ABSTRACT
Several investigations have demonstrated in vitro antibacterial activity of plant extracts. Kalanchoe pinnata is globally used in folk medicine for the treatment of various diseases, such as diarrhea, infections, tuberculosis and fever. Among the enteropathogenic bacteria, enteroaggregative Escherichia coli (EAEC) is an important cause of diarrhea in the world. EAEC is also capable of forming biofilms, conformation that provides antibiotic resistance and a high degree of dispersion and reinfection, which may represent up to 80% of causes of human microbial infections. Thus, it becomes necessary to search for new antimicrobial agents with activity against biofilms. The aim of this study was to evaluate the cytotoxic, antibacterial and antibiofilm in EAEC from aqueous extracts of leaves and the flavonoids quercetin and rutin, occurring in K. pinnata. Leaf aqueous extracts were obtained and evaluated phytochemically. The cytotoxic activity was evaluated in human carcinoma cell lines HEp-2, Caco-2 and T84. The antibacterial activity was evaluated by the macrodilution method and the evaluation of antibiofilm activity was performed in Escherichia coli enteroaggregative (EAEC 042). Aqueous extracts of K pinnata do not show toxicity to Hep-2, but all other cell lines were sensitive to this extract. Instead, the flavonoids quercetin and rutin showed no cytotoxicity with any of the tested cell lines. Quercetin is capable of inhibiting bacterial growth of all tested strains. The aqueous leaf extract and quercetin were able to inhibit EAEC 042 biofilm formation above 50%. The results indicate the potential use of the species in treatment of bacterial infections.
Key words: Antimicrobials, EAEC 042, biofilms, quercetin, Crassulaceae.
Kalanchoe pinnata (Lam.) Pers., also referred by its synonym Bryophyllum pinnatum (Lam.) Kurz., is a species of the Crassulaceae family, commonly known in Brazil as saião-roxo (Oliveira et al., 2003). This plant
is commonly used for various medical applications, such as in the treatment of diarrhea, conjunctivitis, dermatitis, eczema, fever, infections, tuberculosis, respiratory infections, inflammation, among others (Majaz et al., 2011).
The in vitro antibacterial activity of various extracts from K. pinnata had been reported in the literature, like methanolic and alcoholic extracts from leaves, stem and roots. Among the bacterial species susceptible to these extracts are Enterococcus faecalis (Aibinu et al., 2007), Escherichia coli (Pattewar et al., 2013), Klebsiella pneumoniae (Okwu and Nnamdi, 2011), Pseudomonas aeruginosa (Tatsimo et al., 2012), Salmonella typhi (Nwadinigwe, 2011), Shigella dysenteriae (Akinpelu, 2000), Staphylococcus aureus (Biswas et al., 2011), and others. Among the enteropathogenic bacteria, enteroaggregative Escherichia coli (EAEC) is an important cause of diarrhea in the world, being related to cases of diarrhea of persistent diarrhea especially in children, HIV-infected individuals and from travelers in developing countries (Berry et al., 2014). In these countries, the cause of death due to persistent diarrhea in infants between 1 and 11 months is 30%. In children up to 4 years old this rate can reach 69% (Rahman et al., 2014).
In addition to its aggregative ability in human intestinal cells, EAEC is able to form biofilms on surfaces. This configuration provides antibiotic resistance and a high degree of dispersion and reinfection, which may represent up to 80% of causes of human microbial infections (Bueno, 2014). The nature of biofilms is able to protect them from adverse conditions such as desiccation, UV, toxic compounds and antibiotics, favoring the persistence (Abdel-Aziz and Aeron, 2014). Thus, is it important to search for new antimicrobial agents with activity against biofilms.
Much of the antimicrobial activity of plant extracts is related to the action of flavonoids, substances that also have nutraceutical importance, due to the organoleptic properties of food and pharmacological activities on human health (Tapas et al., 2008).
Although not produced by the human metabolism, flavonoids have various pharmacological activities such as anti-allergic, anti-inflammatory and anti-ulcer. Over four thousand substances belonging to the group have been identified, having highlighted in K. pinnata, the compounds of the group of flavonols, such as quercetin and rutin (Lopes et al., 2000).
