Full Length Research Paper
ABSTRACT
The chemical composition and seasonal variation of essential oils (EO) extracted from the aerial parts of the traditional medicinal plant Piper rivinoides were analyzed using gas chromatography (GC) coupled with mass spectrometry (MS) and GC coupled with flame ionization detector (GC-FID) technique, respectively. The wild plants were collected from two different sites in the Atlantic Forest. The analysis allowed us to identify 96.60 to 99.80% of the EO composition. The major compounds with the highest relative percentage for both specimens, regardless of the season, were the bioactive monoterpenes α-pinene and β-pinene, which ranged from 34.78 (summer) to 53.87% (winter) and from 15.24% (autumn) to 47.71% (winter), respectively. The seasonal stability of the major compounds in the two study specimens throughout the year indicates that the phenological cycle influences biosynthesis more than abiotic factors. This type of chemical phenotypic stability is rarely observed in species belonging to the Piperaceae family, which is characterized by high chemical variability. Furthermore, this stability is favorable, and P. rivinoides has the potential to be a source of bioactive compounds.
Key words: 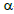 -pinene,
-pinene, 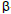 -pinene, Atlantic Forest, aromatic plant, volatile compounds.
-pinene, Atlantic Forest, aromatic plant, volatile compounds.
INTRODUCTION
Piper rivinoides Kunth is a native species of Brazil used for medicinal, ritual, and aromatic purposes. It is popularly known as "Betis-Branco", "Aperta-ruão", "Murta" or "Ruão". P. rivinoides is widely distributed throughout the Brazilian territory, and is described as a glabrous and ciophilous shrub that grows up to 3-6 m tall, has a well-woody trunk, shiny ovate leaves with a symmetrical base, showy and triangular inflorescences, and fruit clusters of the erect spike type(Yuncker, 1972; Flora do Brasil, 2020; Queiroz and Guimarães, 2020).
P. rivinoides has the liturgical name "Èwé Boyí Funfun" according to ethnobotanical surveys in Brazil, and its use in cleansing baths in religious rituals originated in Africa. P. rivinoides has been used medicinally to treat wounds, ulcers, vaginal discharge, bleeding, and even oral problems. Furthermore, this plant is sold in folk markets and collected from wild specimens (Barros, 2010; Mandarino and Gomberg, 2009; Albuquerque et al., 2007).
Previous research with the extracts of this species revealed that the hexane fraction contains a high concentration of bioactive benzofuran neolignans (eupomatenoids and conocarpan) and pharmacological potential against Candida albicans and Leishmania amazonensis (Moreira et al., 2016; Marques et al., 2014).
Literature data on EO, obtained by hydrodistillation from fresh leaves of P. rivinoides, have shown a great chemical diversity rich in monoterpenes, sesquiterpenes, and arylpropanoids, which may vary depending on the collection site (Bernuci et al., 2016; Perigo et al., 2016; Leal et al., 2019). Aspects of the pharmacological potential of these EO indicate their antinociceptive (Souza et al., 2014), antifungal (Bernuci et al., 2016; Leal et al., 2019), antimicrobial (Leal et al., 2019), and cytotoxic (Fonseca et al., 2020; Machado et al., 2021) activity.
Recently, the apoptotic effect of β-pinene (main component) and EO of P. rivinoides in oral squamous cell carcinoma (Machado et al., 2021) was described. This study confirmed the plant’s long history of traditional medicinal use in the treatment of oral problems (Barros, 2010). Essential oil research is of interest in the production of herbal raw materials due to their functions as medicines, cosmetics, and other products. Because of the low cost of extraction, processing is advantageous in low-income areas and allows for large-scale production with low operating costs, which would become profitable through incentive programs and public project funding (Araújo et al., 2021).
Despite the fact that P. rivinoides is widely used in traditional medicine, research on the seasonal variation profile of EO from this plant is limited. It is well known that biotic and abiotic factors influence the large chemical phenotypic plasticity of Piperaceae species, and that these variations in the composition of plant chemistry can lead to changes in their respective biological potential (Monzote et al., 2010; Salehi et al., 2019).
Based on the foregoing, this study aims to evaluate the effect of seasonality on the chemical profile of EO of wild specimens of the medicinal plant P. rivinoides collected from two different sites in fragments of the Atlantic Forest with varying altitudes and climatic patterns. Determining the chemical nature of EO of P. rivinoides in different months over a year and at two different collection sites can help us understand the chemotaxonomics and chemophenetics of the plant and the genus Piper. Furthermore, this study provides tools for determining the best time to collect plant material that can provide an EO with high-quality secondary metabolites and, consequently, biological activities.
