ABSTRACT
Valerian (Valeriana officinalis L.) is a natural anxiolytic widely used in traditional medicine. However reproductive toxic effects have been demonstrated in mice treated with this species. This study evaluated the effect of valerian aqueous suspension on the reproductive system of Wistar rats treated for 53 days. Four groups were studied (n=25): control (1 ml of distilled water) and treated (500, 1000 or 2000 mg/kg) with the aqueous suspension per os/day. On the 54th day, 15 rats/group were killed and the following parameters were assessed: weight of reproductive organs, liver, kidneys and spleen; serum testosterone level, sperm morphology and concentration; testicle and epididymis histomorphometry. Ten other animals/group was analyzed using the dominant lethal test. There were not significant differences in body weight, hematological and biochemical parameters, weights of the liver, kidneys, spleen, reproductive organs and accessory glands between control and treated animals. The treatment with valerian did not alter the tubular and luminal diameters of the seminiferous tubules, the serum testosterone levels and the sperm viability. The sperm concentration in the epididymis cauda was not altered by the treatment although a significant increase in the number of abnormal gametes was observed in the valerian-treated animals at all dose levels when compared to control values. Significant reduction (p<0.05) in the height of the epididymal epithelium and a significant increase in the number of abnormal gametes were observed in the animals treated with the extract at 1000 and 2000 mg/kg.
Key words: Valerian, Valeriana officinalis, rats, reproduction, male, fertility, testis.
For the past 20 years, male human fertility has been vastly investigated in many aspects, including gamete production, hormonal action and toxicity of exogenous substances. In 1992, Carlsen and collaborators, for instance, reported a progressive decrease in sperm concentration in man (Carlsen et al., 1992; Skakkebæk et al., 2006). Indeed, the human ejaculate concentration, which was approximately 133 million spermatozoa/ml in 1940, has been reduced to 66 million/ml in 1990. The World Health Organization (WHO) (2010) estimates that the actual concentration is 15 million/ml (WHO, 2010). The authors explained one reason could be the human exposure to chemical substances.
The production of viable gametes depends on adequate formation and storage conditions in the reproductive organs that are regulated directly by well-adjusted intrinsic factors. Modifications in life style as a result of stress and eating habits, for example, have increased human exposure to physical, chemical and biological agents that may interfere with homeostasis and lead to a fertility deficit (Carlsen et al., 1992; Kimmel et al., 1995; Pflieger-Bruss et al., 2004; Skakkebæk et al., 2006; WHO, 2010; Ontario College of Family Physicians (OCFP), 2012; Vandenberg et al., 2012).
Phytotherapic medicines and their active compounds as well as drugs and medications are among the chemical agents responsible for causing alterations in the organism and thus in the reproductive system. Therefore, regulatory agencies such as the Food and Drug Administration (FDA), WHO and “Agência Nacional de Vigilância Sanitária” (ANVISA, Brazil) have issued rules and protocols for the study of foods, chemical agents in general, phytotherapics medicines and their constituents with the purpose of verifying the absence of reproductive toxicity of foreign substances (OMS, 2002; ICH, 2005; Turolla and Nascimento, 2006).
Valerian, obtained mainly from the roots of Valeriana officinalis L. (Valerianaceae), is a natural agent broadly used as an alternative therapy for sleep disorders and anxiety (Houghton, 1999; Gooneratne, 2008; Becker et al., 2014; Gromball et al., 2014, Gharib et al., 2015). According to US National Library of Medicine of National Institute of Health (NIH), the recommended doses for humans for inability to sleep (insomnia) are 400 to 900 mg of valerian extract up to 2 h before bedtime for as long as 28 days. Toxicological studies involving the genus Valeriana were carried out and they did not reveal the occurrence of systemic (Olaya et al., 2008) and maternal-fetal (Yao et al., 2007) toxicities. Nevertheless, in a study using female mice treated with Valerian extract during pregnancy, Mahmoudian et al. (2012) observed decreased levels of zinc on fetal brain tissue, which could increase the risk of developing disorders in the offspring. Since zinc is necessary for brain development, the authors have warned the use of Valerian by women during gestation. Furthermore, Al-Majed et al. (2006, 2007) detected toxic effects of the Valerian extract on the reproductive system of male Swiss mice, which was evidenced by diminished proteins and nucleic acids concentration, elevated levels of reactive oxygen species (ROS) in the testis, and high incidence of abnormal spermatozoa.