For quercetin, important antimicrobial properties have been identified, such as antileishmanial (Muzitano et al., 2006, Muzitano et al., 2011) and antibacterial (Gatto et al., 2002, Mittal et al., 2014; Prasad et al., 2014), being a metabolite of great interest in antimicrobial studies.
Thus, the objective of this study was to evaluate the cytotoxic activity in different cell lines, antibacterial activity in different strains and antibiofilm in EAEC from aqueous extracts of leaves and the flavonoids quercetin and rutin, occurring in K. pinnata.
Plant material
Samples of K. pinnata were collected in Jacarepaguá (Rio de Janeiro/Brazil) and a voucher specimens was deposited in the Herbarium of the University of the State of Rio de Janeiro (UERJ) under the number HRJ12515.
For the different analyses, samples of K. pinnata were grown in the greenhouse at UERJ. Plants were cultivated under in vivo conditions, in containers using fertilized soil, under sunlight and were watered twice a day. Samples of material were collected in the summer, between the months of November 2014 and February 2015, for the production of extracts and phytochemical analysis.
Phytochemical analysis
For the phytochemical analysis and evaluation of the medicinal potential of K. pinnata, aqueous extracts were prepared from leaves according to the methodology proposed by Muzitano et al. (2011).
This fresh material was mechanically macerated and heated in distilled water at 20% (w/v) for 30 minutes at 50°C. After this period, the material was filtered, frozen at -20 and then lyophilized under pressure of 60 μmHg at -59°C. After lyophilization, the material was resuspended in sterile distilled water at the concentrations required for experimentation.
The flavonoids quercetin and rutin (Sigma-Aldrich) were used as standard substances for analysis, being solubilized 1 mg.mL-1 of substance in methanol (MeOH).
The qualitative analysis for the phytochemical aqueous extracts was performed following the protocol proposed by Barbosa (2001). Groups of secondary metabolites of medicinal interest were evaluated, including flavonoids, alkaloids, saponins, phenols and tannins.
Cytotoxic activity of K. pinnata extracts
In order to evaluate the cytotoxic activity, cell lines that represent cells related to passage of aqueous extract in the gastrointestinal tract were selected. Thus, the cell lines HEp-2 (ATCC CCL23 originating from human larynx carcinoma), Caco-2 (ATCC HTB37, originating from human colon adenocarcinoma) and T84 (ATCC CCL248 originating colon rectal carcinoma) were used in the study.
The aqueous extracts were evaluated at concentrations of 500 and 1000 μg.mL-1 and the flavonoids quercetin and rutin at the concentration of 50 μg.mL-1. The assay was performed in 96-well plates showing confluent layer of cells and cultivated in 100 μL of DMEM or MEM medium per well. In each culture well 100 μL extract or flavonoids solubilized in PBS-D were added, keeping the culture for 24 h at 37°C and 5% of CO2.
In the corresponding period of three hours before the end of cultivation (45h) 5 mg.mL-1 of solution of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was added. After this period, the plate was centrifuged at 3000 rpm for 5 min to deposition of MTT crystals formed. The supernatant was discarded and the crystals diluted by adding 100 μL of DMSO (100%) in each well.
The reading was performed in a microplate reader at 492 nm. As negative controls, cell cultures were used in the absence of treatment and cultivation in the presence of 5% methanol. As a positive control, cells were cultured in the presence of 100% methanol. The experiments were performed in triplicate and repeated three times or more.
Antibacterial activity
In all assays, reference strains were used, obtained mostly from the American Type Culture Collection (ATCC, Rockville, MD, USA) and the other, of the collection of the Department of Microbiology, Immunology and Parasitology, UERJ (Table 1).
The evaluation of the antimicrobial activity of the aqueous extract and flavonoids has been carried out by the method of agar dilution (macrodilution) used by Soberon et al. (2007) with some modifications.
The bacterial strains were grown in 15×100 mm test tubes containing 3 ml of Mueller- Hinton Broth (MHB, Oxoid, Ltd.), incubated at 37°C for 18 h. The extract, at 1000 μg.mL-1 and the flavonoids, at the concentrations of 10 and 100 μg.mL-1 were solubilized in 20 ml of Mueller-Hinton Agar (MHA), not yet solidified, and poured into 90 mm petri dishes. As negative controls of the experiment were used MHA medium and the same medium plus 1 and 10% of MeOH representing the final concentration of solvent after solubilization of quercetin and rutin.