MATERIALS AND METHODS
Plant
Samples of fresh leaves of wild P. rivinoides were collected from two specimens in different regions of the Atlantic Forest in Rio de Janeiro State: (a) Tijuca National Park, Rio de Janeiro/RJ (PRR) (S 22° 57' 09. 94"; E 43° 01 '17. 19", elevation 205 m (permit SISBIO n. 57296-1) and (b) Serra da Tiririca State Park, Niterói/RJ (PRN) (S 22° 97' 18.24"; E 43° 24' 23.78", elevation 28 m (INEA permit n° 07/ 0002.4729/ 2019). Dr. Elsie Franklin Guimares performed the botanical identification of the plant material at the Research Institute of the Botanical Garden of Rio de Janeiro, and the herborized specimens were deposited in the Herbarium of the College of the State of Rio de Janeiro (HRJ) under voucher numbers 13404 for PRR and 13403 for PRN. In each season of 2019, one specimen was collected twice: summer (January and February), autumn (April and May), winter (July and August), and spring (October and November), always in the morning between 9:00 and 10:00 am. Data on abiotic factors, including average temperature (°C), humidity (%), and precipitation (mm), were obtained from the Brazilian Institute of Metrology and Research (INMET) and are presented in Figure 1.
Extraction of essential oil
For 2 h, approximately 100 g of fresh leaves were hydrodistilated in a modified Clevenger apparatus (Oliveira et al., 2014). The EO was separated from the aqueous phase and dried with anhydrous sodium sulfate. The total yield was expressed as a percentage, considering the weight of EO (g) per 100 g of fresh plant material. The EO was stored in sealed amber vials under refrigeration at -20°C until analysis (Oliveira et al., 2014).
Essential oil analysis
Chemical characterization and quantification of the EO of P. rivinoides was performed by gas chromatography (GC) coupled with mass spectrometry (MS) and GC coupled with flame ionization detector (GC-FID). GC-FID analyses were performed in triplicate to increase accuracy. The EO was diluted in dichloromethane (1 mg/mL) [HPLC grade, Tedia, Brazil] before analysis.
GC-MS and GC-FID conditions are as follows: GC-MS analyses were performed using a gas chromatograph HP Agilent GC 6890 coupled to a mass spectrometer Agilent MS 5973N, with an ionization energy of 70 eV (positive mode). EO solution was injected at 1 μL (splitless), with an injector temperature of 270°C and equipped with HP-5MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness) capillary column [Agilent J & W; GC columns (USA)], temperature programming from 60 to 240°C, with an increase of 3°C/min (60 min total run), using helium (~99.99%) as carrier gas at a constant flow rate of 1.0 mL/min and mass range m/z 40-600 atomic mass units (u). GC-FID analyses were performed using a gas chromatograph HP-Agilent 6890 GC-FID. EO solution was injected at 1 μL (splitless) with an injector temperature of 270°C, equipped with HP -5MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness) capillary column [Agilent J & W; GC Columns (USA)], temperature programming from 60 to 240°C, with an increase of 3°C/min (60 min total run), using hydrogen as carrier gas at a constant flow rate of 1.0 mL/min (Oliveira et al., 2014).
Compounds identification and statistical analysis
Retention indices (RI) were calculated from the retention time of a homologous series of saturated aliphatic hydrocarbons (C8-C28, Sigma-Aldrich, Brazil) obtained with CG-FID under the same analytical conditions as EO (Dool and Kratz, 1963). Compounds in the EO were identified by comparing their mass spectra with database records (WILEY 7n, NIST) and by comparing the calculated RIs with those from the literature (Adams, 2017). Furthermore, co-injection with authentic standards was performed wherever possible, as described previously. All data on the percentage of compounds in the EO were reported as mean ± standard deviation for three independent experiments (extraction).
Principal component analysis (PCA) and hierarchical analysis (HCA) graphs were constructed for chemometric analysis, in which the chemical composition of EO was treated as operational taxonomic units. For this purpose, the percentage values (% area) were extracted from the CG data, transformed into a sinusoidal arc of the root of p, and converted into a matrix, excluding unidentified compounds (Catinella et al., 2021). The Euclidean distance was combined with the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method to generate the dendrogram. Multiple comparisons between the main components of EO and abiotic factors (temperature, humidity, precipitation) were performed using Pearson correlation (p < 0.05). All results were processed using STATISTICA version 10 software (StartSoft Inc., Tulsa, USA).
RESULTS AND DISCUSSION
Essential oil yield
A total of 16 colorless samples were obtained from EO. These results are presented in Table 1. The yields found in this study for wild P. rivinoides ranged from 0.16 to 1.36 (w/w) (Table 1), higher than others previously reported in the literature (0.05 (Souza et al., 2014) and 0.63 (Perigo et al., 2016)). Monthly yields varied similarly at both collection sites, with no site dominating (p > 0.05) (Figure 2).
