ABSTRACT
The present study was undertaken to establish the distribution and diversity of enteric bacterial communities along the Omubhira stream and also determine if physico-chemical parameters influence their distribution in water in terms of total coliforms (TC) and Escherichia coli. Stratified random sampling was used and four strata with 15 selected sampling sites identified along the stream. Selection of the sampling sites was in relation to land use activities that are likely to be sources of bacterial contamination to the stream. The results of bacteriological analysis of water quality revealed that water from some of the selected sites of the stream had bacterial loads that exceeded the WHO value/guidelines for water for recreational use. Mean comparison of coliforms counts using a one way ANOVA test revealed that the difference in coliforms among the sampling sites of Omubhira stream was significant (F=18.324, P=0.0005). Pearson product-moment correlation showed that there was a strong positive correlation between Escherichia coli and electrical conductivity which was statistically significant (r=0.413, n=80, p<0.0005), total dissolved solids (r=0.408, n=80, p<0.0005), dissolved oxygen (r =0.446, n=80, p<0.0005) and total coliforms (r=0.983, n=80, p<0.0005). However, there was no relationship between faecal coliforms and temperature, total suspended solids and pH which was not statistically significant; temperature (r =0.185, n=80, p>0.101), total suspended solids (r=-0.118, n=80, p>0.298) and pH (r=-0.089, n=80, p>0.433). The bacteria isolated from water samples in this study included Escherichia coli, Enterobacter spp., Citrobacter spp., Proteus spp., Serratia spp., Shigella spp., Providencia spp. Morganella spp., Salmonellae spp. and Klebsiella spp. Escherichia coli was the most predominant enterobacterial isolate during both the dry and the wet season. Intervention measures including creating awareness, educating residents on hygiene practices on the use of Omubhira stream water and improvement of sanitation should be implemented.
Key words: Enteric bacteria, Omubhira stream, Escherichia coli, total coliforms (TC).
Urban rivers are vulnerable to different urban processes and activities that cause pollution and degradation of the water ecosystem. In the recent past, pollution of water sources in the urban set up with deleterious entero- bacterial communities has been on a steady increase. Major source of these microbes in water is faeces from
human and other mammals (Musyoki et al., 2013)
In Kenya, according to NEMA regulations, all sources of water for domestic uses should comply with stringent standards set out in the first schedule to these regulations. Escherichia coli, Shigella spp., Pseudomonas aeruginosa or coliforms should not be detectable in 250 ml of drinking water (Vail et al., 2003). World Health Organization (WHO) developed microbiological quality guidelines based on intended water uses. The guidelines stipulate that faecal coliforms (FC) should not exceed 10² per 100 ml of water used in irrigation of crops that are eaten uncooked, sports fields, and public parks in unrestricted regions (WASREB, 2006) . Enteric bacteria in the family Enterobacteriaceae reside normally in the guts of many animals, including humans (Wawire et al., 2013). Some members of Enterobacteriaceae such as Escherichia coli, Enterobacter spp., and Serratia spp. are natural inhabitants of the gastro-intestinal tract of human beings and are used as indicators of faecal contamination of the environment. The pathogenic members of Enterobacteriaceae that infect the gastro-intestinal tract of humans include Salmonella spp., Shigella spp., Proteus spp., Campylobacter spp. and E. coli. They get access to the human when ingested through contaminated water, food and oral contact with infected surfaces (WASREB, 2006). This raises public health concern. Diarrhoeal cases have majorly been associated with enteric .bacterial groups, this in turn accounts for a substantial degree of morbidity, and mortality in different age groups worldwide (Obi et al., 2003; Eze and Madumere, 2012).
In Kakamega town, the Omubhira stream, like any other stream or rivers is facing pollution problems due to increased human activities along the stream. The stream traverses formal and informal settlements, agricultural farms, learning institution and wastewater treatment plant. Water from the stream is used extensively for watering livestock, washing, bathing and irrigation of crops namely arrow roots, vegetables, plantain and nappier grass grown along it. Although this stream has been providing water for domestic and agricultural purposes over the years, no studies have however demonstrated the distribution and diversity of enteric bacterial along the stream.
The current study therefore establishes the distribution and diversity of enteric bacterial communities along theOmubhira stream. Information gathered provides baseline data that would be applied in controlling and reducing occurrence of disease burdens among stream users, contamination of agricultural produce and reduce pathogen transmission.