For each treatment, 2 μL of each bacterial suspension in duplicate were plotted on the plate. The growth of colonies was evaluated after 24 h incubation at 37°C. All the material and culture media were sterilized by autoclaving at 121°C for 20 min. The experiments were repeated three times or more.
Evaluation of biofilm formation of enteroaggregative Escherichia coli (EAEC 042)
The evaluation of the growth of biofilms of EAEC 042 was performed based on the spectrophotometric assay of biofilm inhibition, using as reference the working of Namasivayam and Roy (2013) with some modifications.
E. coli suspensions were adjusted and standardized to 0.14 nm of optical density (OD), and added 100 μL of the suspension in 96 well plates. Then the extract and flavonoids was added in triplicate, being the culture maintained in incubation for 18 h at 37°C. The aqueous extract was evaluated at 1000 μg.mL-1 and the flavonoids quercetin and rutin in concentrations of 5 and 50 μg.mL-1.
After incubation, the supernatant was discarded and added 100 μL of crystal violet solution (1% w/v) for 30 min. After this period, the solution was removed and wells were washed with PBS-D, and then added 100 μL of 95% ethanol, keeping incubation for 15 min for solubilization of the crystals. The reading was performed in microplate reader at 570 nm. The calculation of the percentage inhibition of biofilm formation was in accordance with the formula:
Statistical analysis
The experiment with comparisons between controls was performed by the analysis of variance of the means obtained (ANOVA) using the program GraphPad PRISM®, being carried out after Bonferronipost-test, with p<0.05.
Phytochemical analysis
Phytochemical analysis of K pinnata leaf extracts used in the experiments (Table 2) showed the presence of flavonoids, saponins, phenols and tannins.
Cytotoxic activity of K. pinnata extracts
In cell cultures of HEp-2 (Figure 1a) the aqueous extract of leaves showed no toxicity. However, Caco-2 and T84 strains (Figure 1b and c) were more sensitive to the culture in the presence of the extract in a high concentration (1000 μg.mL-1).
The flavonoids quercetin and rutin and the methanol at a concentration of 5% showed no toxicity for the cultivation of different cell lines evaluated.
Antibacterial activity
The aqueous extracts of K. pinnata showed antibacterial activity, reducing the growth of Citrobacter freundii, Bacillus thuringiensis, Shigella sonnei and Escherichia coli K-12 (HB 101) grown in the presence of aqueous leaf extract obtained from plants grown in vivo (Table 2).
At 10 μg.mL-1, rutin was able to reduce the growth of Pseudomonas aeruginosa and C. freundii. Have quercetin in same concentration reduced the growth of P. aeruginosa, C. freundii, E. coli (17-2), Serratia marcescens and B. thuringiensis. When the concentration of these flavonoids is raised to 100 μg.mL-1 in culture, the rutin is able to reduce the growth of all strains with the exception of Enterococcus faecalis, whereas quercetin inhibited the growth of all analyzed strains (Table 3).The controls using methanol are related to the final concentration of solvent in the plate containing quercetin or rutin, due to the fact of these substances are soluble only in this condition. At 10%, methanol in culture showed influence on the growth of P. aeruginosa, C. freundii, E. coli (17-2), E. coli (ATCC 25922) and Staphylococcus aureus.
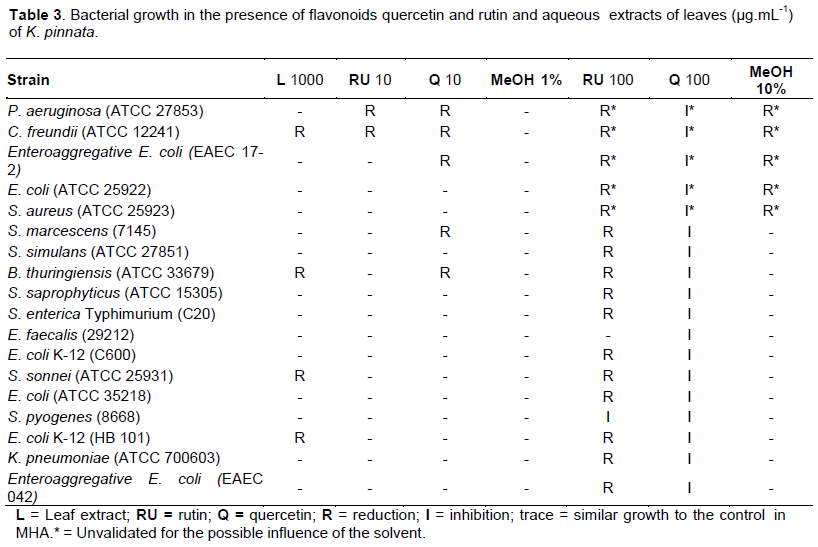
Therefore, results of reduction or inhibition of growth of these strains in the presence of 100 μg.mL-1 of rutin and quercetin cannot be considered due to the possible influence of the solvent in cultivation. Conversely, such as 10% methanol in culture has no influence on the growth of other strains, these results can be validated.