There was a considerable increase in the EO yield in the spring, which coincides with the reproductive phase of the plant (flowering). A tendency to increase yield was also noticed as the temperature decreased. So, a lower quantity of EO was obtained in the hottest months of the year (summer). Similar results were found for other Piperaceae species such as Piper mosenii C. DC and Piper gaudichaudianum Kunth (Del Quiqui et al., 2019). Other reports in the literature also corroborate these findings (El Beyrouthy et al., 2015; Marques et al., 2014).
Pearson's analysis did not show correlations between EO yield and precipitation and temperature. However, there was a moderate correlation between EO yield and humidity for both locations (r = 0.5954 PRN and r = 0.6588 PRR), but significant only for the results of PRN (p < 0.05). According to the literature, in humid environments where water is not a limiting factor, as in rainforests, there is an increase in the rate of plant transpiration, which may explain this correlation. When the rate of metabolism increases the rate of volatilization decreases, then the EO yield is directly affected (Queiroz et al., 2017). AHC of bioclimatic data suggested that general volatile differentiation in P. rivinoides from Rio de Janeiro essentially does not represent an adaptive response to bioclimatic, or geological conditions. Therefore, there was no clear pattern of correlation between climate and EO yield for P. rivinoides, suggesting the biggest connection to the vegetative cycle than climatic variations, a fact previously described for other plants (El Beyrouthy et al., 2015; Shadi and Saharkhiz, 2016).
In the spring, which coincides with the reproductive phase of the plant (flowering), there was a significant increase in EO yield. A tendency to increase yield was also observed when temperatures decreased. Thus, a lower amount of EO was obtained during the hottest months of the year (summer). Similar results were found for other Piperaceae species such as P. mosenii C. DC and P. gaudichaudianum Kunth (Del Quiqui et al., 2019). Other reports in the literature also confirm these results (El Beyrouthy et al., 2015; Marques et al., 2014).
Pearson analysis showed no correlations between EO yield and rainfall and temperature. However, there was a moderate correlation between EO yield and moisture for both sites (r = 0.5954 PRN and r = 0.6588 PRR), but it was significant only for the results of PRN (p < 0.05). The literature states that in humid environments where water is not a limiting factor, such as rainforests, plant transpiration rate increases, which could explain this correlation. When the metabolic rate increases, the rate of volatilization decreases, affecting the EO yield directly (Queiroz et al., 2017). The AHC of bioclimatic data suggests that the overall differentiation in volatility in P. rivinoides from Rio de Janeiro is essentially not an adaptive response to bioclimatic or geological conditions. Therefore, there was no clear pattern of correlation between climate and EO yield in P. rivinoides, suggesting that the greatest link is to the vegetative and reproductive cycles than to climatic variation, a fact previously described for other plants (El Beyrouthy et al., 2015; Shadi and Saharkhiz, 2016).
Chemical profile of the essential oils
The relative percentage of compounds is shown in Table 1. Forty-one compounds were identified in summer, 37 in fall, 24 in winter, and 21 in spring, representing 98.74, 97.56, 97.58, and 98.56%, respectively, of the total compounds identified.
Both EO had a rich fraction of cyclic non-oxygenated monoterpenes and a minority fraction of sesquiterpenes. However, some compounds were not detected in all individuals, with slight qualitative (richness) and quantitative (content) differences. The compounds with the highest relative content for both specimens, regardless of season, were α-pinene and β-pinene, ranging from 34.78 to 53.87% and from 15.24 to 47.71%, respectively. These results are interesting from a pharmacological point of view, since the main difficulty in the preparation of drugs from natural products is the relative complexity of obtaining bioactive compounds in high concentrations and with chemical stability (Araújo et al., 2021; WHO, 2011).
A large relative percentage of non-oxygenated cyclic terpenes (bicyclogermacrene and limonene) was also found in all seasons. The largest relative percentage of α-pinene and β-pinene is like other results obtained in species of the genus Piper such as Piper anonifolium Kunth (45.0% α-pinene and 18.0% 32.2 β-pinene), Piper bredemeyeri Jacq. (20.3% α-pinene and β-pinene), Piper ovatum Vahl. (23.1% α-pinene and 14.2% β-pinene) (Da Silva et al., 2017), Piper solsianum C. DC (22.7% α-pinene and 3.5% β-pinene), and Piper amplum Kunth (18.1% α-pinene and 0.9% β-pinene) (Perigo et al., 2016). These monoterpenes are also described for other species of the Piperaceae family, such as Piper hainanense Hemsl. (7.14% α-pinene and 0.14% β-pinene), Piper pseudofuligineum C. DC. (6.73% α-pinene); Piper laetispicum C. DC. (6.09% α-pinene and 0.1% β-pinene) (Hao et. al., 2018); and Piper albispicum C. DC (14.6% α-pinene and 15.6% β-pinene (Nguyen et al., 2022). However, the percentages are relatively in the minority for these species.
