Full Length Research Paper
ABSTRACT
In order to assess the diversity, regeneration and structure of wild edible fruit trees, a study was carried out in the Laf-Madjam forest reserve and its surroundings. A semi-structured interview with local residents and a floristic inventory of woody plants (8 transects of 1000 m × 20 m each) were carried out. 24 species were cited as being used by the local residents. A total of 2134 individuals subdivided into 69 species, 43 genera and 27 families were counted, including 448 edible fruit individuals divided into 25 species, 19 genera and 14 families. The latter with important IVIs are: Balanites aegyptiaca , Sclerocarya birrea , Ziziphus mauritiana and Hexalobus monopetalus. Diversity is medium in edible fruit trees (HSI=3.10 bits, E= 0.67). Fruit trees with individuals of diameter ≥ 21 cm are in the majority. Inverted "L" and bell-shaped gaits are observed. The regeneration rate of fruit trees is higher in Annona senegalensis , H. monopetalus , and B. aegyptiaca . The involvement of local people in the management of the reserve's fruit trees is important for sustainable management.
Key words : Edible fruits, Moutourwa, sustainable management, structure, phytodiversity.
INTRODUCTION
The Central Africa forests are sources of income for the countries of the sub-region in both the formal and informal sectors (Eba'a et al., 2011). In most countries of the Congo Basin, the exploitation of forest resources remains the main provider of private wage employment, particularly in remote rural areas. Most scientific work recognises many benefits of forests, which are classified under the theme of ecosystem services (M.E.A, 2005; Arrif et al., 2011). These services include reducing air pollutants (Nowak et al., 2006), reducing heat islands (Akbari et al., 2001), enhancing precipitation and CO2 absorption, regulating local climate and mitigating global climate change (Laporte and Cordeau, 2007). They also improve the environmental quality of localities on which people's health depends (Jim and Chen, 2008).
In addition, more than half of the world's population lives in urban areas and uses forest resources to meet their needs (Véron, 2007). These high anthropogenic activities are very accentuated and the direct consequence is the marked deterioration of these resources on green spaces. The phenomenon is more noticeable in developing countries, where the sensitivity of city dwellers to the presence of vegetation becomes weaker as the city becomes more densely built (Rusterholz, 2003). This environmentally damaging situation is also observed in Cameroon (Wolff, 2005). The creation of protected areas in Cameroon is in line with the government's desire to preserve ecosystems in order to maintain biological diversity. The Government of Cameroon's objective in terms of biodiversity conservation is to eventually convert 30% of the national territory into protected areas. These include national parks, nature reserves, and zoos (Tieguhong and Betti, 2008). In Cameroon, the total area of protected areas covers more than 17% of the national territory. They are located in both forest and savannah ecosystems. The Far North Region in particular, characterised by a low vegetation cover and very aggressive, has a vast network of protected areas made up of 5 forest reserves and 3 national parks which are also subject to strong anthropic pressure for the exploitation of resources (Wafo, 2008; Fotsing, 2009). The boundaries of the protected areas have greatly regressed and are poorly managed, and the lack of involvement of local populations in management is noticeable (Barbault, 2007). The reserves in the Far North Region, such as those in the Moutourwa locality, have been the scene of abusive exploitation of resources for decades. The latter, despite its status, is threatened by human activity, further accentuated by the unsustainable exploitation of plant species in general and edible fruit trees in particular.
The dry zones of sub-Saharan Africa are characterized by a long dry season and a short rainy season each year. In addition, the Far North Region of Cameroon is characterized by a population explosion and aridity of the soil. This situation is partly responsible for the scarcity of foodstuffs. The populations living in the vicinity of the Laf-Madjam reserve enter this reserve to collect non-timber forest products (NTFPs) such as edible fruits. This exploitation is anarchic and does not allow for the sustainability of these fruit trees and it is important to evaluate them. The real problem here is the unsustainable use of fruit species in the Laf-Madjam Forest Reserve. Several studies have been conducted in and around the reserve, such as those by Fotsing et al. (2003) and Todou et al. (2016). These authors focused their studies on the state of the reserve and riparian areas and on the floristic diversity in this area. Little is known about the floristic diversity of harvested edible fruit trees in this reserve. This study contributed to the development of edible fruit trees for their effective conservation and sustainable use in the Moutourwa district. Thus, it was a question of inventorying the edible and non-edible fruit trees of the reserve and its surroundings, but also of determining the diversity of fruit species exploited by the local population in the reserve and its surroundings.
MATERIALS AND METHODS
Study site
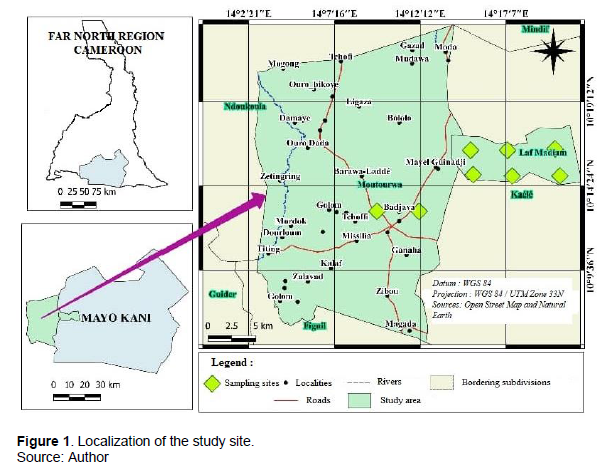
The district of Moutourwa is located in the division of Mayo Kani, Far North Region. It is located between 10° 09' 36 '' - 10° 19' 12 '' North latitude; and between 14° 2' 21'' - 14° 17' 7'' East longitude (Figure 1). The climate of the Moutourwa council is the Sudano-Sahelian type and is characterized by 2 seasons: a long dry season, from October to May and a rainy season, from June to September. The average annual rainfall is 867 mm and the average annual temperature is 27°C, with a maximum of 38°C from March to April and a minimum of 18°C from December and January (Suchel, 1987). Four types of soils with different characteristics that are threatened by the effects of rain and especially wind erosion. The woody and herbaceous vegetation has elements of Sudano-Sahelian savannahs, dry savannahs and Sahelian steppes (Letouzey, 1968). The flora is characteristic of the thorny steppes, consisting of tree and shrub savannahs with a very irregular herbaceous cover.
