ABSTRACT
Species composition, habitat association and altitudinal distribution of rodents and shrews were assessed in Chato Protected Area, Ethiopia, between July, 2015 and March, 2016. The area was stratified into five habitats based on dominant vegetation types and altitudinal zonation. The habitats were Carissa spinarum - Justicia schimperiana, Maytenus gracilipes, Podocarpus falcatus - Pyschotria orophila dominated habitats, riverine and plantation. A total of 254 small mammals comprising five rodent and one shrew species were live trapped from 1862 trap nights. The recorded rodent species were: Stenocephalemys albipes (40.95%), Lophuromys flavopunctatus (23.6%), Arvicanthis sp. (16.9%), Mus mahomet (13%), Mastomys natalensis (4.35%) and a shrew sp. (1.2%). Two of these rodent species (S. albipes and L. flavopunctatus) were the most abundant species that comprised 64.56% of the total; while Crocidura sp. was the least abundant distributed along the centre of the forest. M. gracilipes was dominant at 1,789 to 1,975 m, and was the most diverse habitat and comprised 19.39% of the trap success. P. falcatus- P. orophila was dominant from 1,975 to 2,230 m, and was diverse habitat that comprised 22.7% of the trap success. The plantation supported the least number of rodents.
Key words: Altitudinal variation, Chato Protected Area, distribution, habitat, small mammals.
Rodents belong to mammalian order Rodentia, which consists of about 1750 species world-wide. They account for 28% of the total mammalian fauna in Eastern Africa (Kingdon, 1989). In Africa, rodents are the most ubiquitous and numerous among the mammals. The soricomorph fauna (shrews) were slightly diverse with 140 species (Hutterer and Yalden, 1990).
In Ethiopia, the diverse macro and micro-climatic conditions have contributed to the formation of different ecosystems leading to diversity of life forms of both animals and plants (Senbeta, 2006). It is known that there occur 284 species of mammals of which 39.4% are small mammals (Yalden and Largen, 1992). However, recent data indicate the number has risen to over 300 (Bekele and Yalden, 2013).
Rodents are not uniformly distributed in all habitat types (Shenkute et al., 2006). The distribution of rodents and shrews depends on various factors, largely on the seasonal availability of food and water. In addition, vegetation structure and cover affect the micro-climate and protect small mammals against predators (Hansson, 1999). Their distribution and abundance is influenced by vegetation structure and composition, which reflect the habitat condition (Gebresilassie et al., 2004). Bekele (1996b) has revealed the distribution patterns of 10 species of rodents across different vegetation zones including human habitats in the Menagesha State Forest.
Small mammals consume invertebrates, leaves, fruits and seeds, and play extremely important role as dispersal and pollination agents in different habitats. Thus changes in their abundance and distribution can affect the dynamics of other species as well (Solari et al., 2002). In addition to seed dispersal, rodents and shrews are known to have ecological, economical, social and cultural values (Avenant, 2011). They play an important role in natural communities and they are the main food items for many predators including humans (Davies, 2002).
Small mammals are the most diverse group of mammals in Ethiopia. According to Yalden and Largen (1992), rodents comprise 25% of the Ethiopian mammal fauna, and around 50% of total endemic species. This is due to the diversified topography of the country.
Western lowlands of Ethiopia are under-explored for faunal diversity due to inaccessibility and remoteness of the area. Accelerated human interactions in search of arable land and resettlement have been adversely affecting the natural habitats of this area (Chekol et al., 2012). As a result, the biodiversity resources along with their habitats were rapidly disappearing in the country (Senbeta and Denich, 2006). Therefore, there is a need for further biodiversity assessments focused on the different habitats present in the area, with a particular emphasis on small mammals. The current study aims to investigate the diversity, abundance, habitat associations and distribution of rodents and insectivores in Chato Protected Area, Ethiopia.