It is believed that substances present in valerian such as flavonoids can eventually interfere in the normal functioning of the male reproductive system and may exert estrogenic or antiestrogenic effects on the organism (Bhargava, 1989; Hiremath and Hanumantharao, 1990; Hiremath et al., 1997; Das et al., 2004; Guo et al., 2012). Another example is the valepotriates known to be genotoxic and hence, antiproliferative (Hui-Lian et al., 2003; Lin et al., 2015).
Considering the broad use of this phytotherapic medicine (Houghton, 1999; Gharib et al., 2015), the progressive reduction in human fertility observed in the last decades (Carlsen et al., 1992; Pflieger-Bruss et al., 2004; Skakkebæk et al., 2006), the previous studies that demonstrated the reproductive toxicity of the valerian extract in vivo and in vitro (Hui-Lian et al., 2003; Al-Majed et al., 2006), and the potential deleterious effect of its constituents (Hui-Lian et al., 2003; Das et al., 2004; Guo et al., 2012), this study was aimed at evaluating the occurrence of reproductive toxicity caused by the administration of the aqueous suspension of V. officinalis L. to male Wistar rats during a complete spermatogenic cycle.
The experimental protocol followed the international norms established in the manual about care and use of laboratory animals (National Research Council, 2003) and was approved by the Ethics Committee in Animal Experimentation of the Federal University of Juiz de Fora (UFJF) (protocol number 046/2009).
Valerian extract
The dry extract of valerian was imported by QUIMER® (São Paulo, Brazil) company (registration no 002/2009) and supplied by ORTOFARMA® Company (Matias Barbosa, Brazil), where the physicochemical quality analysis was carried out. The dry extract was stored in dark glass vials at room temperature and kept in a vacuum desiccator to avoid humidity. The extract was suspended in deionized water and diluted to the concentration of 2000 mg/ml, which was used as reference for the calculation of the doses administered to the animals. The suspension was prepared daily according to the number of treated animals.
Experimental assay
One hundred male Wistar rats (Rattus norvegicus Berckenhout, 1769) - 90 days old and weight around 220 g - were obtained from the vivarium of the Reproduction Biology Center at the Federal University of Juiz de Fora. The animals were placed in laboratory cages kept in acclimatized shelves ALESCO® (Monte-Mor, Brasil), with airflow, and under standard laboratory conditions, with a controlled temperature of 22 ± 2°C, and a 12-h light/dark photoperiod. They were fed on rat chow pellets (NUVILAB CR1® - Nuvital Nutrientes Ltda. – Colombo, Brazil) and received water ad libitum. The animals were randomly divided into four groups (n = 25) and treated intragastrically by daily gavage as follows. The doses used were chosen according to previous works and followed the recommendations by the regulatory agencies (OMS, 2002; ICH, 2005; Turolla and Nascimento, 2006; Al-Majed et al. 2006; Al-Majed, 2007; Olaya et al., 2008).
Control group: 1 ml of distilled water;
Treatment group 1: 500 mg/kg of body weight of valerian aqueous suspension;
Treatment group 2: 1000 mg/kg of body weight of valerian aqueous suspension;
Treatment group 3: 2000 mg/kg of body weight of valerian aqueous suspension.
Treatment was carried out for 53 days, which corresponded to the duration of the spermatogenic cycle of the Wistar rat (Aslam et al., 1999; Hess and França, 2008). The animals were observed daily for detection of possible clinical signs of systemic toxicity such as piloerection, diarrhea, sialorrhea, stereotypical movements, alterations in locomotor activity, and reduced food consumption. For two hours after administration of the suspension, the animals were observed again. Body weight was recorded before the beginning of treatment and at weekly intervals until the end of treatment. On the 54th day, 15 animals from each group were anesthetized with a combination of ketamine chloridrate (90 mg/kg) and xylazine chrloridrate (10 mg/kg), administered intraperitoneally, for blood collection by cardiac puncture. Afterwards, the animals were killed by asphyxia caused by rupture of the diaphragm and underwent laparotomy for the removal and weighing of the following organs: liver, kidneys and spleen; testes, left epididymis, seminal vesicle and ventral prostate.