Data collection
Ethnobotanical survey
This phase, carried out in September 2020, consisted of administering a questionnaire using the tools of the Accelerated Participatory Research Method (APRM) and a participatory approach described by Sunderland (1998) and Guedjé (2002). These surveys were complemented by direct field observations. They were conducted with local populations and actors involved in the exploitation of resources. The choice of interviewees was random. This interview targeted all categories of people. In total, 4 villages were selected for this survey (Laf, Moulva, Mayel Guinnadji and Bajava) and 117 people were interviewed. The overall survey approach made it possible to gather the following information the demographic and socio-economic characteristics of the populations of the Laf-Madjam forest reserve, the peasant perception and importance of the forest reserve for the local population, the main human activities practiced in the forest reserve, the most important edible fruit species for the local population, the main NTFPs harvested, the state of the vegetation, the management and development of the vegetation in the area, as well as the priority edible fruit species for the local pharmacopoeia. Data on the use of plant species in all areas were collected.
Floristic inventories
The inventory of plant species was done in 8 transects of size 1000 m × 20 m and consisted of counting all woody individuals present in the different types of plant formations. Following along a line of layons, members of the inventory team scanned the transect to identify, count, measure, and observe individuals of woody species encountered using an inventory form. Each species was identified by its scientific and/or local name. For unidentified species, samples were taken and then pressed to make the herbarium. Subsequently, these species could be identified with the help of appropriate documents such as the flora of Arbonnier (2009), but also by the botanists of the Faculty of Science. In addition to counting, dendrometric measurements (trunk circumference at 1.30 m from the ground and height) were taken for edible fruit trees.
Data processing and analysis
The species composition of the different plant formations was described using the following parameters and formulas: (i) The relative frequency (RFr) (Braun-Blanquet, 1932): RF = (Ai/B) ×100, where Ai=number of plots containing the species i and B=total number of plots sampled; (ii) relative dominance (RDo) (Dona et al., 2016): RDo= πDi²/4, RD = relative dominance; Di = diameter at breast height of the specie i; π=3.14; (iii) relative density (RDe) (Ngom et al., 2013): RDe = Pi×100, where Pi=ni/N, ni is the number of individuals belonged to taxon i and N is total number of individuals of all sampled plots; (iv) relative diversity (RDi): RDi = number of species of one family×100/total number of species; (v) density (stems/ha) (Ngom et al., 2013): D = ni/A where ni is the number of individuals belonging to species i and A is the area in hectares; (vi) basal area (BA) (Devineau, 1984): BA= (∑?Di)²/4, where D is diameter at breast height and S in m²/ha; (vii) importance value index (Cottam et Curtis, 1956): IVI=relative abundance + relative frequency + relative dominance; (viii) regeneration rate (Koulibaly, 2008; Konan, 2009): Tr = (n/N) × 100; n is the total number of juvenile individuals with a diameter less than or equal to 10 cm and N is the total number of individuals in the vegetation unit; (ix) species rarity index (Kokou et al., 2005): RI = (1-(ni/N)) ×100 with ni: the number of records in which species i occurs and N the total number of records. The Fisher test was used to compare some of the different means.
Specific diversity of the site was described using the following indices: Shannon and Weaver index (Frontier and Pichod-Viale, 1995): H' = -Σ ni/N × log2 ni/N With ni is the proportion of a species i to the total number of species (N). Pielou evenness index (Dajoz, 1982): E = H'/log2 N or H'/Hmax, with H' the Shannon diversity and N the species richness. Sorensen’s coefficient of similarity (Dajoz, 1985; Krebs, 1999): Ks= (2c/a+b) × 100, where a is the number of species in a list belonging to site A; b is the number of species in a list belonging to site B; and c is the number of species common to the two sites (A and B).
RESULTS
Medicinal plants reported and diseases treated
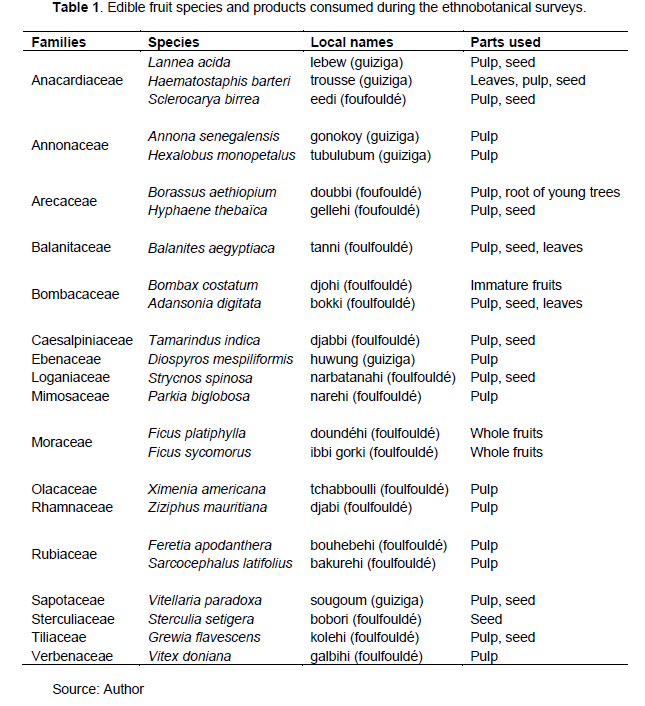
The ethnobotanical surveys among the local populations showed that 24 species divided into 23 genera and 17 families were cited as being used by the local people (Table 1). The most represented families were Anacardiaceae (3 species), Annonaceae, Arecaceae, Bombacaceae, Moraceae and Rubiaceae (2 species each). Some organs cited by these populations are consumed directly while others are first processed before use. These include whole fruits, roots, leaves, seeds and pulp. The results showed that the pulp is the part of the fruit tree most used by the local people.