Study area
Chato Protected Area (CPA) is located in the Horo Guduru Wollega Zone of Oromia Region, Ethiopia. It was part of National Forest Priority Areas (NFPAs) and was known by the name, Chato-Sangi-Dangab Forest. The forest lies approximately between 9Ëš 38’ to 9Ëš 48’ N latitude and 36Ëš 58’ to 37Ëš 20’E longitude along the borders of Jardega Jarte, Abe Dongoro and Horo Districts, 30 km north-west of Shambu which is located at about 314 km west of Addis Ababa. Chato Protected Area was located along altitudinal ranges between 1532 and 2537 masl and covers a total area of 42,000 ha. Plantations of Eucalyptus tree, Juniperus procera and Cupressus lusitanica comprise 18,000 ha of CPA, which correspond to 42.8% of its area (Figure 1).
For this study, we considered five habitat types. Thus, the native forests were categorized into three plant communities (Abdena, 2010), according to their structural composition and use (Table 1). The remaining two habitats correspond to forest plantations and riparian vegetation.
Methods
A representative grid for each vegetation type was established based on possible representation of different habitats as well as easy accessibility. For both live and snap traps during wet (the last week of July and the first two weeks of August) and dry (March) seasons, the same sampling grids were used. Bats were not considered in this study.
Snap trapping grids were established at 200 m away from live trapping grids in each area. Traps of both live and snap trapping grids were separated and placed at 10 m intervals. Each sampling site of live trap constituted an area of 4900 m2 (70 × 70 m). For body measurements (head and body length, tail length, hind foot and ear length) and further studies, 15 snap-traps were used during both wet and dry seasons.
A total of 49 Sherman live traps were used in randomly selected grids of each habitat during both seasons. The traps were baited with peanut butter and checked twice a day, early in the morning hours (6:00 - 8:00h) and late in the afternoon hours (17:00-18:00h). Traps were covered by hay and plant leaves during the dry season. Traps were re-baited as necessary for three consecutive nights.
Trapping and handling of captured rodents and shrews followed the procedures of Gurnell and Flowerdew (1990). A number was assigned to each toe and no two individual animals on the same grid were given the same mark even if they belong to the same species. Following the toe clipping method, a toe per foot was clipped to mark the individuals captured. Known captured animals were identified to their genus level, while others coded for identification in the Zoological Natural History Museum (ZNHM) of Addis Ababa University except Crocidura species which was not snap trapped.
Population number of rodents in each trapping sessions and grids was estimated by capture mark recapture (CMR) method. Shannon-Weaver Diversity Index was used for calculating the rodent species diversity in different habitat types. As;
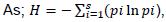
), where: Pi is the relative proportion of species
i in habitat and ln is the natural logarithm.
Abundance of small mammals in each habitat was assessed by trap success during the wet and dry seasons. The percentage of trapped individuals was expressed as,

, where N is the number of individuals captured, Nt is the number of traps and Nn is trap nights. Comparisons of species richness, distribution and habitat association of species in the study area were made by using Chi-square test and SPSS Version 21.0 statistical program.
From a total of 1862 trap nights, 254 individuals representing 5 rodent species and a shrew species were captured during both dry and wet seasons. These were: Stenocephalemys albipes, Lophuromys flavopunctatus, Arvicanthis sp., Mus mahomet, Mastomys natalensis and a Crocidura species. The total trap success was 13.64%. All of the species were recorded from Maytenus gracilipes dominated habitat between 1,789 -1,975 m asl.
Stenocephalemys albipes and L. flavopunctatus were trapped from all habitat types and Arvicanthis sp. was absent from a plantation habitat. S. albipes was the most abundant of all trapped animals that accounted the highest abundance (40.95%). A Crocidura sp. was the least abundant species of the study area while L. flavopunctatus, Arvicanthis sp. and M. mahomet, accounted 23.6, 16.9 and 13%, respectively. Mastomys natalensis was one of the least abundant (4.35%) rodents of the area. Crocidura sp. was trapped only from two habitats (Maytenus gracilipes and P. orophila - P. falcatus dominated habitats) during the wet season, accounting 1.2% of all trapped animals. The total number of animals captured and percentage abundance of rodents and shrews were presented in Table 2.