Antibiofilm activity
The formation of biofilms of enteroaggregative E. coli (EAEC 042) was altered when grown in the presence of aqueous extract of leaves from K. pinnata and quercetin (Figure 2). Although not express inhibiting the growth of this strain in the macrodilution assay (Table 3), the aqueous extracts of the leaves were able to reduce the formation of biofilms of the EAEC 042 exceeding 50%.
This reduction was observed in both tested concentrations (500 and 1000 μg.mL-1).
At the highest concentration tested (50 μg.mL-1), quercetin was able to reduce biofilm formation. For this compound, the inhibitory concentration for 50% of biofilm formation (IC50) was 70.17 μg.mL-1. However, no inhibition was observed in the cultivation in the presence of rutin at concentrations of 5 or 50 μg.mL-1.
The HEp-2 strain was resistant to the presence of the aqueous extract of K. pinnata in cultivation, which was not observed for the other cell lines. The phytochemical analysis revealed the presence of flavonoids in this extract. Once cytotoxicity was not observed to any lineage to the flavonoids quercetin and rutin, the toxicity observed in the leaf extract may not be related to the free flavonoids present in the plant.
The cytotoxic activity of extracts of the species has been attributed to compounds called bufadienolides. These are characteristically present in leaves and stems of K pinnata, being demonstrated high cytotoxicity in human carcinoma lines, especially the compounds bryofilin B and bryofilin A (Milad et al., 2014). The presence of these compounds in the assessed aqueous extract may be related to the sensitivity observed in Caco-2 and T84 lines. This metabolite group can be considered the largest in this activity for this species, also having potential for chemoprevention of cancer (Supratman et al., 2001).
The aqueous extract of leaves showed a reduction in the growth of four of the evaluated strains. The antibacterial activity of aqueous extracts of K. pinnata leaves was also observed in Propionibacterium acnes (Kumar et al., 2013), Alcaligenes faecalis and Bacillus subtilis (Sharma et al., 2014).
As observed for the aqueous extract, in vitro studies have demonstrated the antibacterial activity of methanolic extracts of K. pinnata leaves. This action is observed in strains of genres evaluated in this study, as E. faecalis (Aibinu et al., 2007) E. coli (Pattewar et al., 2013), K. pneumoniae (Okwu and Nnamdi, 2011), P. aeruginosa (Tatsimo et al., 2012), S. typhi (Nwadinigwe, 2011), S. dysenteriae (Akinpelu, 2000), and S. aureus (Biswas et al., 2011).
The antibacterial activity in S. marcescens was also observed in methanol extracts of the plant (Sharma et al., 2014). In this work, the reduction and inhibition activities of this strain were observed only when grown in the presence of quercetin. The flavonoid may be one of the active principles present in methanolic plant extracts, since it shows high solubility in methanol (Megawati and Fajriah, 2013).
Quercetin has noted antibacterial activity at concentrations above 10 μg.mL-1 being able to inhibit the growth of all the strains tested in this study. Besides these strains, quercetin also presents antibacterial activity in strains of Proteus mirabilis, Acinetobacter baumannii, Helicobacter pylori and also methicillin resistant S. aureus (MRSA) when evaluated alone or in combination with oxacillin (Ramos et al., 2006, Ozcelik et al., 2006).