Edible plants used in traditional pharmacopoeia
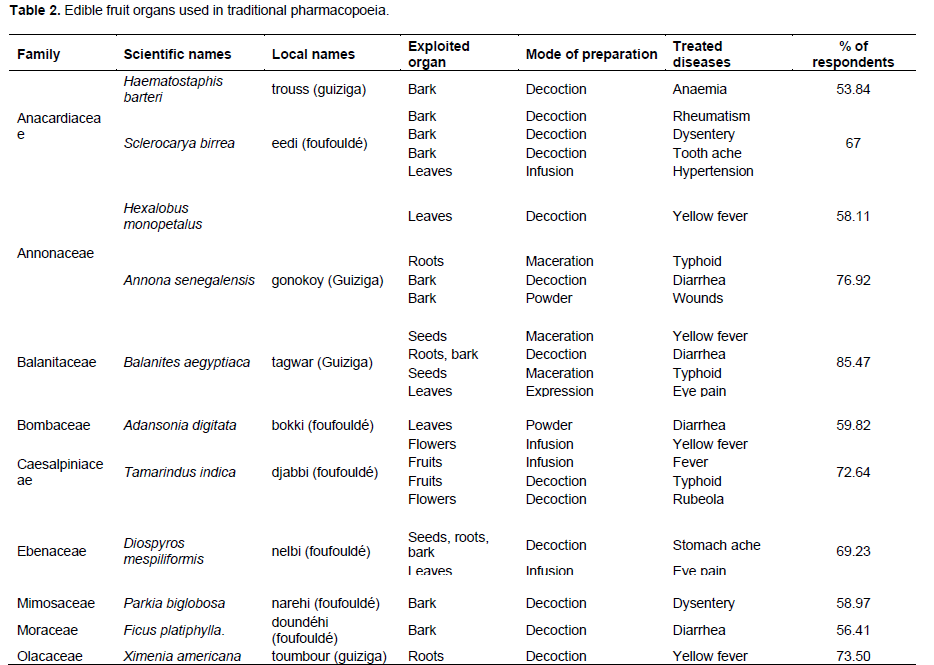
For medicinal purposes, the local population uses several fruit trees. The organs used and the method of preparation vary according to the species and the diseases. The most used species in the traditional pharmacopoeia was Balanites aegyptiaca (85.47%), followed by Annona senegalensis (76.92%), Ximenia americana (73.50%) and Tamarindus indica (72.64%) (Table 2). The most frequent diseases in the study area encountered were: typhoid, diarrhoea and jaundice (1.12% each). The most used method of preparation was decoction (4.48%), followed by infusion and maceration (1.12% each). As for the organs, bark (4.34%) and leaves (1.55%) were the most used in the recipes (Table 2).
Floristic composition of woody plants
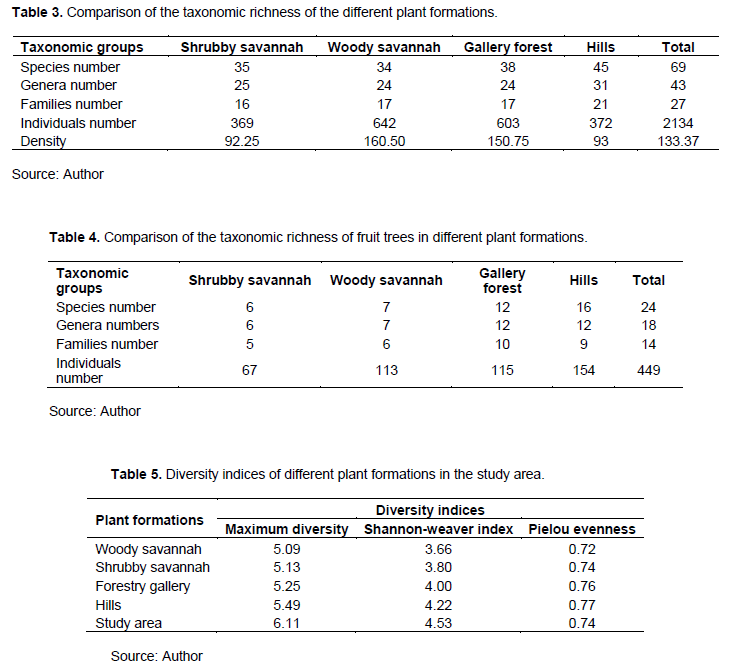
A total of 2134 individuals were inventoried in the Laf-Madjam forest reserve and its surroundings with a total density of 133.37 individuals/ha (Table 3). These individuals are subdivided into 69 species divided into 43 genera and 27 families. The Combretaceae family has the highest number of species (10 species), followed by the Mimosaceae (8 species) and the Anacardiaceae with7 species.

Taxonomic richness of edible fruit trees
A total of 449 individuals of edible fruit trees were recorded and divided into 24 species, 18 genera and 14 families (Table 4). The richest plant formation was hillside with 16 species followed by gallery forest with 12 species.