All species were relatively more abundant during the wet season than the dry season. They showed an increment in number in all habitats except L. flavopunctatus in M. gracileps dominated habitat and S. albipes in plantation habitat. Crocidura sp. was trapped only during the wet season from M. gracilipes and P. orophila - P. falcatus dominated habitats. The species richness of the habitats during the dry and wet seasons was statistically not significant (x2 = 0.00, df=5 and p > 0.05) (Table 2).
The abundance of rodents varied between habitats. The highest abundance for S. albipes, 38.46% and L. flavopunctatus, 38.33% was in P. orophila - P. falcatus dominated habitat. The lowest abundance for S. albipes, 9.61% and L. flavopunctatus, 1.67% was in riverine and C. spinarum - J. schimperiana dominated habitats, respectively (Table 3).
Trap success and diversity index of five habitats with their altitudinal variation were given in Table 4. M. gracilipes dominated habitats, between 1,789 and 1,975 m altitudinal variation was more diverse than others with H' = 1.51 and trap success of 19.39%. The highest trap success was observed in P. orophila - P. falcatus dominated habitat (22.7%). This was the second most diverse habitat with diversity index of 1.32.
From the trapped animals, females comprised 54.3% and males 45.7%. The overall sex ratio of captured rodents from male to female of the study area was1:1.19. The variation was statistically non significant. The structure of rodents and shrews of Chato Protected Area based on age and sex varied between species. From the total captured rodents and shrews, 142 individuals (55.9 %) were adults, 95 individuals (37.4%) were sub-adults and 17 individuals (6.70%) were young. In S. albipes, L. flavopunctatus and Arvicanthis sp., the number of adult individuals were higher than the number of sub-adults and young. The age structure of these rodents was statistically not significant (x2=0.00, df =2 and p> 0.05).
Five species of rodents and one species of shrew were trapped from different vegetation types along altitudes of 1,532 to 2,537 m asl. This may not represent the whole species of the habitat due to heterogeneity and inaccessibility of some areas, but it gives update accounts of rodents and shrews recently present in the forest.
In terms of diversity, P. orophila - P. falcatus dominated habitat was the next more diverse (H'=1.32) habitat than others. The lowest species composition and abundance were recorded in the plantation habitat due to barren ground cover. Similar result was obtained from the study of Bekele (1996a) where young J. procera and C. lusitanica plantations supported fewer species and individuals because of bare ground, no cover and no berries.
The interference of human and other domestic animals was also another disturbing factor for rodents and shrews. Similar report by Bayessa (2010) indicated that modified habitats including plantation forest and cultivation influenced rodent distribution due to availability and quality of food, shelter and rainfall.
S. albipes and L. flavopunctatus were the two most distributed rodent species of the area. Their highest record was from P. orophila-P. falcatus dominated habitat followed by M. gracilipes dominated habitat. In the report of Bekele (1996a) in the Menagesha State Forest, S. albipes was found to be ubiquitous in the forest and was distributed up to 3300 m asl. L. flavopunctatus is one of the most common rodents in the moist areas of East Africa (Clausnitzer and Kityo, 2001), with very wide range of altitude from 500 to 4200 masl (Mulungu et al., 2008). This might be attributed to the diverse feeding habit of the species (Hanney, 1964).
Arvicanthis sp. was the third dominant and widely distributed rodent of the study area. Datiko et al. (2007) also confirmed its wide occurrence in Ethiopia. The highest record of Arvicanthis species was from M. gracilipes and C. spinarum - J. schimperiana dominated habitats. It was frequently trapped from lower areas of riverine habitat and totally absent from plantation habitat. The altitudinal distribution of this rodent was similar to that of Bekele’s (1996a).
M. natalensis is distributed throughout sub-Saharan Africa (Kingdon, 1974). It is also widely distributed over most places in Ethiopia (Yalden et al., 1976). In Chebera Churchura National Park (CCNP), M. natalensis was the most abundant species constituting 29.0% of the total number of captures (Datiko and Bekele, 2013). Even though, in CPA, M. natalensis was the least abundant, it was trapped from C. spinarum – J. schimperiana, M. gracilipes dominated habitats and riverine habitats only. Crocidura sp. was restricted to M. gracilipes and P. orophila – P. falcatus habitats (1,789-2,230 m).