Description of study area
Omubhira stream is a first-order stream that flows in an Easterly direction with its origin situated in Milimani Estate within Kakamega town in Western Kenya. The stream is approximately 0.98 km long and approximately 100 cm wide. Temperature ranges from a minimum of 10.3ºC to a maximum of 30.8ºC with a mean of 20.5ºC. The rainfall ranges between 1250 and 1750 per annum (KARI Kakamega Annual Report, 2011). The community along the stream is majorly engaged in small-scale crop farming, livestock rearing and aquaculture. Omubhira stream joins Lurambi stream at a confluence and both drain into River Isiukhu and eventually into River Nzoia; one of the major rivers draining into Lake Victoria. Important features along the stream include a narrow strip of natural wetland on either side that comprises majorly of the sedges, settlement schemes, fishponds, wastewater treatment plant, farmlands and learning institution (Figure 1).
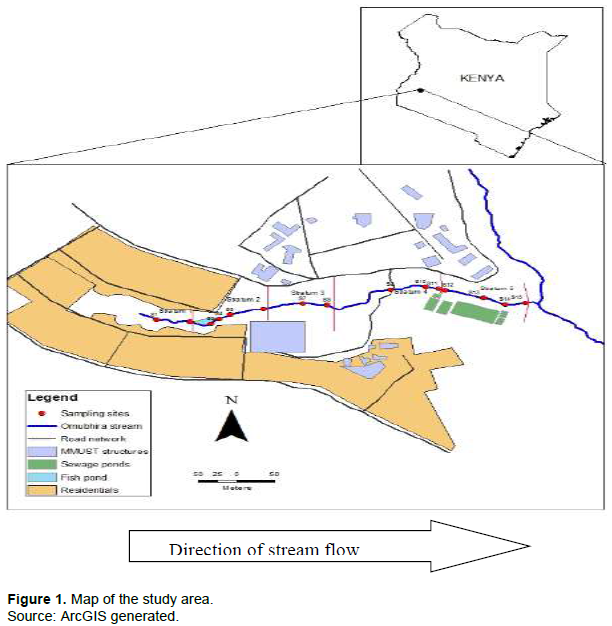
Study design
Stratified random sampling design was applied in this study. The stream was categorised into four strata in relation to the purported sources of bacterial contamination, with three sampling sites per strata. Strata 4(4S) - downstream, strata 3(3S) - lower midstream, strata 2(2S) - upper midstream and strata 1(1S) - upstream (source) coordinates of sampling sites was taken by “Garmin Etrex” (GPS) and charted using the ArcGIS software.
Sample collection
Collection of samples occurred twice in a month. This was conducted randomly between 9 am and 12 noon during the wet season (April to September-2014) and dry season (December- 2014 to February- 2015). Physico-chemical parameters namely: Water temperature, electrical conductivity, ph, dissolved oxygen, total dissolved solids (TDS) and total suspended solids (TSS) were measured in situ at the time of sampling using electrical probes (APHA, 2005). Bacteriological analysis involved determination of the levels of total coliforms and E. coli using the 3 M E. coli/coliforms Petri film count plates. The samples were then transported in ice packed cooler boxes and analyzed within two hours of collection in the Biological Sciences Department - microbiology laboratory within 8 h from the sampling time.
Physico-chemical analysis
Temperature of the water samples was taken at all sampling sites, using a thermometer and recorded in degrees celsius. The thermometer bulb was dipped into the water and allowed to stand for one minute before the reading was taken directly. Electrical conductivity (EC) and total dissolved solids (TDS) were analyzed using Cond/TDS/Salt/Temp meter CTS-406K. The conductivity probe was rinsed with distilled water, immersed into the sample and the reading recorded in a table. PH was analyzed using HI 2211 pH/ORP meter, Hannia instrument. The meter was calibrated by inserting its probe in a standard solution at pH 7.0 then rinsed with distilled water and then at pH 4.0. The probe was then rinsed with distilled water and inserted into the collected sample. The pH was read off above the temperature level displayed on the screen. Dissolved oxygen was analyzed using digital oxygen meter of M.R.C model.
For the determination of total suspended solids (TSS), Whatman GF/C glass microfibre filter papers with 1.2 µm pore size was used. The filter paper was weighed using an electronic digital balance and the initial reading noted. 100 ml of the sample was filtered through and the filter paper oven dried at 50ºC for 1 h. The filter paper was then re- weighed and the final weight of the filter paper gave the value of TSS in grams.