Also present in K. pinnata extracts, rutin demonstrated antibacterial activity for all tested strains. The most significant result for this flavonoid was held at the concentration of 100 μg.mL-1, with the reduction of all tested strains. In some strains, like E. coli and S. aureus, the minimum inhibitory concentration for rutin is presented in amounts exceeding 100 μg.mL-1 (Araruna et al., 2012). This shows the lack of inhibitory activity in low concentrations of rutin, as well as 10 μg.mL-1, evaluated in this study.
The antibacterial activity of aqueous extract of leaves of the species may also be related to the presence of other compounds derived from plant secondary metabolism, such as saponins, phenols and tannins, present in the extract evaluated in this study (Table 2).
The antibacterial activity of saponins isolated from plant extracts has been demonstrated in strains of E. coli, B. subtilis and others, as well as antifungal activity (Edewor et al., 2009, Kannabiran et al., 2009, Maatalah et al., 2012). Similarly, tannins and hydrolyzed tannins are active against the growth of E. coli, B. subitilis, B. cereus and B. licheniformis, Shigella boydii, S. flexneri and others, yeasts and fungi (Banso and Adeyemo, 2007, Lim et al., 2006). Antimicrobial activity of phenolic compounds, such as phenolic acids and coumarins, was observed in strains of E. coli, Bacillus cereus and fungi (Nohynek et al., 2006, Nitiema et al., 2012). The increase and the effectiveness of antibacterial activity of derivatives of phenolic acids are usually related to increasing the alkyl chain in the molecule, also the butyl ester regions being effective (Merkl et al., 2010).
Some phytochemical compounds have the ability to control the establishment and growth of bacterial biofilms. These compounds may act at different stages of biofilm formation, such as in bacterial adhesion, motility and "quorum-sensing", and have the advantage of having a lower probability of bacterial resistance (Borges et al., 2013). Besides acting in antimicrobial activity, plant extracts can be the basis for the synthesis of active compounds. K. pinnata leaf extracts can be used for the synthesis of silver nanoparticles with antibacterial and antibiofilm activity in P. aeruginosa, S. aureus, S. pyogenes and Salmonella enterica Typhi. This activity proves to be higher than that observed for ciprofloxacin (Phatak and Hendre, 2016).
As observed for EAEC, quercetin also shows antibiofilm activity to S. aureus, observed in in vitro assays. This quercetin in plant extracts is able to act in the repression of bacterial genes of cell adhesion and reduces the hemolytic capacity of S. aureus, being suggested for use in inhibiting the formation of recalcitrant biofilms (Lee et al., 2013).
It can be concluded that flavonoids, like quercetin, are active principles of the extracts of Kalanchoe pinnata for antibacterial activity. Thus, phytochemical studies aimed at the research and isolation of flavonoids of the species are promising in the search for new antimicrobial agents.
The aqueous extract of K. pinnata leaves at low concentrations and the flavonoids quercetin and rutin showed no cytotoxicity in tested concentrations. The evaluated material presented antibacterial activity to various strains and potential to inhibit the formation of biofilms of EAEC 042. Quercetin must be considered as one of the active substance of interest responsible for the antibacterial activity.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Aziz SM, Aeron A (2014). Bacterial biofilm: dispersal and inhibition strategies. SAJ Biotechnol. 1:1-10.
Crossref
|
|
|
|
Aibinu IE, Odunayo RA, Adenipekun T, Adelowotan T, Odugbemi T (2007). In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr. J. Tradit. Complement. Altern. Med. 4(3):338-44.
|
|
|
|
|
Akinpelu DA (2000). Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia 71:193-194.
Crossref
|
|
|
|
|
Araruna MKA, Brito SA, Morais-Braga MFB, Santos KKA, Souza TM, Leite TR, Costa JGM, Coutinho HDM (2012). Evaluation of antibiotic & antibiotic modifying activity of pilocarpine & rutin. Indian J. Med. Res. 135:252-254.
|
|
|
|
|
Banso A, Adeyemo SO (2007). Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol. 6:1785-1787.
Crossref
|
|
|
|
|
Barbosa WLR (2001). Manual para análise fitoquímica e cromatográfica de extratos vegetais. Rev. Cient. UFPA. 4:12-18.
|
|
|
|
|
Berry AA, Yang Y, Pakharukova N, Garnett JA, Lee W, Cota E, Marchant J, Roy S, Tuittila M, Liu B, Inman KG, Ruiz-Perez F, Mandomando I, Nataro JP, Zavialov A, Matthews S (2014). Structural insight into host recognition by aggregative adherence fimbriae of enteroaggregative Escherichia coli. PLOS Pathogens 10:1-15.
