Full Length Research Paper
ABSTRACT
Traditional use of herbal medicines implies substantial historical use, and this is certainly true for many products that are available as ‘traditional herbal medicines. The experimental study was conducted between February and May, 2016 at University of Gondar on antibacterial effect of leaf extract of Pterolobium stellatum. The purpose of the present study was to test the antimicrobial effect of P. stellatum extracted leaves against some standard pathogenic bacteria. The collected plant leave sample was extracted with the solvent ethanol, methanol, chloroform and distilled water. Finally, the antibacterial effect of the extract was tested with some bacteria species (Escherichia coli, Pseudomonas species, Salmonella species, Shigella species, Staphylococcus aureus and Streptococcus pyogenes) then the inhibition zone; the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) were determined. The extract of ethanol and methanol solvents showed high anti-bacterial activity on both Gram negative and Gram positive bacteria. The higher and statistically significant (P<0.05) inhibition was seen in ethanol extract for all bacteria and the highest inhibition was shown against Shigella spp. (21.33±1.52) whilst the lower inhibition was statistically significant (P<0.05) with chloroform extract. Both the MIC and MBC of the test extract were effective at the lowest concentration.
Key words: Antibacterial, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), Pterolobium stellatum, sensitivity test.
INTRODUCTION
Pathogenic microorganism borne illnesses are a continuous threat to public health (Yashphe et al., 2006; Araujo et al., 2015). Bacterial species present the genetic ability to acquire and transmit resistance against currently available anti-bacterial since there are frequent reports on the isolation of bacteria that are known to be sensitive to routinely used drugs and became multi-resistant to other medications available on the market (Chandra, 2013; Kouadio et al., 2020). Antimicrobial resistance is a challenge of microorganism against an antimicrobial drug efficacy that was originally effective for treatment of infections caused by it (Adesokan et al., 2007; Yhiler et al., 2019). Interest in the use of natural plant-derived products versus chemicals as antimicrobials is increasing significantly (Suppakul et al., 2003; Santos et al., 2016). Further, various bacteria have developed resistance to certain antibiotics, and thus, other forms of bactericidal agents are required (Oussalah et al., 2006; Kadaikunnan et al., 2015).
Since the time immemorial medicinal plant as whole or their parts are being used in treating all types’ of diseases (Jamuna et al., 2011; Marathe et al., 2013). Medicinal plants are used as natural resources for the treatment of various diseases since a long time ago (Mitiku et al., 2014; Elizabeth et al., 2015) and have been the main source for new drug development (Kumara et al., 2009; Ayandele et al., 2018). The data of plant usage for treatment in many forms provide a major focus in global health care, as well as contributing substantially to the drug development process (Maikai et al., 2009; Mitiku et al., 2014). Medicinal plants contain physiologically active principles that over the years have been exploited in traditional medicine for the treatment of various ailments as they contain anti-microbial properties (Miyakis et al., 2011; Araujo et al., 2015).
It is believed that plants which are rich in a wide variety of secondary metabolites, belonging to chemical classes such as tannins, terpenoids, alkaloids, and polyphenols are generally superior in their anti-microbial activities (Pandian et al., 2006; Hemalatha and Dhasarathan, 2010; Marathe et al., 2013). Therefore, the strength of biological activities of a natural product is dependent on the diversity and quantity of its antimicrobial constituents (Cos et al., 2006; Liu, 2006; Elizabeth et al., 2015). Furthermore, natural products, either pure compounds or as standardized plant extracts, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity (Chakraborty, 2009; Meenakumari et al., 2011; Idris and Abubakar, 2016). This has urged microbiologists all over the world for formulation of new antimicrobial agents and evaluation of the efficacy of natural plant products as a substitute for chemical antimicrobial agents (Alikhan et al., 2012; Alfatah et al., 2013; Mimura et al., 2020).