Enumeration of the total coliforms, faecal coliforms and E. coli levels in water
Levels of total coliforms and E. coli were enumerated using the 3 M E. coli/coliforms petrifilm count plates. Serial dilution of 1:10 dilution of sample collected was prepared using saline solution (0.85%). Briefly, the petrifilm plate was placed on level surface with top film lifted and using a pipette, 1 ml of sample was placed onto the center of bottom film. The top film was then rolled down to avoid entrapping air bubbles making sure the top film does not drop. With flat side down, spreader was placed on top film over innoculum and pressure gently applied on spreader to distribute innoculum over circular area before gel formed. Spreader was lifted and a minimum of one minute given for gel to solidify.
Plates were placed with clear side up in stacks of no more than 20. The incubator was humidified to minimize moisture loss. The plates were incubated for 24 h ± 2 h at 37°C.
Petrifilm plates were counted on a standard colony counter or other illuminated magnifier. Typical coliforms colonies appear pink/red whereas the E. coli appear dark blue. The colonies were enumerated, characterized and recorded. The results were expressed as the number of total coliforms and E. coli in 100 ml of water.
Isolation, purification and characterization of bacterial isolates
Colonies were purified by sub-culturing using streaking method. Characteristic E. coli and other coliforms colonies from the petrifilm plate were picked using a sterile wire loop and streaked onto Mac-Conkey Agar (HIMedia Lab. Pvt. Mumbai, India).
The inoculated Mac-Conkey plates were then incubated at 37°C overnight. Four distinct lactose and non-lactose fermenters colonies based on morphological characteristics were further picked and streaked onto nutrient agar (HIMedia Lab. Pvt. Mumbai, India) and incubated at 37°C. Preliminary characterization was performed using morphological characteristics as described by (Holt et al., 1994). Morphological identification of the isolate was done under the dissecting and compound microscope to observe cell size, shape and arrangement characteristics after classical staining of bacteria (Bartholomew, 1962). Biochemical tests that were also conducted included; citrate utilization using Simmons Citrate Agar test all from HIMedia Lab. Pvt. Mumbai, India, , motility, indole and lysine (MIL) test and Triple Sugar Iron agar (TSI Agar test) for H2S production and sugar utilization test. The biochemical tests were used to classify the isolates to genera level (Cheesbrough, 2002).
Statistical analysis
Data was subjected to analysis by majorly using Correlation analyses and ANOVA analyses. Statistical Package for the Social Sciences (SPSS) version 20 for Windows was used to calculate means and standard deviations and the data tabulated. Pearson product moment correlation procedure was used to perform correlation analysis and determine whether there were significant relationships between different physicochemical parameters, total coliforms and E. coli levels.
To check whether there was any significant difference between the values of physico-chemical parameters at different stations, one way Analysis Of Variance (ANOVA) was performed.
General overview
Assessment of Omubhira stream environment during study revealed that sampling locations were mostly frequented by the nearby residents and livestock as watering points and especially strata four (4S) that represents downstream. Observations also revealed that some people living in the stream basin and downstream use stream to water their crops with a number of fishponds constructed along the stream.
Physico-chemical water quality gradients along the Omubhira stream course in Kakamega town
Data obtained from all samples was in triplicates. Calculation of mean and the average for each stratum was calculated. The results were presented in tables. Table 1 shows the overall averaged mean values of physico- chemical and bacteriological characteristics of the examined water samples. Results obtained for most of the physico-chemical parameters conformed to the NEMA and WHO standards for drinking water quality except for the total coliforms and E. coli that were above the recommended standards.