Species diversity
The Shannon-Weaver diversity index value was 4.53 bits for the study area and varied from 3.66 bits for the woody savannah to 1.22 for the hills (Table 5). The Pielou’s evenness index was 0.74 for the study site and varied from 0.78 for woody savannah to 0.77 for the hills.
For edible fruit trees, the Shannon diversity index calculated in all formations was 3.10 bits. This value varied from 1.10 in the shrubby savannah to 2.03 bits in the hills (Table 6). The Pielou evenness index was 0.67 in the study area. This value varied from 0.43 for the shrubby savannah to 0.50 for the hills and the tree savannah.
Sorensen's coefficient of similarity for all woody plants was ≥ 50% for all plant formations (Table 7) while for fruit trees only (Table 8), these values were ≤ 50% all plant formations.
Rarity index and species importance value index
The calculation of the rarity index of the species encountered in the different formations of Moutourwa and its surroundings shows that 2 species have a rarity index higher than 80%, Diospyros mespiliformis and Gardenia ternifolia rarity index of 87.5%. Among the species, Sclerocarya birrea was found in all 8 transects and had a rarity index of zero (RI = 0). The high abundance of certain species could be considered as a response of the rural society which considers them as a priority species(Sigaud and Eyog-Matig, 2001).
The ecological importance of the species here is assessed by the high value of their IVI. The species with high IVI values were: B. aegyptiaca (72.56), S. birrea (37.54), Ziziphus mauritiana (23.36) and Hexalobus monopetalus (22.38) (Table 9).
Density and basal area of fruit trees
The highest density value (38.24 stems/ha) and the lowest value (16.77 stems/ha) and the highest basal area (1.95 m2/ha) and the lowest value (0.94 m2/ha) were found, respectively in the hills and shrubby savannah (Table 10). Analysis of variance of absolute density and basal area showed a highly significant difference between formation types (p=0.05).
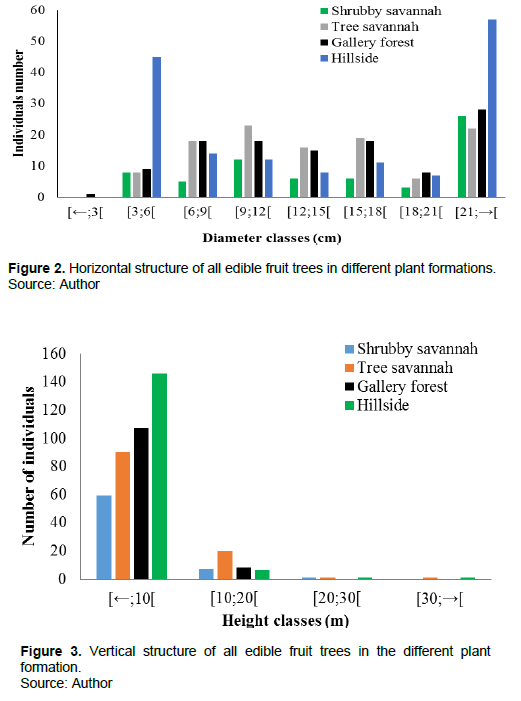
Horizontal structure of edible fruit trees
The distribution of individuals by diameter class in the study area shows some structural divergences clearly discriminated by the number of stems. In the present study, the structure of edible fruit trees in the savannah shrubland and edible fruit trees in the hillside showed 'inverted L' curves (Figure 2). The structure of edible fruit trees in the forest gallery and in the tree savannah showed a bell-shaped curve.
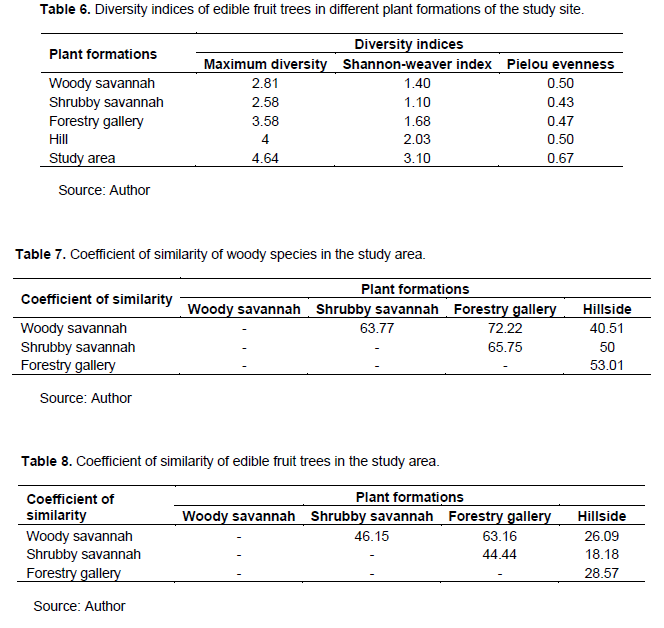
Vertical structure of edible fruit trees
The vertical structure of the plant population showed "L" curves with high proportion of individuals in the class height [←; 10[- in the different formations (Figure 3). This structure showed high proportion of young plants and low proportion of adult individuals.
Regeneration of species
A total of 141 individuals grouped into 11 species were recorded in the different formation types. In the shrubby and tree savannahs, the highest rate is observed in B. aegyptiaca (44 and 68.75%, respectively) (Table 11). In the forest gallery, the highest value is found in Z. mauritiana (66.67%), whereas in the hill, the highest regeneration rate is observed in A. senegalensis (74.04%).