Pterolobium stellatum is a tall, scrambling or climbing shrub with woody rope-like stems. Young plants are densely covered with hairs on stem and leaves. The stem has hooking prickles in pairs at the nodes and scattered ones between the nodes. Leaves are compound with 7 to 15 pairs of leaflets. The leaf axis is armed on the lower side with paired reflexed prickles. The flowers are small and sweetly scented with a pale yellowish-white color. The seed pods are broadly winged with a red to scarlet color when young, becoming brown with age (Afolayan and Aliero, 2006). Considering the vast potentiality of plants as sources for antimicrobial drugs with reference to antibacterial agents, a systematic investigation was undertaken to screen the local flora for their antimicrobial activities. Then, this study has investigated in-vitro antibacterial activity of P. stellatum leaves against some bacterial species.
MATERIALS AND METHODS
Study site description
Gondar, the historical town in the country, is located to the Northern and about 747 km far from Addis Ababa, Capital of Ethiopia. Geographically, Gondar is bounded by 12°35’ 07’’ North latitude and 37°26’ 08’’ East longitude and its altitude varies in between 2000 and 2200 m above sea level. Gondar has a humid subtropical mild summer climate that is mild with dry winters, mild rainy summers and moderate seasonality. This climate is usually found in the highlands of some tropical countries. According to the Hold-ridge life zones system of bioclimatic classification Gondar is situated in or near the subtropical dry forest biome. The annual average temperature is 19.1°C. Average monthly temperatures vary by 4°C (7.2°F) (Gondar Agriculture and Rural Development Office).
Plant leaves collection and identification
The medicinal plant P. stellatum was selected for this study on the base of data obtained from local people and literatures because this plant is traditionally used for wound treatment. Fresh and healthy leaves of P. stellatum were collected from University of Gondar at Atse Tewodros and Maraki campus garden on the winter season. The voucher specimens were identified by Mr. Abiyu Eniyew at University of Gondar, herbarium of botany laboratory and the voucher specimen PLS No. 0113/17 was deposited. Figure 2 shows the plant collected from University of Gondar garden.
Preparation of crude extract of plant materials
All the necessary chemicals, media and equipment for this study were obtained from Microbiology Laboratory of Department of Biology, University of Gondar. The leaf of the plant was washed with running tap water and finally with sterile distilled water. Then, it was dried in an open air, protected from direct exposure to sunlight, to prevent degradation of active ingredients (Girish and Satish, 2008). The plant material was ground using grinding machine (Kika-werke-GM.BH, Germany) and passed through mesh sieve to obtain a fine powder. From the sieved powder sample 50 g of the extract was mixed with 500 ml, that is, 1:10 ratio, of extracting solvent (Chloroform, Ethanol, Methanol, and Water) then shake with mild shaking for 24 h on a shaker. The extract was filtered using filter paper (Whatman Paper No. 1) and the solvent were evaporated on the rotary evaporator under reduced pressure at 78, 61, 65 and 93°C, respectively. The extracts were dried at room temperature (Farrukh et al., 2010).
Standard antibiotics
Gentamicin obtained from University of Gondar teaching hospital pharmacy was used as positive control and distilled water, chloroform, ethanol and methanol solvents were used as negative controls for the anti-bacterial susceptibility test. Since all of the negative controls had 0.00 inhibition zone, all presented as negative control.
Test bacterial strains
Standard Staphylococcus aureus, Escherichia coli, Shigella species, Pseudomonas species, Salmonella species, and Streptococcus pyogenes species were obtained from University of Gondar teaching referral hospital. These bacterial strains are isolated and laboratorically identified culture collection for test and researches in the institution. Since all test strains are clinically isolated and identified; they have no identifying codes, that is, ATTCC strains.
Preparation of McFarland and turbidity standard for inoculation
Standardization of the density of isolated inoculums for susceptibility test was done by the methods described in Erturk (2006). In order to determine the active place of test organisms, each isolates was grown in 5 ml of Muller-Hinton broth (MHB) in separate test tube for each bacterial strain for 24 h in incubator. Samples from exponential phase were taken to adjust the inoculums density with 0.5 McFarland and Turbidity prepared by adding a 0.5 ml of BaCl2 solution into 99 to 95 ml of H2SO4 (Erturk, 2006). The turbidity of the inoculums was adjusted.