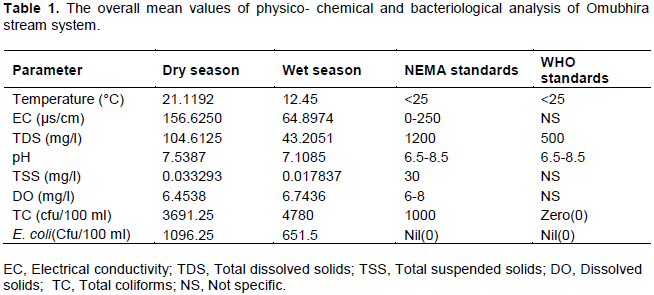
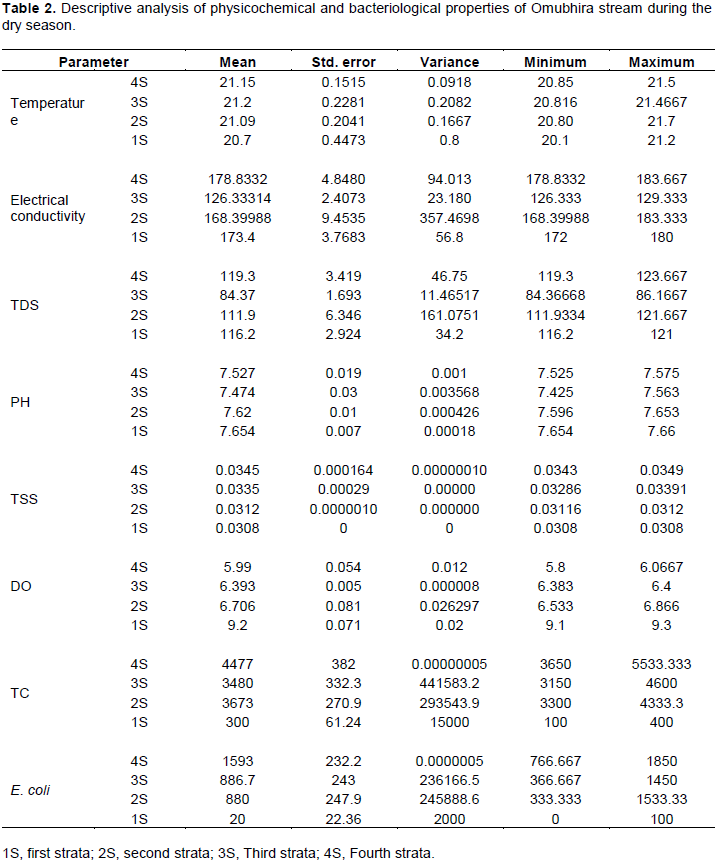
Table 2 shows the descriptive analysis of the physicochemical and bacteriological properties of Omubhira stream during the dry season in regard to the strata along the stream. The physic-chemical conditions in the four strata conformed to the NEMA and WHO standards for water with 3S recording the lowest mean value of electrical conductivity and the total dissolved solids. Bacteriological analysis however indicated that the mean values of total coliforms and E. coli were above the recommended standards with 4S recording the highest values while 1S the lowest mean values with a minimum of zero value.
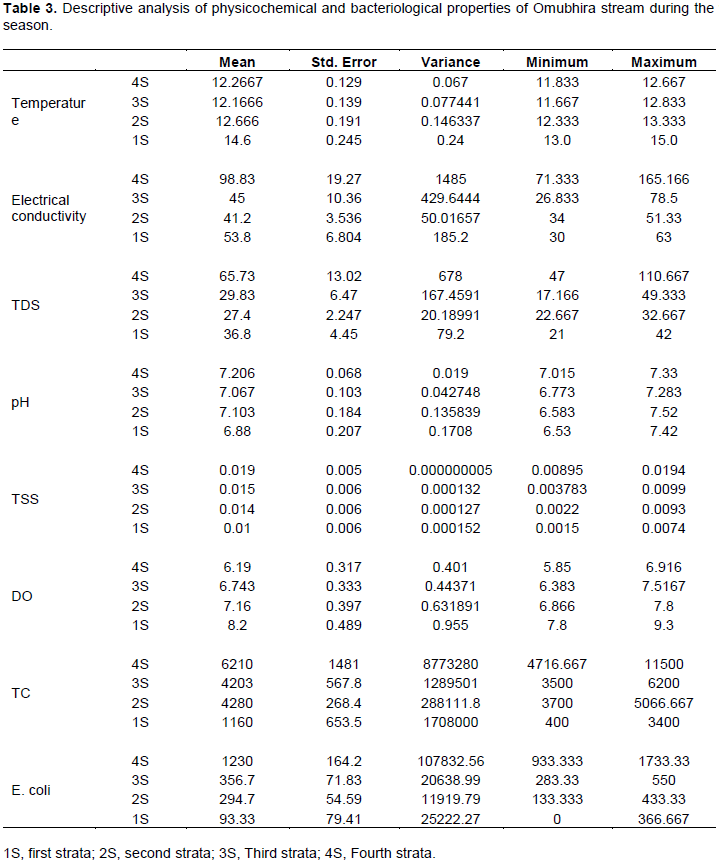
Table 3 indicates the descriptive analysis of physicochemical and bacteriological properties of Omubhira stream during the rainy season in regard to the strata along the stream. The physico-chemical properties conformed to the NEMA and WHO standards for water with fourth stratum (4S) recording the highest mean value for electrical conductivity and total dissolved solids. However, bacteriological analysis indicated that the mean values for total coliforms and faecal coliforms (E. coli) were far above the recommended standards for drinking water with 4S recording the highest values while 1S recording the lowest mean value with a minimum of zero value.