DISCUSSION
Medicinal plants reported and diseases treated
The high use of the pulp by the local people can be explained by the fact that fruits in the form of pulp are the most represented in the study site. Moreover, the fruits most suitable for preservation are in pulp form. These different results corroborate those of Todou et al. (2017) who found 50 species used by local populations in the Mandara Mountains in Cameroon. Nyakabwa (1994) in the DRC also found that the pulp is the most used part of the fruit.
Edible plants used in traditional pharmacopoeia
The interest in leaves and bark can be explained by the fact that these plant organs are the seat par excellence of biosynthesis and even storage of secondary metabolites which is responsible for the plant's pharmaco-biological properties (Nacoulma Ouedraogo, 1996). Furthermore, Bitsindou (1986) attests that the high frequency of use of leaves is due to the ease of harvesting. These results are similar to those obtained by Ngbolua et al. (2019) who showed that leaves and bark were the most used. In addition, Saotoing et al. (2011) obtained 34% of the leaves commonly used in recipes. On the other hand, Gueye (2012) found in their respective works a high frequency of use of barks and roots. In general, all organs of medicinal plants relieve and cure diseases or ailments but uncontrolled use of certain organs such as roots and bark could cause damage to the plant and even its ecosystem. So, it would be better to use good harvesting techniques to preserve it for us and even for future generations.
Floristic composition of woody plants
The highest number of species, genera and families is found in the hills while the lowest number is found in the shrubby and tree savannas. This difference may be due to the existence of permanent anthropic actions in the shrub and tree savannahs while the hills are difficult to access. The dominance of Combretaceae can be explained by the fact that it is a characteristic family of savannahs and easily adapt to water stress. Oumarou (2012) has shown that the Combretaceae family represents the most diverse and abundant family in the savannahs. Considering the high number of species and individuals inventoried in the Laf-Madjam forest reserve and its surroundings, it can be said that this locality is rich in woody species. These results are similar to those of Todou et al. (2016) who found 75 species, divided into 54 genera and 28 families in the same locality. Similarly, Teicheugang (2000) recorded 75 species, divided into 46 genera and 24 families in the Zamay reserve. Froumsia et al. (2019) also found 66 species in 46 genera and 26 families in the unprotected Sudano-Sahelian zone in Cameroon. The similarity of these results may be due to similar microclimatic factors and anthropogenic pressures.
Taxonomic richness of edible fruit trees
The variation in species richness in the different plant formation observed can be explained by climate variation(Sarr, 2008), interspecific competition, resource availability and the level of disturbance (Palmer, 1994). It is also observed following the existence of permanent anthropic actions in the shrubby and tree savannahs while the hill is difficult to access. This specific richness was similar to the work of Todou et al. (2017) who found 38 wild edible fruit species grouped into 29 genera and 19 families in the Far North of Cameroon. These results showed a significant wealth of edible fruit trees in the Laf-Madjam forest reserve, hence the need to valorise them in order to use them rationally.
Species diversity
Based on the calculated diversity indices, the study area had high diversity because the Shannon index was high and the Pielou evenness is near to 1. These values indicate a homogeneity of the stand. In the different vegetation formations, it can be seen that diversity is important everywhere because the Shannon index varies from 3.66 to 4.22 with the hill having a higher diversity.
Considering the values of the different diversity indices, it appears that the diversity of edible fruit species is moderate in the study area, but in each plant formation it is found to be very low, slightly less in the hills. This index is different from that found by Mapongmetsem et al. (2016) in the agroforests of the peri-urban area of the city of Bafia in the Central Cameroon Region (3.33 bits). In terms of Pielou equitability, it is 0.67 across the study area (0.50 for hill and tree savannah, 0.47 for forest gallery and 0.43 for shrub savanna). These values are lower than those of Todou et al. (2016) who found 0.82 in the uncultivated plain of Moutourwa located in the Sahelo-Sudanese sector of Cameroon. These results suggest that edible fruit species are equitable in the study area. Sorensen's coefficient of similarity for all woody plants was ≥ 50% for all plant formations and confirmed that these plant formations belong to the same plant communities while for fruit trees only, the values were ≤ 50% all plant formations and confirmed that these plant formations belong to different plants communities.
Rarity index and species importance value index
In the North Cameroon Region, 55 indigenous edible fruits were identified by Mapongmetsem et al. (2012). Among these fruits, B. aegyptiaca was one of them. These 55 fruits were among the most prized and traded in the region. Similarly, Kristensen and Balslev (2003) in Gourounsi, Burkina Faso, note that the availability of most fruits coincides with a decline in food supplies. At this time, the availability and consumption of wild fruits is a considerable contribution to household diets. Furthermore, Guinko and Pasgo (1992) state that wild fruits contribute to a varied diet in terms of vitamin intake. These results suggest that the consumption of these fruits therefore saves on very low agricultural produce to ensure year-round feeding. For this reason, it would be important to preserve the reserve's highly endangered fruit trees.
Density and basal area of fruit trees
These results are confirmed by Birnbaum (2017) who indicated that savannah tree formations are subject to anthropic influences. The high human pressure in the formations could explain the low presence of breeding individuals due to abusive logging. Comita et al. (2007) agree, saying that the higher the number of breeding individuals, the higher the overall density.