Preparation of culture media
Muller Hinton agar (MHA) media was used for sensitivity. The media was prepared and treated according to manufactures guidelines. 38 g of MHA was mixed with 1 L of distilled water and settled in hot plate then autoclaved separately enclosed under 15 psi pressures at 121°C for 15 min. The medium was later dispensed into 70 mm sterile agar plates and left to set. The agar plates were incubated for 24 h at 37°C confirming their sterility when no growth occurred after 24 h the plates were considered sterile.
Agar well diffusion
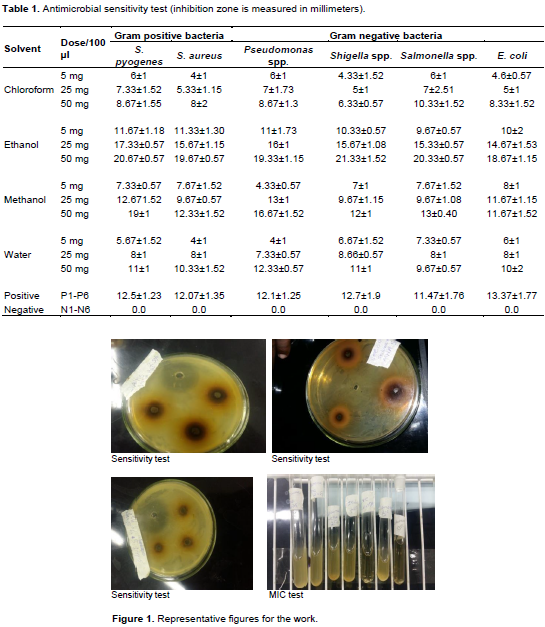
Bacterial strains were tested in MHA media by making (6 mm) well in the media using a steric-borer. Inoculums from exponential growth of each bacteria isolates were mixed using vortex. The turbidity of the reconstituted organisms was adjusted to 0.5 McFarland standards. Both the standard and bacterial suspensions were agitated on vortex mixer medially prior to use. From these suspensions a volume of 100 µl bacteria were inoculated by using micro-pipette. After inoculating the bacterial isolates, the plates were allowed to dry for 5 min after which the crude extracts and the controls were dispensed into each well. The plates were incubated at 37°C for 24 h. The inhibition zone sizes were measured in millimeters compared to standard Gentamicin (Theuretzbacher, 2011).
Minimum inhibitory concentration (MIC)
The MIC of the extract was determined by MBH dilution technique (Zied et al., 2011). First the leaves of the crude extracts were prepared in different concentrations (6.25, 12.5, 25 and 50 mg/100 mL). Broth containing test tubes was tightly closed, arranged in test tube rank and autoclaved under 15 psi pressures at 121oC for 15 min. The broths were allowed to cool until the temperature is equitable to room temperature. The extracts with different concentrations (100 mL) and the test bacteria [1 × 10-8 CUF/ml] was aseptically introduced. The inhibition of growth was observed after 24 h incubation at 37°C. The presence of growth was evaluated by comparing the negative control, positive control and culture containing test tubes. The lowest concentration of compound that showed antimicrobial activity against test organisms was recorded as MIC value (Igoji et al., 2005).
Minimum bactericidal concentration (MBC)
Broth containing test tubes that did not show any bacterial growth at MIC was used to determine MBC. Small volumes of these broths are streaked onto the surface of MHA medium by sterile wire loop. The medium was incubated at 37°C for 24 h. The least concentration of plant extracts that effectively inhibit bacterial growth on the agar plate was recorded as MBC of the extracts (Igoji et al., 2005).
Data analysis
The data collection instrument was experimental through basic laboratory technique. Data like susceptibility was analyzed using SPSS software package version 20.00. Microsoft Excel was employed for analysis of MIC and MBC.