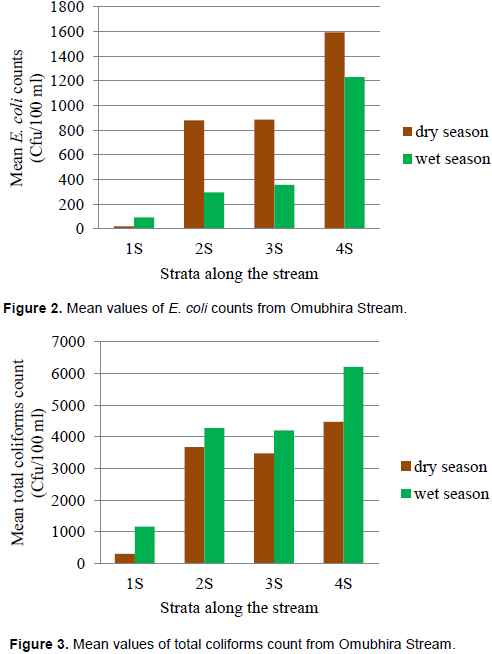
Figure 2 shows the mean values of faecal coliforms (E. coli) counts during the study periods. The values range from 20- 1593 Cfu/100 ml during the dry season and 93-1229 Cfu/100 ml during the wet season. Fourth stratum (4S) recorded the highest counts in both seasons while first stratum (1S) recorded the least in both season with a minimum of zero (0) coliforms count. Generally, the mean coliforms counts were higher than the WHO standard (≤ zero Cfu/100 ml) for water for recreational use.
Figure 3 shows the mean values of total coliforms counts during the study periods. The values range between 300 and 4476 Cfu/100 ml during the dry season and 1160 to 6209 Cfu/100 ml during the wet season. Fourth strata (4S) recorded the highest in both seasons while first strata (S4) recorded the least in both season. The total coliforms counts were above the recommended WHO and NEMA standard for water for recreational use.
During the wet season, Pearson product-moment correlation showed a relationship between E. coli and some physico-chemical properties of Omubhira stream. There was a strong, positive correlation between E. coli and electrical conductivity. However, there was no relationship between E. coli and temperature, dissolved oxygen and pH since there was no statistical significance.
During the dry season, Pearson product-moment correlation showed that there was a strong relationship between E. coli and dissolved oxygen, total dissolved solids and electrical conductivity (Table 4).
Figures 4 and 5 shows the different microorganisms recovered and identified from stream water in both the wet and dry season. These included Escherichia coli, Enterobacter spp., Citrobacter spp., Salmonellae spp., Providencia spp., Proteus spp., Klebsiella spp., Shigella spp., Morganella spp. and Serratia spp. Microorganisms recovered were present in both wet and dry season accordingly except for Citrobacter freundii and Proteus spp. Escherichia coli was the most predominant enterobacterial isolate in both seasons and across all the study sites. This showed that there was a common source of these microorganisms.
The physico-chemical parameters of water in Omubhira stream exhibited significant variations in the dry and wet seasons. Furthermore, there were spatial variations of the physico- chemical parameters from upstream to downstream.
All the physico-chemical parameters studied were within the maximum permissible limit as per the WHO standards (WHO, 2006) and NEMA regulations for water for recreational use. Electrical conductivity, TDS and TSS had higher values in the dry season than in wet season.
However, strata four (4S) that is situated at the lower reaches of the stream had significantly high mean levels
of electrical conductivity and TDS than in the other three sampling strata. High levels of electrical conductivity and total dissolved solids could have been due to the high organic load, which in this study was from the wastewater effluent discharge and livestock watering points. This corresponds to a study on Nyangores stream, Mara river basin whereby the levels of electrical conductivity, total suspended solids and total dissolved solids increased downstream with the low temperature, conductivity, TSS and BOD levels recorded in upstream station increasing downstream due to the increased run-off from agricultural activities and sewage effluents (Gichana et al., 2014)
The temperature values obtained attributed mainly to the atmosphere and weather conditions of the study area. Water temperature variation at the various sampling sites could have been influenced by the differences in the quantity of water present in the site and due to the presence of vegetation shielding water source from direct radiation.
High temperatures during the dry season could have been influenced by factors that majorly include solar radiation and turbidity. Bacteriological and physico-chemical studies of the rural catchments of the Lake Victoria showed high temperature levels during the dry season (Ouma et al., 2016). In general, the entire Omubhira stream system surface water samples had temperature means that were within the permissible limit in both seasons and averagely suitable for survival of most tropical aquatic organisms.
Increased turbidity increases water temperature (Ogendi et al., 2015). This was observed during the dry season whereby defecation by livestock directly into the water during feeding and watering increased the amount of suspended solids which absorbed heat from solar radiation and thus increasing temperature
During this study, it was observed that compared to other types of water sources, this water source was mainly located in high altitude areas where they are on most occasions sheltered by trees. Such an environment is likely to experience cool air, which influences water temperature.