Sperm analysis
Two samples of sperm were collected from the epididymal secretion of the right epididymis cauda. One of these samples was placed in a phosphate buffer saline (PBS) drop heated at 34 to 36°C and later diluted 300 times (20 µl in 6 ml of distilled water) for homogenization. From this homogenate, a sample was taken and transferred to a hemocytometer with improved double Neubauer ruling for counting the number of spermatozoa under an optic microscope. Counts for four large corner squares from both sides of the hemocytometer were taken and the total concentration was estimated according to the formula:
Number of sperm/ml = 300 × 104 × average of the quadrants from both sides of the hemocytometer
The other sample was used for the evaluation of sperm morphology. A sperm smear was prepared and stained by use of the Shorr method. Sperm (n = 200) were classified in normal and abnormal under the BX41TF optic microscope, Olympus® (Tokyo, Japan) (1000×) (WHO, 2010).
Histomorphometric analysis
The left epididymis and the right testicle were used for this analysis. The epididymis was fixed in Bouin for 24 h, severed in half, separating the head and cauda, and then transferred to a solution of 30% ethyl alcohol for three days. The testicle was fixed in Davidson′s modified fixative for 24 h, sectioned and transferred to formalin for three days. To conduct a light microscope examination, the organs were embedded in paraffin, sectioned at 5 μm thickness for Gomori trichrome staining (Creasy, 2003). The height of the epididymal epithelium was determined from eight measurements per tubule in 10 transversal tubules/animal (Figure 1) taken from the caput, corpus and cauda of the epididymis. The average epithelium height per tubule was calculated and five epididymides per group were analyzed, totaling 50 averages per group. The tubular and luminal diameter of the seminiferous tubules were estimated by the average obtained between the highest and lowest measures of diameter found in the tubular transverse sections. Five testicles per group and 30 tubules per animal were analyzed, totalling 150 averages per group. The histometric analyses were carried out using the optic microscope (AXIOPHOT HBO50), with camera (AXIOCAM ICc3) and automated digital measurement software (Axiovision Release, version 4.7) - ZEISS® (Jena, Germany).

Blood analysis
The biochemical, hematological and hormonal analyses were performed in a blood sample of approximately 5 ml. The complete blood count was performed in automated cell counter pocH-100iV Diff, Sysmex® (Kobe, Japan), while the biochemical analysis was performed in the automated apparatus Labmax Progress (Labtest®), and kits Labtest® (Lagoa Santa, Brazil) for the dosing of serum creatinine, aspartate aminotransferase, alanine aminotransferase and cholesterol. Serum testosterone level was assayed using the Microreader ELISA plate ASYS HITECH GMBH® (Engendorff, Austria) and the kit Testosterone EIA Kit Caymman Chemicals® (Ann Harbour, USA).
Functional viability test of sperm
At the end of treatment, the 10 remaining animals from each group were evaluated with respect to the functional viability of the gametes using the dominant lethal test. During one week each male was mated with one female in proestrus of proven fertility as evidenced by previous gestation. The presence of spermatozoa in the vaginal smear indicated successful mating and was considered as day one of gestation. Pregnant females were euthanized on the 15th day of gestation as previously described and the following parameters were analyzed:
1. Mean fetus and placenta weight;
2. Number of live fetuses;
3. Number of implants and resorptions;
4. Number of corpora lutea;
5. Presence of external malformations (limbs, face and neural tube closure).
For the evaluation of sperm functional viability, the male was considered the treatment unit and for each male the following indexes were estimated:
1. Mating (inseminated females/mated females × 100);
2. Gestation (females with implants/mated females × 100);
3. Fertility (females with implants/inseminated females × 100);
4. Implantation (number of implants/number of corpora lutea ×100);
5. Pre-implantation loss (number of corpora lutea – number of implants/ number of corpora lutea × 100);
6. Post-implantation loss (number of resorptions/number of implants × 100) (Yakubu and Afolayan, 2009)
Statistical analysis
The data were analyzed using the parametric test one-way analysis of variance (ANOVA) followed by the Dunnett′s test. The Kruskal-Wallis test followed by the Mann-Whitney test (p ≤ 0.01) was used for the analysis of non-normal distributed data. For non-homogeneous variance data, the Dunnett 3 test was used and the Chi-square test was employed for the analysis of proportions. For the analysis of pre- and post-implantation losses, the proportions were transformed into Arc-sin and the variables were analyzed using ANOVA and Dunnett′s test pos hoc (Sokal and Rohlf, 1995). The level of significance considered was α = 0.05. The tests were performed using SPSS for Windows® (Statistical Package for the Social Sciences) version 13 (Chicago, USA).
No clinical signs of toxicity were observed. The variation in body weight during the experimental procedure and the hematological and biochemical parameters are shown in Figure 2 and Table 1, respectively. None of the comparisons were statistically significant. Table 2 shows the weight of the liver, kidneys (average weight of right and left kidneys) and spleen of control and valerian-treated animals. There was no significant difference