RESULTS
Antibacterial sensitivity test
Antimicrobial activity of P. stellatum leaves extract was evaluated based on the diameter of clear inhibition zone in millimeters. If there is no inhibition zone, it is assumed that there is no anti-microbial activity. Table 1 shows the diameter of zone of inhibition of bacterial growth at varying concentration of the leaf extract in chloroform, distilled water, ethanol figure and methanol after 24 h incubation. The extract of ethanol and methanol solvents shows high anti-microbial activity on both Gram negative and Gram positive bacteria. Higher inhibition was seen in ethanol extract for all bacteria: S. pyogenes (20.67±0.57), E. coli (18.67±1.15), S. aureus (19.67±0.57), Pseudomonas spp. (19.33±1.15), Salmonella spp. (20.33±0.57) and the highest inhibition was seen against Shigella spp. (21.33±1.52) and the lower inhibition was with chloroform extract (8.67±1.15), (8.33±1.52), (8±2), (8.67±1.3), respectively and with Shigella spp. (6.33±0.57) except Salmonella (9.67±0.57) which was in distilled water (Figure 1).
Minimum inhibitory concentration (MIC in mg/μl)
MIC of all crude extract against the test organisms were performed using broth dilution method. All the test organisms were inhibited by all extract solvents with the range of 6.25 to 50% and the obtained result of MIC rangedfrom 6.25 to 12.5%. Chloroform extract result showed MIC of 6.25 against E. coli, Pseudomonas spp. and Salmonella spp. and 12.5 was for the rest tested organisms. The result of chloroform and other solvents are shown in Table 2.
Minimum bactericidal concentration (MBC in mg/μl)
The MBC values were performed using agar well diffusion method from the MIC for all test organisms. The range was obtained from 6.25 to 12.5%. MBC of S. aureus for all extracted solvents was 12.5 but the other testes organisms were with different value (Table 3).
DISCUSSION
The bacterial resistances against multiple antibiotic are of great concerns to both veterinary and human medicine
worldwide and have been posing serious problems in the treatment of infectious diseases (Madubuike et al., 2018). Antibiotics which are widely used for the treatment of infectious diseases are under constant threat due to the emergence of antibiotic resistant pathogens (Marathe et al., 2013; El-Banna and Qaddoumi, 2016). Many studies were conducted and information is available on P. stellatum. In East Africa, fresh leaves of P. stellatum were ‘chewed or a decoction is drunk to treat tuberculosis and related respiratory diseases (Pandian et al., 2006) used for weight loss or athletic performance enhancement (Girish and Satish, 2008). In Kenya, a root decoction is used by the Maasai against stomach-ache. Juice of the roots is swallowed to treat snake-bites. In Malawi, a root infusion is drunk by women against infertility (Hassan et al., 2007). Tea from the leaves is used to treat fever; packets of leaves are burned under the bed of colic sufferers (Salatino et al., 2007).
Agar diffusion methods are the highly recommended method in antibacterial testing (El-Banna and Qaddoumi, 2016). In this study, anti-microbial activity of P. stellatum was conducted; this antimicrobial activity was recorded and analyzed based on the inhibition zone. The current study clearly indicated that chloroform, distilled water, ethanol, and methanol extract of P. stellatum could be able to inhibit the tested bacteria. This result was in line with the previous report by Dhiman et al. (2011). The plant extract shows antibacterial activity against the test organisms due to the plant active ingredients that inhibit bacterial growth (Meenakumari et al., 2011). However, the degree of their inhibition pattern is different; this may be because of the difference in bacterial strain and the kind of solvent used (Zied et al., 2011; Alikhan et al., 2012). The variation in effectiveness of the extracts’ concentration against the isolates under study may be attributed to its phytochemical composition couple with better membrane permeability gradient of the bacterial organisms for chemicals and their possible metabolism (Madubuike et al., 2018).