This study showed negative correlation between temperature and faecal coliforms and therefore is in agreement to the assessment of physico-chemical properties and sewage pollution indicator bacteria in surface water of River Gomti (India) whereby temperature showed significant negative correlation with faecal coliforms, total coliforms and biological oxygen demand (Anukool and Shivani, 2011).
Electrical conductivity values were higher in the dry season when compared to the rainy/wet season. This is consistent with the findings in River Moiben, Kenya whereby electrical conductivity values were higher in dry season (Masese et al., 2009). This was due to the high solute concentrations in dry season because of evapo-transpiration losses from the channel. Moreover, run-off is supplied from ground water reservoirs, where water has a long residence time and solute release is prompted. However, during the rainy season, run-off is generally flows much more rapidly to the stream channel, has less opportunity for solute pick up and therefore has lower dissolved solids content.
The values of electrical conductivity observed during sampling periods are however within the range prescribed by WHO (WHO, 2006). The correlation coefficient for the electrical conductivity and dissolved solid values is 0.99 which implies that the presence of the total dissolved solids is a major contributing factor to the electrical conductivity of water. However, relatively high conductivity of water observed at some sampling sites could have been due to the effect of released effluent from sewage treatment plant (Gichana et al., 2014)
This study showed significant correlation between conductivity and coliforms and therefore is in agreement to a study of physico-chemical properties and sewage pollution indicator bacteria in surface water of River Gomti (India) whereby results revealed that EC showed significant positive correlation with faecal coliforms, total coliforms and biological oxygen demand (Anukool and Shivani, 2011).
Oxygen availability in an aquatic ecosystem is an indication of the systems health and general well-being and dissolved oxygen usually reflects the physical and biological processes prevailing in the water (Cohen and Hillel, 1972). Dissolved oxygen values for the fourth stratum (4S) of 5.99 and 6.12 mg/l in dry and wet season respectively indicated that there was slight pollution occurrence at this sampling location. The decreased dissolved oxygen levels downstream could have been attributed to the high organic load and slightly due to increased water temperature that decreases solubility of oxygen in water (Gichana et al., 2014). DO values of 9.2 and 8.2 for the dry and wet season respectively recorded at the first stratum (1S) which is the source of the stream indicated that the waters at this sampling location were high quality water. Dissolved oxygen concentrations above mug/l in all the studied strata implies that the water is not stressful to fish growth since fish kills are usually observed at below 5 mg/l concentrations and thus suitable for fish farming.
TDS values in this study were within the prescribed limit given by WHO and the NEMA. This could not interfere with the osmo-regulation of fresh water organisms in the stream. Excess sediment can harm the water quality since high level of solids in water increases water density and affect osmo-regulation of fresh water organisms thereby reducing the solubility of gases such as oxygen.
The TSS levels were high for the dry season than for the wet season. This could have been due to the stampede of the stream basin by the livestock that are directly watering and feeding just by the stream banks during the dry season. However, low values of TSS during the wet season could account for the reason why the entire appearance of the water samples was clear, not turbid and having no odour.
The Omubhira stream had a measured pH ranging from 6.88 to 7.654 .The pH of water samples indicated that it was within the range set by WHO. pH influences the survival of aquatic organisms in the water bodies since their metabolic activities are pH dependent and drastic changes in pH can have detrimental effects on stream health (Ouma et al., 2016). The presence of total and faecal coliforms counts in the stream water indicated contamination by raw sewage or defecations in the bush in the catchment or rather defecation by livestock during watering. This was similar to the findings in the study of Nyanchwa- Riana River (South West Kenya) (Ogendi et al., 2015).
The average mean for total coliforms and E. coli (faecal coliforms) counts were far above the NEMA and WHO recommended standards for bacteria for portable and recreational water. Moreover, the four strata representation of the stream also recorded TC and E.coli counts that were above the recommended NEMA and WHO standards for drinking water. Generally, higher mean counts for TC were recorded in the wet season than in the dry season while E. coli (faecal coliforms) mean counts were higher in the dry season than in the wet season.