Mean trap success of the current study area was 13.73%, which is high compared to Bekele (1996a) on Menagesha Forest (9.1%), Kassa and Bekele (2008) on Wando Genet (12.7%). There are also other places that have higher trap success in Ethiopia, Tsegaye (1999) on Entoto Natural Park (62.8%).
Total number of captures varied between seasons and the highest number of individuals was trapped during the wet season. The abundance of rodents was based on their reproduction time which can be affected by availability of food, shelter and moisture. The time of reproduction also varied from species to species. Similarly, Datiko et al. (2007) and Geleta (2010) stressed quality of food resource and shelter within habitats playing crucial role on the onset of breeding in many small mammal species.
In the present study, out of the total number of captured individuals, adults comprised the largest number (55.9%). This result goes in line with the study of Shanker (2001) who reported that adults and sub-adults have relatively larger home ranges than young individuals of the same species. As a result, the total number of capture for each age group varied. In most places of CPA, the abundance of female rodents was more than that of males. Similar findings were reported by Bekele (1996a), Datiko et al. (2007) and Datiko and Bekele (2013) in different parts of the country.
The authors have not declared any conflict of interests.
The authors are grateful to Addis Ababa University for providing financial assistance and Oromia Wildlife and Forest Office Shambu branch for providing the necessary facilities.
REFERENCES
|
Abdena F (2010). Floristic composition and structure of vegetation of Chato Natural Forest in Horo Guduru Wollega Zone, Oromia National Regional State, west Ethiopia. M.Sc. Thesis, Addis Ababa University, Addis Ababa.
|
|
|
|
Avenant NL (2011). The potential utility of rodents and other small mammals as indicators of ecosystem 'integrity' of South African grasslands. Wildlife Research 38:626-639.
Crossref
|
|
|
|
|
Bayessa B (2010). Species composition, distribution, abundance and habitat association of rodents in forest and farmlands around Tepi, southwest Ethiopia. M.Sc. Thesis, Addis Ababa University, Addis Ababa.
|
|
|
|
|
Bekele A (1996a). Rodents of the Menagesha State Forest, Ethiopia, with an emphasis on the endemic Praomys albipes (Ruppel 1884). Tropical Zoology 9:201-212.
Crossref
|
|
|
|
|
Bekele A (1996b). Population dynamics of the Ethiopian endemic rodent Praomys albipes in the Menagesha State Forest. Journal of Zoology London 238:1-12.
Crossref
|
|
|
|
|
Bekele A, Yalden DW (2013). The Mammals of Ethiopia and Eritrea. Addis Ababa University Press, Addis Ababa.
|
|
|
|
|
ChekoL T, Bekele A, Balakrishnan M (2012). Population density, biomass and habitat association of rodents and insectivores in Pawe area, northwestern Ethiopia. Tropical Ecology 53:15-24.
|
|
|
|
|
Clausnitzer V, Kityo R (2001). Altitudinal distribution of rodents on Mt. Elgon. Tropical Zoology 14:95-118.
Crossref
|
|
|
|
|
Datiko D, Bekele A, Belay G (2007). Species composition, distribution and habitat association of rodents from Arbaminch forest and farmlands, Ethiopia. African Journal of Ecology 45:651-657.
Crossref
|
|
|
|
|
Datiko D, Bekele A (2013). Species composition and abundance of small mammals in Chebera-Churchura National Park, Ethiopia. Journal of Ecology and the Natural Environment 5:95-102.
Crossref
|
|
|
|
|
Davies G (2002). African Forest Biodiversity: A field Survey Manual for Vertebrates. Cambridge: Earthwatch pp. 120-126.
|
|
|
|
|
Gebresilassie W, Bekele A, Belay G, Balakrishnan M (2004). Microhabitat choice and diet of rodents in Maynugus irrigation field, northern Ethiopia. African Journal of Ecology 42:315-321.