Antibacterial activity difference between extracts may be attributed to the fact that different compounds from the plant material get extracted in solvents of different polarities (Marathe et al., 2013; Elizabeth et al., 2015). In our study, ethanol extracts of this plant showed highest inhibition zones compared to the positive control (Gentamicin) while the negative control had no antimicrobial activity. The result was in accordance with previous report by Calderon et al. (2012), in which they are widely used to obtain crude extracts of phytochemicals from plant materials in the herbal medicine industry for therapeutic applications. Chloroform extract has shown the lowest inhibition zone against all bacteria’s except Salmonella spp. which was given the lowest inhibition zone with distilled water. All the plant extracts showed minimum inhibition zones at the concentration of 5 mg/ml against all bacteria in agar well diffusion method and the highest was 50 mg/ml. This result was in accordance with the previous results by Biruhalem et al. (2011).
The antimicrobial activity of the crude extract may be attributed to a specific compound or a combination of compounds (Marathe et al., 2013; Elizabeth et al., 2015). These bio-actives can be alkaloids, flavonoids, coumarins, saponins and steroids compounds of plant origin known to have antibacterial activity (Marathe et al., 2013). The obtained data showed that all the solvent plant extracts were active against all the test organisms such as Staphylococcus aureus, Shigella spp., Salmonella spp., Pseudomonas spp., S. pyogenes and E. coli. The ethanol extract against all test organism (Gram-positive and Gram-negative bacteria) showed maximum inhibition zone than the other. This is due to the antibiotic active compounds of the plant leave extracted by ethanol is highly effective on all tested organisms (Dhiman et al., 2011). The antimicrobial activities analysis of the crude extract revealed the presence of some of phytochemicals active coumpounds including: flavonoids, steroids, triterpenes, tannins, saponins and alkaloids (Madubuike et al., 2018).
Most of the Gram positive bacteria are highly sensitive than Gram negative (Selvamohan et al., 2012; Michael et al., 2013). It is an established fact that the Gram-positive and negative bacteria react differently to antibacterial agents due to the differences in their cell wall component (peptidoglycan) and the ability of these agents to penetrate them (Idris and Abubakar, 2016; Elizabeth et al., 2015; Ko and Stone, 2020). The other possible explanation is that the presence of some bioactive compounds in the extract might be responsible for the extracts higher effect against Gram-positive than Gram-negative bacterium (Lima et al., 2006 as cited in Santos et al., 2016; Ko and Stone, 2020). But in our results both of them were sensitive; this may be due to the fact that plant extract is active against both Gram negative and positive bacteria.
There is a difference when compared with positive control in 5 mg concentration of chloroform extract. E. coli inhibition zone has a significant bacterial difference when compared with other bacteria of 25 mg concentration of chloroform extract. E. coli and S. pyogenes with 50 mg concentration have a difference when compared with others in chloroform extract. Ethanol with 5 mg concentration has a significant dose difference within the group. E. coli has a difference in the positive control in all extract of distilled water.
Determining the appropriate concentration required to inhibit and kill the organism and determination of MIC and MBC is crucial in antibacterial experiment, respectively (Idris and Abubakar, 2016). In this study, the MIC results range from 6.25 to 12.5 mg/μl. Methanol and chloroform in concentration of 6.25 mg/μl inhibit E. coli and the other strains with 12.5 mg/μl. Methanol, ethanol and chloroform inhibited S. aureus with 12.5 mg/μl, whereas distilled water with 6.25 mg/μl. Shigella was inhibited with concentration of 6.25 mg/μl for all extracts except with chloroform (12.5 mg/μl). The MBC results of all extracts against the tested organisms showed similar range with MIC. S. aureus of all solvent extract has the range of 12.5% but different in others. Pseudomonas spp. has a significant extract difference with 25 mg of all solvent extract except in distilled water. This result was agreed with the results stated by Surjeet et al. (2011).