Crossref
|
|
|
|
|
Biswas SK, Chowdhury A, DAS J, Karmakar UK, Shill MC, (2011). Assessment of cytotoxicity and antibacterial activities of ethanolic extracts of Kalanchoe pinnata linn. (family: Crassulaceae) leaves and stems. Int. J. Pharm. Sci. Res. 2:2605-2609.
|
|
|
|
|
Borges A, Abreu AC, Malheiro J, Saavedra MJ, Simões M (2013). Biofilm prevention and control by dietary phytochemicals, in: Mendez-Vilas A (Ed.), Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Center, Badajoz. pp. 32-41.
|
|
|
|
|
Bueno J (2014). Anti-biofilm drug susceptibility testing methods: looking for new strategies against resistance mechanism. J. Microbial Biochem. Technol. 1:1-9.
|
|
|
|
|
Edewor TI, Ibikunle GJ, Usman LA (2009). Phytotoxic and antimicrobial screening of saponin isolated from ethanolic leaf extract of Xylopia aethipioca. Sci. Focus 14:507-512.
|
|
|
|
|
Gatto MT, Falcocchio S, Grippa E, Mazzanti G, Battinelli L, Nicolsi G, Lambusta D, Saso L (2002). Antimicrobial and Anti-Lipase Activity of Quercetin and Its C2-C16 3-O-Acyl- Esters. Bioorg. Med. Chem. 10:269-272.
Crossref
|
|
|
|
|
Kannabiran K, Mohankumar T, Gunaseker V (2009). Evaluation of antimicrobial activity of saponin isolated from Solanum xanthocarpum and Centella asiatica. Int. J. Nat. Eng. Sci. 3(1):22-25.
|
|
|
|
|
Kumar S, Malik DK, Kumar R (2013). Antimicrobial effects of Mangifera indica, Bombax ceiba, Syzygium cumini and Kalanchoe pinnata against acne-inducing bacteria. Asian J. Exp. Biol. Sci. 4:645-647.
|
|
|
|
|
Lee JH, Park JH, Cho HS, Joo SW, Cho MH, Lee J (2013). Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 29:491-499.
Crossref
|
|
|
|
|
Lim SH, Darah I, Jain K (2006). Antimicrobial activities of tannins extracted from Rhizophora apiculata barks. J. Trop. Forest. Sci. 18:59-65.
|
|
|
|
|
Lopes RM, Oliveira TT, Nagem TJ, Pinto AS (2000). Flavonoides. Biotec. Cienc. Des. 17:18-22.
|
|
|
|
|
Maatalah MB, Bouzidi NK, Bellahouel S, Merah B, Fortas Z, Soulimani R, Saidi S, Derdour A (2012). Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulata. J. Biotechnol. Pharm. Res. 3:54-57.
|
|
|
|
|
Majaz QA, Tatiya AU, Khurshid M, Nazim S, Siraj S (2011). The miracle plant (Kalanchoe pinnata): A phytochemical and pharmacological review. Int. J. Res. Ayurveda Pharm. 2(5):1478-1482.
|
|
|
|
|
Megawati AD, Fajriah S (2013). 3',4'-Dimethoxy Quercetin, a flavonol compound isolated from Kalanchoe pinnata. J. Appl. Pharm. Sci. 3:88-90.
|
|
|
|
|
Merkl R, Hradkova I, Filip V, Smidrkal J (2010). Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 28:275-279.
|
|
|
|
|
Milad R, El-Ahmady S, Singab AN (2014). Genus Kalanchoe (Crassulaceae): a review of its ethnomedicinal, botanical, chemical and pharmacological properties. Eur. J. Med. Plants 4:86-104.
Crossref
|
|
|
|
|
Mittal AK, Kumar S, Banerjee UC (2014). Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 431:194-9.
Crossref
|
|
|
|
|
Muzitano MF, Tinoco LW, Guette C, Kaiser C, Rossi-Bergmann B, Costa SS (2006). The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry 67:2071-2077.