Increase in the relative percent content of b-pinene in January (44.30%) and July (44.39%) and decrease in the percent content of limonene in the same months (7.08% and 3.07%, respectively) were highlighted in the Serra da Tiririca-Niterói/RJ collecting area (PRN). This variation was not observed in the specimens from the Tijuca/RJ collecting area (PRR), which even showed greater uniformity in the content of α-and β-pinene and greater variation in the content of limonene (2.32% in February to 9.09% in January).
Chemical analysis in the different seasons showed that the intraspecific variation in the EO of leaves of P. rivinoides collected in the two study areas was very low (Figure 3A-B). PCA analysis showed that it is not possible to distinguish the two areas or the four seasons with respect to the biosynthesis of compounds present in EO. This is confirmed by the Euclidean distance formed in the dendrograms (Figure 4A and B), where clusters can only separate samples with a higher content of α-pinene from samples with a higher content of β-pinene and/or limonene, without separating the two study areas. This implies that P. rivinoides exhibits some stability of chemical phenotype in the two different collection areas. Although many studies have reported variation in the chemical profile of EO from the same plant species in different collection areas (Marques et al., 2019; Queiroz et al., 2017; Morshedloo et al., 2018), it is also possible that the variation (chemotype) is small, implying that intrinsic biotic factors are more influential than abiotic factors (Elechosa et al., 2017; Souza et al., 2017).
The results of the present study differ slightly from other results available in the literature, which describe a volatile fraction for P. rivinoides composed exclusively of monoterpenes, with a α-pinene (73.2%) and a lower β-pinene (5.2%) for samples collected in the state of São Paulo/Brazil (Perigo et al., 2016). In another study with wild-collected P. rivinoides in the state of Paraná/ Brazil, the sesquiterpenes bicyclogermacrene (11.8%) and α-humulene (10.0%) were registered as the maincomponents, with a lower relative proportion of α-pinene (4.4%) and and β-pinene (3.7%) (Bernuci et al., 2016). On the other hand, this study agrees with the studies of Souza et al. (2014), who describe as main constituents the monoterpenes α-pinene(32.9%) and b-pinene (20.9%), besides limonene (3.22%). It is worth noting that the collection sites of the present study are fragments of the Atlantic Forest, as well as the specimen cultivated by Souza et al. (2014), whose specimen originated from the Atlantic Forest, suggesting a possible ecotype or chemotype for this species.
It is also notable that metabolites from the acetate-mevalonate or methylerythritol phosphate pathway in the EO are exclusive to both plants studied in the present study. Secondary metabolites from, for example, shikimate was not detected, although they are common in Piperaceae and have been identified in other EO samples of P. rivinoides. The EO of this plant, collected from the Bom Jesus Biological Reserve in the Brazilian state of Paraná, had a chemical composition completely different from the others previously described. The arylpropanoid E-isoelemicin was the main constituent (40.81%), followed by non-oxygenated monoterpenes (16.88%) and non-oxygenated sesquiterpenes (12.45%). In this study, the compound α-pinene, β-pinene and limonene were not detected (Leal et al., 2019).
The presence of α-pinene and β-pinene in a high relative percentage in EO of P. rivinoides from Rio de Janeiro state is significant because studies conducted with these terpenes have shown various biological activities, including activity against infectious bronchitis virus (IBV) with IC50 of 0.98 ± 0.25 and 1.32 ± 0.11 mM for α-pinene and β-pinene, respectively (Yang et al., 2011).
In addition α-pinene showed a dose-dependent growth inhibitory effect on hepatocellular carcinoma cell lines (BEL -7402) with an inhibition rate greater than 79% for all concentrations tested (Chen et al., 2015). A large apoptotic potential or cytotoxic effect against tumor cell line has been reported in the literature for β-pinene (Fonseca et al., 2020; Machado et al., 2021). An assay evaluating nematocidal activity against Bursaphelenchus xylophilus described that among 97 compounds evaluated, the monoterpenes (+)-α-pinene and (-)-α- pinene showed the highest inhibition with 83% and 80%, respectively (IC50 0.64 and 18.03 mM, respectively). For (+)-β-pinene and (-)-β-pinene, the inhibitory effect was moderate at 39% and 35%, respectively (IC50 was not described) (Kang et al., 2013).