Active components in plants may provide potential sources of new drugs for the safe and effective treatment of microbial diseases (de Oliveira Santos et al., 2016). Our investigation clearly indicates that leaves of P. stellatum contain a great potential of anti-microbial component which has contributed a great role in pharmaceutical industries and healing various disease. The high potency observed in our study is therefore a rapid response call for further analysis of this plant using higher molecular techniques to ascertain its safety in the management of human and animal diseases (Madubuike et al., 2018).
CONCLUSION
The present work demonstrated that P. stellatum leaves extract have the antimicrobial potential on all tested bacteria: S. aureus, Shigella spp., Salmonella spp., Pseudomonas spp., S. pyogenes and E. coli with various solvents: methanol, ethanol, chloroform and distilled water between 5, 25 and 50 mg dose difference. Ethanol and methanol extracts showed high antimicrobial activities than chloroform and distilled water. Ethanol extract has high anti-bacterial effect than others extract and chloroform has less effect. Both the MIC and MBC of the test extract were effective with the lowest concentration. Further studies like isolation and analyzing the specific antibacterial principle, effectiveness of other parts of the plant, the toxicity and isolation of the bioactive compounds are needed to better evaluate the antibacterial potential of the plant.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors express their sincere gratitude to Department of Biology, University of Gondar for allowing them to use the facility of microbiology laboratory.
REFERENCES
|
Adesokan A, Akanji A, Yakubu M (2007). Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. African Journal of Biotechnology 6(22):2502-2508. |
|
|
Afolayan A, Aliero A (2006). Antimicrobial activity of Solanum tomentosum. African Journal of Biotechnology 5(4):369-372. |
|
|
Alfatah A, Christina Y, Saad M (2013). Antimicrobial Activity of Four Medicinal Plants Used by Sudanese Traditional Medicine. Journal of Forest Products and Industries 2(1):29-35. |
|
|
Alikhan A, Ahmed Q, Khan A (2012). Antibacterial activity of leaves extracts of Trifolumale xandrium against pathogenic bacteria causing tropical diseases. Asian Pacific Journal of Tropical Biomedicine 2(3):189-194 |
|
|
Araujo AR, Quelemes PV, Perfeito ML, de Lima LI, Sá MC, Nunes PH, Joanitti GA, Eaton P, dos Santos Soares MJ, de Almeida JR (2015). Antibacterial, antibiofilm and cytotoxic activities of Terminalia fagifolia Mart. extract and fractions. Annals of clinical microbiology and antimicrobials 14(1):1-10. |
|
|
Ayandele AA, Adewoyin AG, Olatunde SK (2018). Detection of antibiotic resistant bacteria and sterol concentration in hand dug wells cited near pit latrine in Southwestern Nigeria. Journal of Microbiology and Antimicrobials 11(2):11-21. |
|
|
Biruhalem T, Mirutse G, Abebe A, Jemal S (2011). Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pacific Journal of Tropical Biomedicine 1(5):370-375. |
|
|
Calderon P, Gagnon D, Wendakoon C (2012). Evaluation of Selected Medicinal Plants Extracted in Different Ethanol Concentrations for Antibacterial Activity against Human Pathogens. Journal of Medicinally Active Plants 1(2):60-68. |
|
|
Chakraborty S (2009). Anti-microbial activity of Chlorophytum borivilianum leaves. Indian Drugs 46:571-579. |
|
|
Chandra M (2013). Antimicrobial Activity of Medicinal Plants against Human Pathogenic Bacteria. International Journal of Biotechnology and Bioengineering Research 4:653-658. |
|
|