Horizontal structure of edible fruit trees
The general diametric distribution of edible fruit trees in the Laf-Madjam reserve showed that individuals with a diameter ≥21 cm were in the majority. It shows an "inverted L" distribution in some plant formations and a bell-shaped distribution in others. This type of distribution is similar to that obtained by Mapongmetsem et al. (2011) in Vitellaria paradoxa in the high Guinean savannahs. In the first case, this type of distribution shows that individuals with a large diameter are in the majority; this can be explained by anthropogenic actions such as bush fires and grazing, as juvenile species are destroyed at the expense of adults. In the second case, there is a dominance of individuals with a small diameter; this may show the good regeneration of these species. This may also be due to the strong anthropic pressure on this group and therefore the death of adult plants as a result of poor management or overexploitation. Thus, numerous studies carried out in the Sahelian and Sudanian zones have also confirmed this structural variability of species (Rabiou et al., 2015; Idrissa et al., 2017; Amadou et al., 2020). According to Sandjong et al. (2018), adaptations to ecological conditions, competition for resources and exploitation would underlie this structural variability. The low presence of adult individuals with a diameter greater than 15 cm was observed in species with an inverted J-shape that embodies secure regeneration. This indicates that large individuals are heavily exploited as highlighted by Idrissa et al. (2017). The 'inverted J' shape type has been observed in T. indica (Fandohan et al., 2011), Dialium guineense (Assongba et al., 2013), Prosopis africana (Houètchégnon et al., 2015) and Lophira lanceolata (Lankoandé et al., 2017). The predominance of young individuals can be explained by the relationship between the temperament of the species and their diameter distribution. However, the survival of these young individuals is problematic due to bushfires and overgrazing. On the contrary, species that are resistant to bushfires have a high proportion of mature individuals (Nkongmeneck et al., 2010) in their distribution, such as species with a bell-shaped distribution. This type of distribution is characteristic of mono-specific stands with very low regeneration potential.
Vertical structure of edible fruit trees
These results are in agreement with those of Sani (2009), in a revegetated site and a degraded site in the department of Mirriah and confirm one of the characteristics of savannah ecosystems. However, the L-shaped structure is a sign that the whole ecosystem is in a state of degradation as concluded by Jiagho et al. (2016) who worked in Waza National Park. In addition, Tchobsala et al. (2010) found the same structure in the peri-urban area in Ngaoundéré, Cameroon. These results are also in line with those of Faber-Langendeon and Gentry (1991), who obtain decreasing distributions of structural parameters in a hut field and justify that these distributions are characteristic of formations containing mature stands with many small individuals and a low representation of large trees.
Regeneration of species
This result can be justified by the adverse effects of man whose actions impact on the survival of many seedlings. In addition, several authors report that the regeneration of many woody species is made difficult by the harmful action of bush fires and grazing (Mbaiyetom et al., 2021, Nangndi et al., 2021). The species with a high regeneration rate were: A. senegalensis, H. monopetalus, B. aegyptiaca and Z. mauritiana, which together represent more than 80% of the regeneration rate of edible fruit trees. However, it should be noted that some species, although well present at the adult stage, have a low regeneration rate, or even none at all, at the seedling stage, while others are only present at the seedling stage and absent at the adult stage. According to Froumsia (2013), three quarters of seedlings live only 3 months at most. The results showed that the higher regeneration rate, the better the regeneration. The decrease in the overall regeneration rate of the species in the forest reserve would be attributed to the high density of species in this location negatively influencing the survival of natural regeneration due to insufficient light. Several research studies have indicated that the high regeneration rate is recorded in species suitable for suckering and layering (Douh et al., 2014).
CONCLUSION
The availability of edible fruit trees was highlighted in the Laf-Madjam forest reserve and its surroundings. The inventory revealed that Combretaceae, Mimosaceae and Anacardiaceae are the most represented families in the plant formations studied. The diversity of edible fruit species is moderate in the study area, but very low in some plant formations, somewhat less so in the hills. The highest basal area is observed in the hills and the lowest in the shrubby savannah. The highest rate of natural regeneration is found in B. aegyptiaca in the shrubby and tree savannahs, the highest value in the forest gallery is found in Z. mauritiana and in the hill it is observed in A. senegalensis. The analysis of diametric structure of the edible fruit trees in the Laf-Madjam reserve shows us that individuals with a diameter ≥21 cm is the most represented. It shows an "inverted L" distribution in some plant formations and a bell shape in others. The vertical structure of the plant population in the different formations, as well as in the formations as a whole, shows L-shaped curves. Strategies need to be developed to ensure conservation, survival of natural regeneration and to enable low-cost propagation activities of wild edible fruit species to improve their availability.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors thank traditional and administrative authorities as well as the local populations of Laf-Madjam for their collaboration during the fieldwork.