Studies with EO of Baccharis reticulata Ruiz & Pav. α-pinene, dillapiole and β-pinene, showed excellent antibacterial potential against multidrug-resistant strains of Staphylococcus aureus (MICs ≥256 μg/mL), Pseudomonas aeruginosa (MICs ≥1024 μg/mL), and Escherichia coli (MICs ≥ 1024 μg/mL). The authors concluded that the effect was related to the presence of α-pinene in high concentrations in the sample (Freitas et al., 2020). Other studies also highlight the antimicrobial potential of terpinene and β- pinene monoterpenes (Freitas et al., 2020; Nguyen et al., 2022; Huong et al., 2019; Leite et al., 2007).
There are numerous other studies in the literature describing the biological potential of α and β-pinene monoterpenes (Medeiros et al., 2017; Pereira et al., 2017; Da França et al., 2015; Rivera-Yañez et al., 2017).
All in all, it is evident that the EO medicinal plant P. rivinoides from fragments of the Atlantic Forest in Rio de Janeiro State has shown potential for biological activities, such as the previously published apoptotic effect of β- pinene on oral squamous cell carcinoma (Machado et al., 2021). However, the activity of an essential oil must be evaluated rather than the activity of some of its constituents to avoid antagonisms or synergies between them. For this reason, the overall relationship between the potential uses of this plant and its bioactivity is still very relative and further studies are needed to confirm its bioactivity.
Furthermore, it is also worth highlighting the significant increase in the levels of limonene in PRN (3.07 - 14.05%) and linalool in PRR (2.32 - 9.09%). This is very important because pure limonene and high levels EO of this compound are bradycardia and antiarrhythmic (Nascimento et al., 2019), antifungal (Viriato, 2014; Cai et al., 2019), molluscicidal (Barros et al., 2020), antibacterial and antibactericidal (Yatoo et al., 2021; Hazrati et al., 2020), antioxidant (Yatoo et al., 2021; Hazrati et al., 2020), antiparasitic activities, among others (Vieira et al., 2018). Antimicrobial (Herman et al., 2016), insecticidal (Estrada et al., 2019), anti-inflammatory (Wu et al., 2014), cytotoxic (Chen et al., 2019), antidepressant (Dos Santos et al., 2018), larvicidal (Fujiwara et al., 2017), anesthetic, and sedative (Pereira et al., 2018; Mirghaed et al., 2016) effects have been reported for linalool.
Pearson analyses were also performed to evaluate the correlation of the main compounds and chemical classes with temperature (°C), humidity (%), and precipitation (mm). The results are described in Table 2.
The monoterpens α-pinene and β-pinene, and limonene do not appear to be affected by climatic factors at either collection site. The biosynthesis of monoterpenes and sesquiterpenes also does not seem to be affected by climatic abiotic factors evaluated for Serra da Tiririca State Park (PRN). Other results were found for the specimen from Tijuca National Park (PRR), where the percentage content of monoterpenes showed a negative correlation with temperature (r = -0.7813; p < 0.05), while the content of sesquiterpenes was positively correlated with temperature (r = 0.7236; p < 0.05) and precipitation (r = 0.6055; p < 0.05). For the other components of EO, there was a moderate positive correlation between bicyclogermacrene content and temperature for both sites. However, it is known that interactions resulting from biotic factors with this species at this site may be an important factor in this analysis (Salazar et al., 2016; Whitehead et al., 2021).
CONCLUSION
The results presented here highlight the biological potential of EO, which was extracted from P. rivinoides being suitable for medicinal use in the treatment of various problems due to its chemical composition, which is consistent with its traditional medicinal use. It is suggested that P. rivinoides EO has low chemical phenotypic variability in both areas, despite environmental and seasonal differences. This chemical stability of EO is little observed in Piperaceae species that show great phenotypical chemistry variability. This is a good thing because it means that the EO's biological activities will continue to be active even in different months with different abiotic influences, making P. rivinoides a potential source of bioactive compounds. The seasonality of chemical variations suggests that the phenological cycle has a greater influence on biosynthesis than abiotic factors. Based on the results presented and the literature, we suggest possible chemotypes or ecotypes for P. rivinoides. However, a more in-depth study is needed to confirm them by examining EO samples from different regions of Brazil in the field and in cultivation.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors thank CAPES, FAPERJ, PROEP - CNPq (407845/2017-8) for their financial support.
