(1):29-37.
Crossref
|
|
|
|
|
Wang M, Huang W, Wang Y, Zhang Y, Zhang M, Wu S, Sandford AJ, He J (2018). Association between tobacco smoking and drug-resistant tuberculosis. Infection and Drug Resistance 11:873-887.
Crossref
|
|
|
|
|
World Health Organization, Global Tuberculosis Control: WHO Report 2017, WHO, Geneva, Switzerland.
|
|
|
|
|
Yeboah-Manu D, Asante-Poku A, Ampah KA, Kpeli G, Danso E, Owusu-Darko K, Bonsu FA (2012). Drug susceptibility pattern of Mycobacterium tuberculosis isolates from Ghana; Correlation with Clinical Response. Mycobacacterial Disease 2:107.
|
|