Crossref
|
|
|
|
|
Geleta M (2010). Species Richness, Abundance and Habitat Preference of Rodents in Komto Protected Forest, Western Ethiopia. M.Sc. Thesis, Addis Ababa University, Addis Ababa.
|
|
|
|
|
Gurnell J, Flowerdew JR (1990). Live Trapping Small Mammals: A Practical Guide. The Mammal Society, London.
|
|
|
|
|
Hanney P (1964). The harsh-furred rat in Nyasaland. Journal of Mammals 45:345-356.
Crossref
|
|
|
|
|
Hansson L (1999). Intraspecific variation in dynamics: Small rodents between food and predation in changing landscapes. Oikos 85:159-169.
Crossref
|
|
|
|
|
Hutterer R, Yalden DW (1990). Two new species of shrews form a relict forest in Bale Mountains, Ethiopia. In: Vertebrates in the Tropics, Peters, G. and R. Hutterer (Eds.), Museum Alexander Koenig, Bonn. pp. 63-72.
|
|
|
|
|
Kassa D, Bekele A (2008). Species composition, abundance, distribution and habitat association of rodents of Wondo Genet, Ethiopia. SINET: Ethiopian Journal of Science 31:141-146.
Crossref
|
|
|
|
|
Kingdon J (1974). The East African Mammals: an Atlas of Evolution (Hares and Rodents). Academic Press, London.
|
|
|
|
|
Kingdon J (1989). Island Africa. Academic Press, Princeton.
|
|
|
|
|
Mulungu LS, Mankundi RH, Massawe AW, Machangu RS, Mbije NE (2008).Diversity and distribution of rodents and shrew species associated with variations in altitude on Mount Kilimanjaro, Tanzania. Mammalia 72:178-185.
Crossref
|
|
|
|
|
Senbeta F (2006). Biodiversity and Ecology of Afromontane Rainforests with Wild Coffea arabica L. Populations in Ethiopia. Ecology and Development Series No. 38. Center for Development Research, University of Bonn.
|
|
|
|
|
Senbeta F, Denich M (2006). Effects of wild coffee management on species diversity in the Afromontane rain forests of Ethiopia. Forest Ecology Management 232:68-74.
Crossref
|
|
|
|
|
Shenkute M, Mebrate A, Balakrishnan M (2006). Distribution and abundance of rodents in farmlands: A case study in Aleltu Woreda, Ethiopia. SINET: Ethiopian Journal of Science 29:63- 70.
Crossref
|
|
|
|
|
Shanker K (2001). The role of competition and habitat in structuring small mammals communities in a tropical montane ecosystem in southern India. Journal of Zoology London 253:15-24.
Crossref
|
|
|
|
|
Solari S, Rodriguez J, Vivar E, Velazco M (2002). A framework for assessment and monitoring of small mammals in lowland tropical forest. Environmental Assessment 76:89-104.
Crossref
|
|
|
|
|
Tsegaye B (1999). Species composition, distribution and population dynamics of rodents of Entoto Natural Park. M.Sc. Thesis, Addis Ababa University, Addis Ababa.
|
|
|
|
|
Yalden DW, Largen MJ (1992). The endemic mammals of Ethiopia. Mammalian Revolution 22:115-150.
Crossref
|
|
|
|
|
Yalden DW, Largen MJ, Kock D (1976). Catalogue of the mammals of Ethiopia 2. Insectivora And Rodentia: Pubblicazioni Del Centro Di Studio Per La Faunistica Ed Ecologia Tropicali DEL CNR: CXI. Monitore Zoologico Italiano. Supplemento 8:1-118.
Crossref
|
|
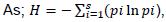 ), where: Pi is the relative proportion of species i in habitat and ln is the natural logarithm.
), where: Pi is the relative proportion of species i in habitat and ln is the natural logarithm. , where N is the number of individuals captured, Nt is the number of traps and Nn is trap nights. Comparisons of species richness, distribution and habitat association of species in the study area were made by using Chi-square test and SPSS Version 21.0 statistical program.
, where N is the number of individuals captured, Nt is the number of traps and Nn is trap nights. Comparisons of species richness, distribution and habitat association of species in the study area were made by using Chi-square test and SPSS Version 21.0 statistical program.