REFERENCES
|
Akbari H, Huang J, Martien P, Rainer L, Rosenfeld A, Taha H (2001). Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Solar Energy 70(3):295-310. |
|
|
Amadou MK, Ismaila D, Rabiou H (2020). Floristic composition, diversity and structure of forest food species in the Sikasso region in southern Mali. European Scientific Journal 16(12):156-178. |
|
|
Arbonnier M (2009). Trees, shrubs and lianas of West Africa (3rd edition). CIRAD MNHN-IUCN, Paris 573 p. |
|
|
Arrif T, Blanc N, Clergeau P (2011). Urban green network, a Nature-Urban report in geography and ecology, Cybergo. European Journal of Geography 17:5-18. |
|
|
Assongba YF, Djègo GJ, Sinsin B (2013). Distribution of the habitats of Dialium guineense (wild) (Fabaceae: Caesalpinioideae) in the eastern phytodistricts of southern Benin. Scientific Bulletin of the National Institute for Environment and Nature Conservation 12:1-16. |
|
|
Barbault R (2007). « Espaces protégés : stratégie dépassées ou dispositifs d'avenir ? » Pour les sciences 35(9):14-17. |
|
|
Birnbaum P (2017). Spatialisation de la diversité et de la structure: un enjeu pour la conservation des forêts tropicales. Biodiversité et Ecologie. Université de Montpellier. France 79 p. |
|
|
Bitsindou M (1986). Enquête sur la phytothérapie traditionnelle à Kindamba et Odzala (Congo) et analyse de convergence d'usage des plantes médicinales en Afrique Centrale. Mémoire Doc (ined.), Université libre de Bruxelles, Belgique 482 p. |
|
|
Comita LS, Condit R, Hubbell SP (2007). Developmental changes in habitat associations of tropical trees. Journal of Ecology 95(3):482-492. |
|
|
Douh C, Daïnou K, Loumeto JJ, Fayolle A, Doucet JL (2014). Explorer la banque de graines du sol pour mieux comprendre la dynamique de régénération des forêts tropicales africaines (synthèse bibliographique). Biotechnologie, Agronomie, Société et Environnement 18(4):558-565. |
|
|
Eba'a RA, Lescuyer G, Jonas NP (2011). Etude de l'importance économique et sociale du Secteur forestier et faunique dans les Etats d'Afrique Centrale 9 p. |
|
|
Faber-Langendoen D, Gentry AH (1991). The Structure and diversity of Rain Forests at Bajo Calima, Choco Region, Western Colombia. Biotropica 23(1):2-11. |
|
|
Fandohan B, Assogbadjo AE, Glèlè Kakai R, Sinsin B (2011). Geographical distribution, tree density and fruit production of Tamarindus indica L. (Fabaceae) across three ecological regions in Benin. Fruits 66(2):53-62. |
|
|
Fotsing J, Ntoupka M, Boubaoua A (2003). Etat de la réserve forestière de Laf et Perspective d'aménagement et de gestion de l'espace. In jamin J.Y. SEIN Y. Boukar L. et Floret C. (Eds), Savane africaine: des espaces en mutation, des acteurs face à de nouveaux défis. Actes du colloque, Garoua, Cameroun 10 p. |
|
|
Fotsing J, Verburg P, Wouter TG, Cheylan JP, Tchuente M (2009). Un modèle intégré pour explorer les trajectoires d'utilisation de l'espace en Zone de Savanes Sèches du Cameroun. Savanes africaines en développement : innover pour durer, Garoua, Cameroun 12 p. |
|
|
Froumsia M (2013). Impact des activités anthropiques sur le couvert ligneux de la réserve de Kalfou, Cameroun. Thèse doctorat Université de Yaoundé I 151 p. |
|
|
Froumsia M, Djosebe A, Souare K, Hamawa Y, Todou G, Tchobsala (2019). Structure, dynamics and impact of the exploitation of the woody plants of woodlands in the Sudano-sahelian zones, North Cameroon. International Journal of Advanced Research in Biological Sciences 6(3):201-220. |
|
|
Gueye M, Cisse A, Diatta CD, Diop S, Koma S (2012). Étude ethnobotanique des plantes utilisées contre la constipation chez les Malinkés de la communauté rurale de Tomboronkoto, Kédougou, Sénégal. International Journal of Biology and Chemistry Science 6(2):778-779. |
|
|
Guinko S, Pasko LJ (1992). Haversting and marketing of edible products from local woody species in Zitenga, Burkina Faso. UNASYLVA n°168. Aride Zone Forestry 43(1):47-56. |
|
|
Idrissa S, Habou R, Machi II, Mahamane A, Mahamane S (2017). Biodiversity and Structure of Woody Plants of Sahelian Rangelands of Baban Rafi, Niger. International Journal of Biology 9(4):1-9. |
|
|
Jiagho ER, Zapfack L, Banoho LPRK, Tsayem-Demaze M, Corbonnois J, Tchawa P (2016). Diversité de la flore ligneuse à la périphérie du Parc National de Waza (Cameroun). Vertigo 16(1). |
|
|
Jim CY, Chen WY (2008). Assessing the ecosystem service of air pollutant removal by urban trees in Guangzhou (China). Journal of Environmental Management 88(4):665-676. |
|
|
Kristensen M, Balslev H (2003). Perceptions, use and availability of woody plants among the Gourounsi in Burkina Faso. Biodiversity and Conservation 12(8):1715-1739. |
|
|
Lankoandé B, Ouédraogo A, Boussim JI, Lykke AM (2017). Natural stands diversity and population structure of Lophira lanceolata Tiegh. ex Keay, a local oil tree species in Burkina Faso. West Africa. Agroforestry Systems 9(1):85-96. |
|
|
Laporte G, Cordeau JF (2007). The dial-a-ride Problem: models and algorithms. Annals of Operations Research 153(1):29-46. |
|
|
Letouzey R (1968). Etude phytogéographique du Cameroun. Eds. Paul Lechevalier, Paris 511 p. |
|
|
Mapongmetsem PM, Guidawa F, Noubissie TJB, Nkonmeneck AB, Bidou HSS, Ronald B (2016). Propagation végétative de Vitex doniana sweet à partir des sections de racines. Bois et Forêts des Tropiques 327(327):29-37. |
|
|
Mapongmetsem PM, Kapchie VN, Tefempa BH (2012). Diversity of local fruit trees and their contribution in sustaining the rural livelihood in the Northern Cameroon. Ethiopian Journal of Environmental Studies and Management 5(1):32- 46. |