Crossref
|
|
|
|
|
Muzitano MF, Bergonzi MC, De Melo GO, Lage CLS, Bilia AR, Vincieri FF, Rossi-Bergmann B, Costa SS (2011). Influence of cultivation conditions, season of collection and extraction method on the content of antileishmanial flavonoids from Kalanchoe pinnata. J. Ethnopharmacol. 133:132-137.
Crossref
|
|
|
|
|
Namasivayam SKR, Roy EA (2013). Antibiofilm effect of medicinal plat extracts against clinical isolate of biofilm of Escherichia coli. Int. J. Pharm. Pharm. Sci. 5:486-489.
|
|
|
|
|
Nitiema LW, Savadogo A, Simpore J, Dianou D, Traore AS (2012). In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 3:183-187.
|
|
|
|
|
Nohynek LJ, Alakomi HL, Kahkonen MP, Heinomen M, Helander IM, Oksman-Caldentey KM, Puupponen-Pimia RH (2006). Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 54:18-32.
Crossref
|
|
|
|
|
Nwadinigwe AG (2011). Antimicrobial activities of methanol and aqueous extracts of the stem of Bryophyllum pinnatum (Crassulaceae). Afr. J. Biothechnol. 10:16342-16346.
Crossref
|
|
|
|
|
Okwu ED, Nnamdi UF (2011). A novel antimicrobial phenanthrene alkaloid from Bryopyllum pinnatum. J. Chem. 8(3):1456-1461.
|
|
|
|
|
Oliveira LFG, Gilbert B, Villas Bôas GK (2013). Oportunidades para inovação no tratamento da leishmaniose usando o potencial das plantas e produtos naturais como fontes de novos fármacos (Opportunities for innovation in the treatment of leishmaniasis using the potential of plants and natural products as sources of new drugs). J. Fitos Electron. 8(1):33-41.
|
|
|
|
|
Ozcelik B, Orhan I, Toker G (2006). Antiviral and antimicrobial assessment of some selected flavonoids. Z. Naturforsch 61:632-638.
|
|
|
|
|
Phatak RS, Hendre A (2016). Green synthesis of silver nanorods using aqueous extract of kalanchoe pinnata fresh leaves and its synergistic effect with ciprofloxacin and antibiofilm activities. Int. J. Pharm. Pharm. Sci. 8:168-174.
|
|
|
|
|
Pattewar SV, Patil DN, Dahikar SB (2013). Antimicrobial potential of extract from leaves of Kalanchoe pinnata. Int. J. Pharm. Sci. Res. 4:4577-4580.
|
|
|
|
|
Prasad VGNV, Krishna BV, Swamy PL, Rao TS, Rao GS (2014). Antibacterial synergy between quercetin and polyphenolic acids against bacterial pathogens of fish. Asian Pac. J. Trop. Dis. 4:326-329.
Crossref
|
|
|
|
|
Rahman AE, Moinuddin M, Molla M, Worku A, Hurt L, Kirkwood B, Mohan SB, Mazumder S, Bhutta Z, Raza F, Mrema S, Masanja H, Kadobera D, Waiswa P, Bahl R, Zangenberg M, Muhe L (2014). Childhood diarrhoeal deaths in seven low- and middle-income countries. Bull. World Health Organ. 92:664-671.
Crossref
|
|
|
|
|
Ramos FA, Takaiashi Y, Shirotori M, Kawaguchi Y, Tsuchiya K, Shibata H, Higuti T, Tadokoro T, Takeuchi M (2006). Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) Skin. J. Agric. Food Chem. 54:3551-3557.
Crossref
|
|
|
|
|
Sharma A, Bhot M, Chandra N (2014). In vitro antibacterial and antioxidant activity of Bryophyllum pinnatum (Lam.) Kurz. Int. J. Pharm. Pharm. Sci. 6:558-560.
|
|
|
|
|
Soberon JR, Sgariglia MA, Sampietro DA, Quiroga EN, Vattuone MA (2007). Antibacterial activity of plant extracts from northwestern Argentina. J. Appl. Microbiol. 102:1450-1461.
Crossref
|
|
|
|
|
Tapas AR, Sakrkar DM, Kakde RB (2008). Flavonoids as nutraceuticals: a review. Trop. J. Pharm. Res. 7:1089-1099.
Crossref
|
|
|
|
|
Tatsimo SJN, Tamokou JD, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P (2012). Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 5:1-6.
Crossref
|
|