Cos P, Vlietinck A, Vanden B, Maes L (2006). Anti-infective potential of natural products: How to develop a stronger in vitro 'proof-of concept'. Journal of Ethnopharmacology 106:290-302. |
|
|
Dhiman A, Nanda A, Ahmad S, Narasimhan B (2011). In vitro antimicrobial activity of methanolic leaf extract of Psidium guajava. Journal of Pharmacy and Bio-allied Sciences 3:226-229. |
|
|
El-Banna N, Qaddoumi SS (2016). Antimicrobial activity of Bacillus cereus: Isolation, identification and the effect of carbon and nitrogen source on its antagonistic activity. Journal of Microbiology and Antimicrobials 8(2):7-13. |
|
|
Elizabeth AA, Tukur AM, Racheal AM (2015). Antimicrobial activity and phytochemical screening of the fruit pulp of Dialium guineense (Velvet Tamarind) on some microbial isolates. Journal of Microbiology and Antimicrobials 7(4):33-41. |
|
|
Erturk O (2006). Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia 61:275-278. |
|
|
Farrukh H, Bashir A, Ishfaq H, Ghulam D, Parveen S, Sadiq A (2010). Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of polygonaceae. African Journal of Biotechnology 9:5032-503. |
|
|
Girish H, Satish S (2008). Antibacterial Activity of Important Medicinal Plants on Human Pathogenic Bacteria-a Comparative Analysis. World Applied Sciences Journal 5:267-271. |
|
|
Gondar Agriculture and rural development office, on 29/05/2020 |
|
|
Hassan S, Lawal M, Muhammad B, Umar R, Bilbis L, Faruk U, Ebbo A (2007). Antifungal Activity and Phytochemical Analysis of Column Chromatographic Fractions of Stem Bark Extracts of Ficussycomorus L. (Moraceae). Journal of Plant Sciences 2:209-215. |
|
|
Hemalatha N, Dhasarathan P (2010). Multi-Drug Resistant Capability of Pseudomonas aeruginosa Isolates from Nasocomal and Non-Nasacomal Sources. Journal of Biomedical Sciences 2:236-239. |
|
|
Idris A, Abubakar U (2016). Phytochemical and antibacterial investigations of moringa (Moringa oleifera) leaf extract on selected bacterial pathogens. Journal of Microbiology and Antimicrobials 8(5):28-33. |
|
|
Igoji J, Tor-Anyiin T, Igoji N (2005). Traditional medicine practice amongst the Igede people of Nigeria. African Journal of Traditional. Complementary and Alternative Medicine 2:134-152. |
|
|
Jamuna A, Ravishankar V, Pradeepa VS (2011). Evaluation of the antimicrobial activity of three medicinal plants of South India. Malaysian Journal of Microbiology 7:14-18. |
|
|
Kadaikunnan S, Rejiniemon TS, Khaled JM, Alharbi NS, Mothana R (2015). In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Annals of Clinical Microbiology and Antimicrobials 14:9. |
|
|
Ko WC, Stone GS (2020). In vitro activity of ceftazidime avibactam and comparators against Gram-negative bacterial isolates collected in the Asia-Pacific region as part of the INFORM program (2015-2017). Annals of Clinical Microbiology and Antimicrobials 19(14). |
|
|
Kouadio IA, Yapi PA, Sanogo M (2020). Bactericidal activities of the leaf extract of Spondias mombin L. plant used for culinary and therapeutically purposes in Ivory Coast. Journal of Microbiology and Antimicrobials 12(1):1-16. |
|
|
Kumara M, Agarwala R, Deyb K, Raib V, Johnsonc B (2009). Antimicrobial Activity of Aqueous Extract of Terminalia chebula Retz. on Gram positive and Gram negative Microorganisms. International Journal of Current Pharmaceutical Researches 1:56-60. |
|
|
Liu H (2006). Introduction to Traditional Herbal Medicines and their Study, in Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies. John Wiley & Sons 2011. |
|
|
Madubuike SA, Mailafia S, Ode OJ, Okpo N (2018). Phytochemical screening and antibacterial susceptibility of Escherichia coli O157:H7 isolates on Acacia ataxacantha leaves. Journal of Microbiology and Antimicrobials 10(1):1-8. |
|
|