REFERENCES
|
Adams RP. (2017). Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4.1ª ed. Allured Publishing Corporation. |
|
|
Albuquerque UP, Monteiro JM, Ramos MA, Amorim ELC (2007). Medicinal and magic plants from a public market in northeastern Brazil. Journal of Ethnopharmacology 110(1):76-91. |
|
|
Araújo SJD, Madureira MT, Nascimento LP, Maciel MCB (2021). Sistema Nacional de Redes Fito: uma abordagem sobre o desafio da produção de matérias primas vegetais com qualidade. Revista Eletrônica TECCEN 14(2):17-27. |
|
|
Barros AJ (2010). Ossaim O Orixá E Nossos Chás Volume Único. Clube de Autores (managed), 333, São Paulo. |
|
|
Barros PR, Costa LJDD, Sousa DA, Everton GO, Reis JB, Louzeiro HC, Mouchrek-Filho VE (2020). Study of the chemical composition, toxicity and molluscicidal activity of the essential oil Citrus sinensis (L.) Osbeck. Revista Colombiana de Ciências e Química Farmaceuticas 49(1):28-43. |
|
|
Bernuci K, Iwanaga CC, Fernandez-Andrade CMM, Lorenzetti FB, Torres-Santos EC, Faiões VDS, Gonçalves JE, Amaral W, Deschamps C, Scodro RBL (2016). Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 21(12):1698. |
|
|
Cai R, Hu M, Zhang Y, Niu, C, Yue T, Yuan Y, Wang Z (2019). Antifungal activity and mechanism of citral, limonene, and eugenol against Zygosaccharomyces rouxii. LWT 106:50-56. |
|
|
Catinella G, Badalamenti N, Ilardi V, Rosselli S, De Martino L, Bruno M. (2021). The essential oil compositions of three Teucrium taxa growing wild in Sicily: HCA and PCA analyses. Molecules 26(3):643. |
|
|
Chen Y, Weng Y, Zhou M, Meng Y, Liu J, Yang L, Zuo Z (2019). Linalool-and α-terpineol-induced programmed cell death in Chlamydomonas reinhardtii. Ecotoxicology and Environmental Safety 167:435-440. |
|
|
CHEN W, LIU Y, LI M, MAO J, ZHANG L, HUANG R, JIN X, YE L (2015). Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing g2/m cell cycle arrest. Journal of Pharmacological Sciences 127(3):332-338.. |
|
|
Da França RKA, Amorim LV, Dias CN, Moraes DFC, Carneiro SMP, Amorim CF (2015). Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. Journal of Ethnopharmacology pp. 16032-16040. |
|
|
Da Silva JK, Da Trindade R, Alves NS, Figueiredo PL, Maia JGS, Setzer WN. (2017). Essential Oils from Neotropical Piper Species and Their Biological Activities. International Journal of Molecular Sciences 18(12):2571. |
|
|
Del Quiqui EM, Deschamps C, do Amaral W, Sipriano RR, Machado MP (2019). Yield and Chemical Composition of Essential oil of Piperaceae in one Segment of the Semi deciduous Forest of Paraná State, Brazil, in Seasonal Sampling Research and Science 6(5): 355-367. |
|
|
Dool HVD, Kratz PDA (1963). A Generalization of retention index system including linear temperature programmed Gas-Liquid Partition Chromatography. Journal of Chromatography 11:463-471. |
|
|
Dos Santos ÉRQ, Maia CSF, Junior EAF, Melo AS, Pinheiro BG, Maia JGS (2018). Linalool-rich essential oils from the Amazon display an antidepressant-type effect in rodents. Journal of Ethnopharmacology 212:43-49. |
|
|
El Beyrouthy M, Cazier F, Arnold NA, Aboukais A (2015). Seasonal Variation in Yield and Composition of Essential Oil from Satureja cuneifolia Ten. Growing Wild in Lebanon. Journal of Essential Oil-Bearing Plants 18(4):908-916. |
|
|
Elechosa MA, Lira PDL, Juárez MA, Viturro CI, Heit CI, Molina AC, Martínez AJ, Lopéz S, Molina AM, van Baren CM, Bandoni AL (2017). Essential oil chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochemical Systematics and Ecology 74:19-29. |
|
|
Estrada JLT, Moscoso KEP, Salas IF, Achee NL, Grieco JP (2019). Spatial repellency and other effects of transfluthrin and linalool on Aedes aegypti and Aedes albopictus. Journal of Vector Ecology 44(1):89-93. |
|
|
Flora do Brasil (2020). Piper in Flora do Brasil. 2020. Jardim Botânico do Rio de Janeiro. |
|
|
Fonseca ACC, de Queiroz LN, Felisberto JS, Ramos YJ, Marques AM, Wermelinger GF, Pontes B, Moreira DL, Robbs BK (2020). Cytotoxic effect of pure compounds from Piper rivinoides Kunth against oral squamous cell carcinoma. Natural Product Research pp. 1-5. |
|
|