|
|
Mapongmetsem PM, Nkongmeneck AB, Romgoumi G, Dongock ND, Dongmo B (2011). Impact des systèmes d'utilisation des terres sur la conservation de Vitellaria paradoxa Gaertn. F. (Sapotaceae) dans la région des savanes soudano-guinéennes. International Journal of Environmental Studies 68(6):851-872. |
|
|
Mbaiyetom H, Avana TML, Tchamba NM, Wouokoue TJB (2021). Diversité floristique et structure de la végétation ligneuse des parcs arborés de la zone soudanienne du Tchad. International Journal of Biological and Chemical Sciences 15(1):68-80. |
|
|
MEA (2005). Ecosystem Wealth and Human Well-being. Island Press 135 p. |
|
|
Nacoulma-OG (1996). Plantes médicinales et Pratiques médicales traditionnelles au Burkina Faso : cas du plateau central. Thèse de Doctorat d'Etat ès Sciences Naturelles, Université de Ouagadougou, tome II, 285 p. |
|
|
Nangndi B, Avana TML, Wouokoue TJB, Etchike DAB, Wolwai DT, Fonkou T (2021). Floristic and structural diversity of woody vegetation in the Sudano-guinean zone of Larmanaye, Chad. Journal of Ecology and the Natural Environment 13(3):63-72. |
|
|
Ngbolua KN, Inkoto CL, Mongo NL, Ashande CM, Masens YB, Mpiana PT (2019). Étude ethnobotanique et floristique de quelques plantes médicinales commercialisées à Kinshasa, République Démocratique du Congo. Revue marocaine des Sciences agronomiques et vétérinaires 7(1):118-128. |
|
|
Nkongmeneck BA, Kemeuze VA, Mapongmetsem PM, Ibrahima A, Jiofack RT (2010). Distribution des Combretums en rapport avec l'aridité au Cameroun. International Journal of Environmental Studies 67(1):41-50. |
|
|
Nowak DJ, Hoehn RE, Crane DE, Stevens JC, Walton JT (2006). Assessing Urban Forest, Effects and Values: Chicago's Urban Forest. Resource Bulletin, NRS-37. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station 27 p. |
|
|
Oumarou S (2012). Caractéristiques de la végétation ligneuse et impact des traitements sylvicoles dans la dynamique évolutive des forêts naturelles : cas de la forêt classée du Nazinon dans le Centre - Ouest du Burkina-Faso, Mémoire de fin du cycle à l'Institut du développement rural à l'Université Polytechnique de Bobo-Dioulasso. 91 p. |
|
|
Rabiou H, Diouf A, Bationo BA, Segla KN, Adjonou K, Kokuste AD, Radji R, Kokou K, Mahamane A, Saadou M (2015). Structure des peuplements naturels de Pterocarpus erinaceus Poir. dans le domaine soudanien, au Niger et au Burkina Faso. Bois et Forêts des Tropiques 3(325):71-83. |
|
|
Rusterholz HP (2003). Biodiversité en milieu urbain : Protection de la nature en milieu urbain et rôle des espaces verts affectés à un entretien extensif. Institut pour la protection de la nature, du paysage et de l'environnement, Paris 24 p. |
|
|
Sandjong SRC, Ntoupka M, Vroumsia T, Ibrahima A (2018). Caractérisation structurale de la végétation ligneuse du Parc National de Mozogo-Gokoro (Cameroun). Flora et Vegetatio Sudano-Sambesica 21:7-24. |
|
|
Sani R (2009). Caractérisation biophysique des ressources dans un site reverdi et un site dégradé dans le département de Mirrah. Mémoire de fin d'études d'ingénieur des eaux et forêt, Université de Abdou Moumouni, Niger 2. 53 p. |
|
|
Saotoing P, Vroumsia T, Tchobsala, Tchuenguem FFN, Njan NAM, Messi J (2011). Medicinal plants used in traditional treatment of malaria in Cameroon. Journal of Ecology and the Natural Environment 3(3):104-117. |
|
|
Sarr MA (2008). Variabilité climatique en Afrique de l'Ouest. Dynamique des espaces végétaux à partir d'images satellites. Exemple du Bassin versant du Ferlo (Sénégal). Journée climatologique-Nantes pp. 57-76. |
|
|
Sigaud P, Eyog-Matig O (2001). Situation des ressources génétiques forestières de la zone sahélienne et nord-soudanienne. Plan d'action sous-régional pour leur conservation et utilisation durable. Note thématique sur les ressources génétiques forestières. Document de travail FGR/2F. FAO. 111 p. |
|
|
Suchel JB (1987). Rainfall patterns and regimes rainfall in Cameroon. Doc. Geographic tropical, No 5, CEGET-CNRS, Talence 287 p. |
|
|
Tchobsala, Amougou A, Mbolo M (2010). Impact of wood cuts on the structure and floristic diversity of vegetation in the peri-urban zone of Ngaoundere, Cameroun. Journal of Ecology and Nature Environment 2(11):235-258. |
|
|
Tieguhong CJ, Betti JL (2008). Forest and Protected Areas in Cameroon: Progress has been significant but Challenges remain. International Tropical Timber Organisation (ITTO) Tropical Forest Update pp. 6-9. |
|
|
Todou G, Doudou K, Vroumsia T (2017). Diversity and local transformation of indigenous edible fruits in Sahelian domain of Cameroon. Journal of Animal and Plant Sciences 26(2):5289-5300. ISSN 2071-7024. Available at: |
|
|
Todou G, Froumsia M, Souaré K, Nnanga JF (2016). Woody plants diversity and type of vegetation in non-cultivated plain of Moutourwa, Far North, Cameroon. Journal of Agriculture and Environment for International Development 110(2):217-227. |
|
|
Veron J (2007). Half of the world's population lives in cities. Population and Societies 435:4. |
|
|
Wafo TG (2008). The protected areas of the Far North Cameroon between conservation policies and local practices. Doctoral thesis in Geography-Planning-Environment, University of Orléans. 332 p. |
|
|
Wolff A (2005). The problem of the urban environment seen by an ecologist. The case of Ile de France. In City and environment, Paris: Ellipses edition pp. 204-223. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0