Maikai V, Maikai B, Kobo I (2009). Antimicrobial Properties of Stem Bark Extracts of Ximenia americana. Journal of Agricultural Sciences 1(2):2-9. |
|
|
Marathe NP, Rasane MH, Kumar H, Patwardhan A, Shouche YS, Diwanay SS (2013). In vitro antibacterial activity of Tabernaemontana alternifolia (Roxb) stem bark aqueous extracts against clinical isolates of methicillin resistant Staphylococcus aureus. Annals of Clinical Microbiology and Antimicrobials 12:26. |
|
|
Meenakumari S, Verma S, Absar A, Chaudhary A (2011). Antimicrobial susceptibility pattern of clinical isolates of Pseudomonas aeruginosa in an Indian cardiac hospital. International Journal of Engineering Sciences Technology 3(9):7117-7124. |
|
|
Michael PD, Guy H, Loneragan H, Scott M, Singer RS (2013). Antimicrobial Resistance: Challenges and Perspectives. Comprehensive Reviews in Food Science and Food Safety 12:234-248. |
|
|
Mimura W, Fukuda H, Akazawa M (2020). Antimicrobial utilization and antimicrobial resistance in patients with haematological malignancies in Japan: a multi-centre cross-sectional study. Annals of Clinical Microbiology and Antimicrobials 19:7. |
|
|
Mitiku M, Ali S, Kibru G (2014). Antimicrobial Drug Resistance and Disinfectants Susceptibility of Pseudomonas aeruginosa isolates from Clinical and Environmental Samples in Jimma University Specialized Hospital, Southwest Ethiopia. American Journal of Biomedical and Life Sciences 2(2):40-45. |
|
|
Miyakis S, Pefanis A, Tsakris A (2011). The challenges of antimicrobial drug resistance in Greece. Clinical Infectious Diseases 53(2):177-184. |
|
|
Oussalah M, Caillet S, Saucier L, Lacroix M (2006). Antimicrobial effects of selected essential oils on the growth of a Psuedomonas putidastrain isolated from meat. Meat Science 73(2):236-244. |
|
|
Pandian M, Banu G, Kumar G (2006). A study of antimicrobial activity of Alangium salviifolium. Indian Journal of Pharmacology 38(3):203-204. |
|
|
Salatino A, Salatino M, Negri G (2007). Traditional uses, Chemistry and Pharmacology of Croton species (Euphorbiaceae). Journal of the Brazilian Chemical Society 18:11-33. |
|
|
Santos LB, de Souza Leitao C, de Mendonca Cavalcante A, da Silva MA, Porfirio Z, Santana AE. (2016). Antimicrobial analysis of copaiba oil extract from Passiflora cincinnata and endodontic substances. Journal of Microbiology and Antimicrobials 8(6):34-38. |
|
|
Selvamohan T, Ramadas V, Shiba S (2012). Antimicrobial activity of selected medicinal plants against some selected human pathogenic bacteria. Advances in Applied Science Research 3(5):3374-3381. |
|
|
Suppakul P, Miltz K, Sonneveld B, Bigger W (2003). Antimicrobial properties of basil and its possible application in food packaging. Journal of Agricultural and Food Chemistry 51(11):3197-3207. |
|
|
Surjeet K, Lincy J, Mathew G, Vivek B (2011). Antimicrobial Activity of Methanolic Extract of Rumex Nepalensis Leaves. International Journal of Pharmacy and Pharmaceutical Sciences 3(4):240-242. |
|
|
Theuretzbacher U (2011). Resistance drives antibacterial drug development. Current Opinion in Pharmacology 11(5):433-438. |
|
|
Yashphe J, Segal R, Breuer A, Erdreich-Naftali G (2006). Antibacterial activity of Artemisia herba-alba. Journal of Pharmaceutical Sciences 68(7):924-925. |
|
|
Yhiler NY, Bassey EB, Paul I, Francis UM, Anne A, Ejeko AO (2019). Antimicrobial resistance pattern in Salmonella enterica from clinical and poultry sources in Calabar, Nigeria. Journal of Microbiology and Antimicrobials 11(2):5-10. |
|
|
Zied Z, Adel K, Ines C, Riadh B, Ahmed B, Hafedh M, Néji G (2011). The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids in Health and Disease 10(1):161-170. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0