Freitas PR, de Araújo ACJ, dos Santos Barbosa CR, Muniz DF, da Silva ACA, Rocha JE, Coutinho HDM (2020). GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Industrial Crops and Products 145:112106. |
|
|
Fujiwara GM, Annies V, de Oliveira CF, Lara RA, Gabriel MM & Betim FC (2017). Evaluation of larvicidal activity and ecotoxicity of linalool, methyl cinnamate and methyl cinnamate/linalool in combination against Aedes aegypti. Ecotoxicology and Environmental Safety 139:238-244. |
|
|
Hao CY, Fan R, Qin XW, Hu L, Tan LH, Xu F, Wu BD (2018). Characterization of volatile compounds in ten Piper species cultivated in Hainan Island, South China. International Journal of Food Properties 21(1):633-644. |
|
|
Hazrati S, Govahi M, Sedaghat M, Kashkooli AB (2020). A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior). Industrial Crops and Products 157:112942. |
|
|
Herman A, Tambor K, Herman A (2016). Linalool affects the antimicrobial efficacy of essential oils. Current Microbiology 72(2):165-172. |
|
|
Huong LT, Hung NH, Dai DN, Tai TA, Hien VT, Satyal P, Setzer WN (2019). Chemical compositions and mosquito larvicidal activities of essential oils from piper species growing wild in Central Vietnam. Molecules 24(21):3871. |
|
|
Kang JS, Kim E, Lee SH, Park IK (2013). Inhibition of acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus, by phytochemicals from plant essential oils. Pesticide Biochemistry and Physiology 105(1):50-56. |
|
|
Leal AL, Machado AJT, Bezerra CF, Inácio CES, Rocha JE, Sales DL (2019). Chemical identification and antimicrobial potential of essential oil of Piper rivinoides kunth (BETIS-WHITE). Food and Chemical Toxicology 131:110559. |
|
|
Leite AM, de Oliveira LE, Souza EL, Diniz MDFFM, Trajano VN, Medeiros IA (2007). Efeito inibitório de eugenol, beta-pineno e alfa-pineno sobre o crescimento de bactérias Gram-positivas potencialmente causadoras de endocardite infecciosa. Revista Brasileira de Ciências Farmaceuticas 43(1):121-126. |
|
|
Machado TQ, Felisberto JRS, Guimarães EF, Queiroz GAD, Fonseca, ACCD, Ramos YJ, Moreira DL, Roobs BK (2021) Apoptotic efect of β-pinene on oral squamous cell carcinoma as one of the major compounds from essential oil of medicinal plant Piper rivinoides Kunth. Natural Product Research 36(6):1636-1640. |
|
|
Mandarino AC, Gomberg E (2009). Água e ancestralidade Jeje-Nagô: possibilidade de existências. Textos de História 17(1):143-162. |
|
|
Marques A, Paiva R, Kaplan MA, Moreira DL, Barreto AL, Curvelo A (2014). Bioactive neolignans from Piper rivinoides Kunth (Piperaceae). Planta Médica 80(16):P1L106. |
|
|
Marques APS, Bonfim FPG, Dantas WFC, Puppi RJ, Marques MOM (2019). Chemical composition of essential oil from Varronia curassavica Jacq. accessions in different seasons of the year. Industrial Crops and Products 140:111656. |
|
|
Medeiros VM, do Nascimento YM, Souto AL, Madeiro SAL, de Oliveira Costa VC, Silva SMP, Tavares JF (2017). Chemical composition and modulation of bacterial drug resistance of the essential oil from leaves of Croton grewioides. Microbial Pathogenesis 111:468-471. |
|
|
Mirghaed AT, Ghelichpour M, Hoseini SM (2016). Myrcene and linalool as a new anesthetic and sedative agents in common carp, Cyprinus carpio-Comparison with eugenol. Aquaculture 464:165-170. |
|
|
Monzote L, Cardoso MDG, Batista LR, Rodrigues LMA, Figueiredo ACDS (2010). Chemistry, cytotoxicity, and antileishmanial activity of the essential oil from Piper auritum. Memorias do Instituto Oswaldo Cruz, Rio de Janeiro 105(2):168-173. |
|
|
Moreira DL, de Paiva RA, Marques AM, Borges RM, Barreto ALS, da Rocha Curvelo JA, Cavalcanti JF, Romanos TV, Soares RMA, Kaplan MAC (2016). Bioactive Neolignans from the Leaves of Piper rivinoides Kunth (Piperaceae). Records of Natural Products 10(4):472-484. |
|
|
Morshedloo MR, Maggi F, Neko HT, Aghdam MS (2018). Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Industrial Crops Products 111:1-7. |
|
|
Nascimento GA, Souza DS, Lima BS, Vasconcelos CM, Araújo AA, Durço AO, Quintans-Junior LJ, Almeida JR, Oliveira AP, Santana-Filho VJ, Barreto AS (2019). Efeitos bradicardicos e antiarritmicos do D-Limoneno em ratos. Arquivos Brasileiros de Cardiologia 113(5):925-932. |