High flows in streams tend to increase bacterial counts due to run-offs (Muhibbu et al., 2011) However, high levels of E. coli counts were recorded in the dry season compared to the wet season. The high levels of E. coli could have been due to increased turbidity from suspended particles during stampede by livestock during watering and feeding at the stream banks, which facilitate the survival and growth of coliforms bacteria as they are protected from ultra violet radiation and attack by bacteriophage (Medema et al., 2003). Moreover, the high counts in the dry season compared to the wet season of E. coli could be associated with rainfall occurrences that diluted and weakened the effects of point source pollution. While also increasing the contribution of non- point sources or diffuse pollution through land run-off from agricultural fields and leaches from refuse dumps. (Muhibbu et al., 2011). The high mean E. coli counts during the dry season could be associated with non- human warm-blooded animals’ origin since domestic animals especially cows were a common precincts of sampling locations considered as watering and feeding points. This is in agreement with findings in the study of patterns and sources of faecal pollution in the heavily impaired river Njoro watershed (Kenya) (Jenkins, 2008). Moreover, E. coli counts were associated with humans as a result of the open defecation evident along the stream and sewage treatment plants discharge point (Jenkins, 2008).
Concentrations of coliforms counts studied shows a strong increase from the upstream (source) (S1) to downstream (4S). This indicates that there was input of raw sewage or animal waste at certain points along the stream transect. Zero E. coli counts at the source of the stream (S1) during various sampling occasions may be an indication that stream source is clean, safe and not contaminated. Generally, the findings of the total coliforms (TC) and E. coli counts revealed that the human activities on most occasions increased the bacterial load of the water. Although total coliforms organisms may not always be directly related to the presence of faecal contamination or pathogens in the drinking water, this study found that all water samples contained both total coliforms and E. coli.
In addition, the Omubhira stream is not well protected from direct access by animals, because most of the animals move along the laggas in search of water, salt licks and pasture. In the process, they deposit a lot of organic wastes directly into the stream or rather on the laggas floor. When it rains, the seasonal floods wash off bacteria and organic water into the Omubhira stream hence contaminating them (Musyoki et al., 2013)
However, presence of a significant difference in TC and E.coli counts in Omubhira stream samples from the four strata suggests the level of hygiene in the different sampling sites.
According to Kavka et al. (2006), although some community members enclose their streams to protect them from direct faecal contamination by livestock, the high population of livestock and wildlife that visit the stream beds at different times exposes the stream to some contamination implying that each morning, these streams have to be cleaned before drawing drinking or livestock water. The fourth strata (4S) that represents the downstream had the highest loads of total coliforms and E. coli, while the first stratum had the least and at some occasions zero counts. Microbiological studies of river Danube showed similar characteristic with high levels of faecal pollution being particularly downstream with the main sources of pollution being raw discharges, discharges from wastewater treatment plants, impaired tributaries and impact by diffuse sources. It is therefore clear that should the water be qualified as portable, Omubhira stream must be fully protected from pollutants accordingly from its source in the first stratum to downstream at the fourth stratum.
Various bacterial isolates of public health concern were also identified from stream water samples in this study. E. coli were the most predominant enterobacterial isolate during both wet and dry seasons and across all the study sites. It is evident that the occurrence of pathogenic organism in stream water indicates the contamination of stream water with human or animal wastes and thus of public health significance (Shitu et al., 2008). Though these bacteria are naturally found in the intestinal tract, in soil and water, they can cause primary and opportunistic infections in humans and animals (Cheesbrough, 2002). Most are faecal-oral route transmitted and cause number of diseases from diarrhoeal, urinary tract infections, inflammation and ulceration of intestinal tract, enteric fever to chest infections. However, Serratia spp., though found mostly in soil and water, has been reported to cause pulmonary and urinary infection.
Although the physico-chemical water quality of Omubhira stream was within the acceptable limits as per NEMA and WHO standards, the bacteriological water quality was above the recommended standards levels. This implies that the application of physico-chemical water quality analysis of water alone cannot be used to determine the safety of water as portable and suitable for recreational purposes. The widespread practice of cattle watering in the Omubhira stream appears to be one of the major causes of gross faecal pollution in the watershed. Defecation by cattle while drinking water directly from the stream and the transport of the faecal matter during major rainfall- run-off events of accumulated cattle faecal deposits from contributing areas along stream are likely to explain large spikes in faecal contamination during peak run-off .This may pose a health risk to several communities which rely on the receiving water body primarily as their source of domestic water. Therefore, control of human activities to prevent faecal matter from entering water body is the key to avoiding bacterial contamination. Though the bacterial levels render stream water unfit for human consumption before treatment, the water can be used for other purposes depending on the particular use.
The authors have not declared any conflict of interests.
The author extends their thanks to the microbiological department for the effort during microbial analysis.