|
|
Oliveira GL, Vieira TM, Nunes VF, De Ruas MO, Duarte ER, Kaplan MAC, Martins ER (2014). Chemical composition and efficacy in the egg-hatching inhibition of essential oil of Piper aduncum against Haemonchus contortus from sheep. Revista Brasileira de Farmacognosia 24:288-292. |
|
|
Pereira I, Severino P, Santos AC, Silva AM, Souto EB (2018). Linalool bioactive properties and potential applicability in drug delivery systems. Colloid Surfaces B: Biointerfaces 171:566-578. |
|
|
Pereira NLF, Aquino PE, Júnior JG, Cristo JS, Vieira-Filho MA, Moura FF, Ferreira NMN, Silva MKN, Nascimento EM, Correira FMA, Cunha FAB (2017). Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. Journal of Photochemistry and Photobiology B: Biology 174:144-149. |
|
|
Perigo CV, Torres RB, Bernacci LC, Guimaraes EF, Haber LL, Facanali R, Vieira MAR, Quecini V, Marques MOM (2016). The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern. Industrial Crops and Products 94:528-539. |
|
|
Queiroz GA, Guimarães EF (2020). Peperomia (Piperaceae) no Leste Metropolitano do Rio de Janeiro, Brasil. Rodriguésia P 71. |
|
|
Queiroz TB, Pereira NNDJ, Silva JCRL, Fonseca FSAD, Martins ER (2017). Influence of water regime on initial growth and essential oil of Eucalyptus globulus. Ciência Rural 47(3). |
|
|
Rivera-Yañez CR, Terrazas LI, Jimenez-Estrada M, Campos JE, Flores-Ortiz CM, Hernandez LB, Cruz-Sanchez T, Garrido-Fariña GI, Rodriguez-Monroy MA, Cannales-Mrtinez MM (2017). Anti-Candida activity of Bursera morelensis Ramirez essential oil and two compounds, α-pinene and γ-terpinene-an in vitro study. Molecules 22(12):2095. |
|
|
Salazar D, Jaramillo A, Marquis RJ (2016). The impact of plant chemical diversity on plant-herbivore interactions at the community level. Oecologia 181(4):1199-1208. |
|
|
Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwar ZK, khan T, Sharifi-Rad J, Ozleyen A, Turkdonmez E (2019). Piper species: A comprehensive review on their phytochemistry, biological activities, and applications. Molecules 24(7):1364. |
|
|
Shadi A, Saharkhiz MJ (2016). Changes in Essential oil Contents and Chemical Compositions of Artemisia sieberi at Different Phenological Growth Stages. Analytical Chemistry Letters 6(3):249-256. |
|
|
Souza S, Valverde S, Costa N (2014). Chemical composition and antinociceptive activity of the essential oil of Piper mollicomum and Piper rivinoides Journal of Medicinal Plants 8(22):788-793. |
|
|
Souza TS, Silva FMF, Menini L, Lima SJRC, Parreira LA, Cecon PR, Ferreira A (2017) Ferreira Essential oil of Psidium guajava: Influence of genotypes and environment. Scientia Horticulturae 216:38-44. |
|
|
Vieira AJ, Beserra FP, Souza MC, Totti BM, Rozza AL (2018). Limonene: Aroma of innovation in health and disease. Chemico-Biological Interactions 283:97-106. |
|
|
Viriato A (2014). Terpenoides com atividade antifúngica para Candida Berkhout, causadoras de infecções hospitalares. O Mundo da Saude, 38(1):40-50 |
|
|
Whitehead SR, Schneider GF, Dybzinski R, Nelson AS, Gelambi M, Jos E, Beckman NG (2021) Fruits, frugivores, and the evolution of phytochemical diversity. Oikos pp. 1-16. |
|
|
Wu Q, Yu L, Qiu J, Shen B, Wang D, Soromou LW & Feng H (2014). Linalool attenuates lung inflammation induced by Pasteurella multocida via activating the Nrf-2 signaling pathway. International Immunopharmacology 21(2):456-463. |
|
|
World Health Organization (WHO) (2011). Quality control methods for herbal materials 187 p. |
|
|
Yatoo GN, Wani H, Shah SA, Zargar MI, Rather MA, Banday JA (2021). Gas Chromatographic-Mass Spectrometric Analysis, antibacterial, Antioxidant and Antiproliferative Activities of the Needle Essential Oil of Abies pindrow growing wild in Kashmir, Indian Journal of Microbiology 158:105013. |
|
|
Yang Z, Wu N, Zu Y, Fu Y (2011). Comparative anti-infectious bronchitis virus (IBV) activity of (-)-pinene: effect on nucleocapsid (N) protein. Molecules 16(2):1044-1054. |
|
|
Yuncker TG (1972). The Piperaceae of Brazil. Hoehnea 2:19-366. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0