REFERENCES
|
American Public Health Association (APHA) (2005). Standard methods for the examination of water and wastewater. 20th Edition. Published jointly by the American Public Health Association, American Water Works Association, and Water Environment Federation. Washington, D.C.
|
|
|
|
Anukool S, Shivani S (2011). Assessment of Physico-Chemical properties and sewage pollution indicator bacteria in surface water of River Gomti in Uttar Pradesh. International Journal of Environmental Sciences 2(1):325.
|
|
|
|
|
Cheesbrough M (2002). District Laboratory Practice in Tropical Countries- Part 2. Cambridge University Press.
|
|
|
|
|
Cohen J, Hillel IS (1972). Coliforms, Faecal Coliforms, and Faecal Streptococci as indicators of water pollution, Environmental Health laboratory. Hebrew University. Israel.
|
|
|
|
|
Eze SO, Madumere IC (2012). Physicochemical and microbiological analysis of water bodies in Uturu, Abia State-Nigeria. Asian Journal of Natural and Applied Sciences 1(4):58-65.
|
|
|
|
|
Gichana ZM, Njiru M, Raburu PO, Masese FO (2014). Effects of Human Activities on Microbial Water Quality in Nyangores stream, Mara River basin. International Journal of Scientific and Technology Research 3(2):153-157.
|
|
|
|
|
Jenkins MW (2008). Gross fecal pollution of a rural watershed in Kenya: Research identifying cattle as a major source in the River Njoro Watershed. Research brief, 08-01. Available at:
View
|
|
|
|
|
Kavka GG, Kasimir GD, Farnleitner AH (2006). Microbiological water quality of the River Danube (km 2581-km 15): Longitudinal variation of pollution as determined by standard parameters. Available at:
View
|
|
|
|
|
Masese FO, Muchiri M, Raburu PO (2009). Macroinvertebrate assemblages as biological indicators of water quality in the Moiben River, Kenya. African Journal of Aquatic Science 34(1):15-26.
Crossref
|
|
|
|
|
Medema GJ, Shaw S, Waite M, Snozzi M, Morreau A, Grabow W (2003). Catchment characterization and source water quality. In Assessing Microbial Safety of Drinking Water. World Health Organization Geneva, Switzerland. pp. 111-158.
|
|
|
|
|
Muhibbu-Din OI, Aduwo AO, Adedeji AA (2011). Study of physiochemical parameter of effluent impacted stream in Obafemi Awolowo University, Ile-Ife, Nigeria. Published Msc Thesis.
|
|
|
|
|
Musyoki MA, Suleiman MA, Mbithi JN, Maingi JM (2013). Waterborne Bacterial Pathogens in surface waters of Nairobi River and Health Implications to communities downstream Athi River.
View
|
|
|
|
|
Obi CL, Potgieter N, Bessong PO, Matsaung G (2003). Scope of potential bacterial agents of diarrhoea and microbial assessment of quality of river water sources in rural Venda communities in South Africa. Water Science and Technology 47(3):59-64.
Crossref
|
|
|
|
|
Ogendi GM, Getabu AM, Onchieku JM, Babu JM (2015). An Assessment of Some Physical, Chemical and Biological Characteristics of Nyanchwa- Riana River Flowing through Kisii Town in South West Kenya. International Journal of Applied Science and Technology 5(2):68-79.
|
|
|
|
|
Ouma SO, Ngeranwa JN, Juma KK, Mburu DN (2016). Seasonal variation of the physicochemical and bacteriological quality of water from five rural catchment areas of lake victoria basin in Kenya. Journal of Environmental Analytical Chemistry 3:170.
|
|
|
|
|
Shitu OB, Olaitan JO, Amusa TS (2008). Physico-Chemical and Bacteriological Analyses of Water Used for Drinking and Swimming Purposes in Abeokuta, Nigeria. African Journal of Biomedical Research 11:285-290.
|
|
|
|
|
Vail JH, Morgan R, Merino CR, Gonzales F, Miller R, Ram JL (2003). Enumeration of waterborne Escherichia coli with Petrifilm plates. Journal of Environmental Quality 32(1):368-373.
Crossref
|
|
|
|
|
Water Service Regulatory Board (WASREB) (2006). Drinking Water Quality and Effluent Monitoring Guidelines, Water Service Regulatory Board, Kenya. Available at:
View
|
|
|
|
|
Wawire SA, Miruka DO, Nelson N, Ofulla A (2013). Antimicrobial susceptibility patterns of Enterobacteriaceae isolated from domesticated animals and the environment in Lake Victoria, Kenya. Ecohydrology and Hydrobiology 13(4):246-252.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2006). Guidelines for drinking-water quality. Vol. 1, Recommendations: addendum, 3rd ed. Geneva: World Health Organization. Available at:
View
|
|