between the weights of these organs in the valerian-treated and control animals. The weight of the reproductive organs and accessory glands is shown in Table 3, showing no significant difference between control and treated animals. The testicular histometric parameters of control and treated animals are presented in Table 4. The treatment with valerian did not alter the tubular and luminal diameter of the seminiferous tubules when comparing to the diameter of control animals. The height measurements (µm) of the epididymal tubular epithelium of valerian-treated and control animals are shown in Figure 3. The treatment with valerian significantly reduced the height of the epithelium in the animals that received the highest doses (1000 and 2000 mg/kg).
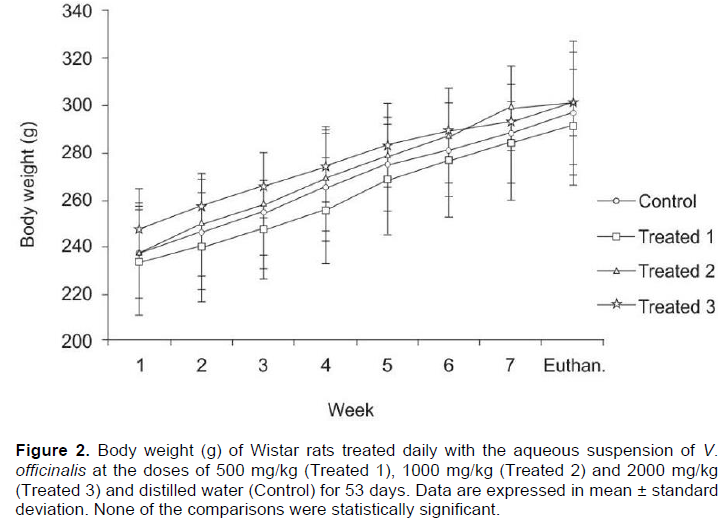
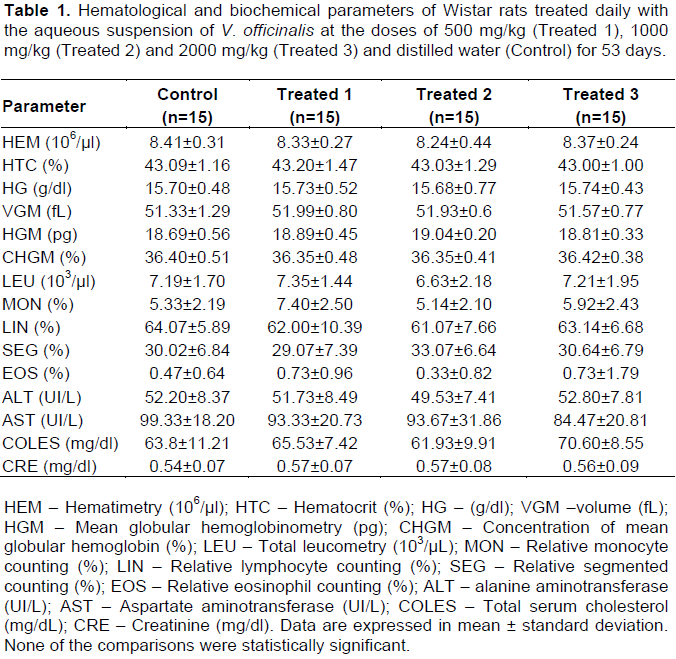
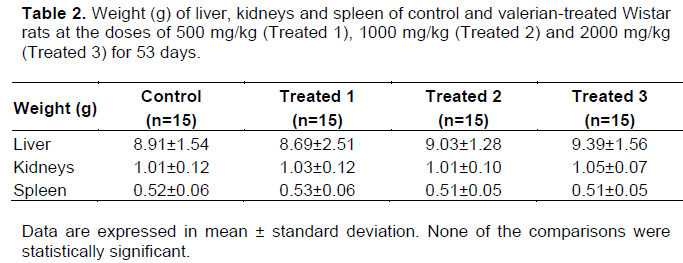
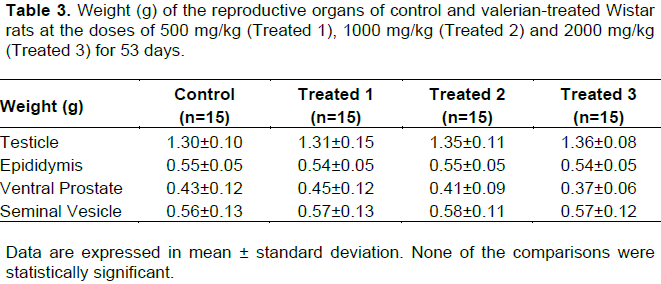
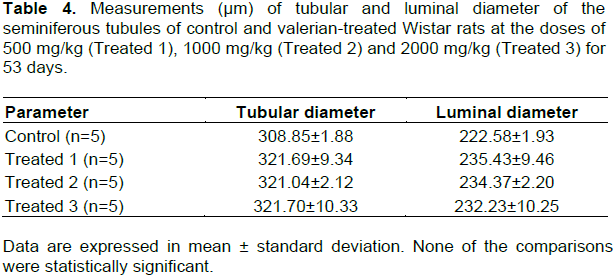
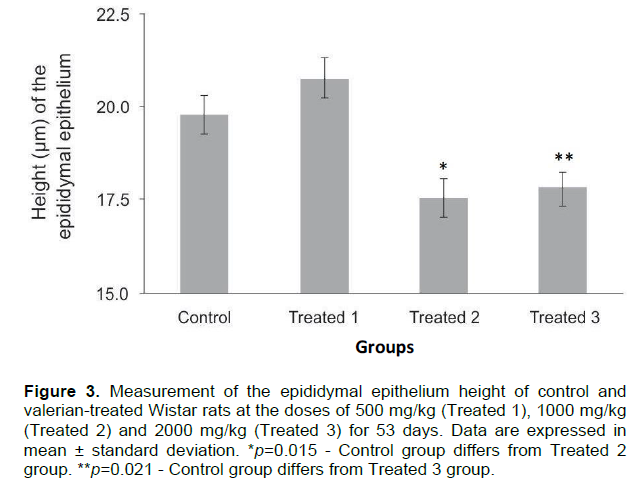
Figure 4 shows transversal sections of distinct areas of the epididymal tubule of control and valerian-treated animals, pointing out the reduced height of the epithelium of the animals treated with the doses of 1000 and 2000 mg/kg. The serum testosterone levels were measured from seven animals per group and they
have been not significantly altered by the treatments with Valerian extract when compared to control values as indicated in Figure 5. The sperm analysis is presented in Table 5. A significant increase in the number of abnormal gametes was observed in the valerian-treated animals at all dose levels when compared to control values. However, the sperm concentration in the epididymis cauda was not altered by the treatment. The gamete functional viability was assessed using the lethal dominant test and the results presented in Tables 6 and 7 show that the treatment of the males did not interfere with sperm viability and the reproductive parameters in the female.
It is well known that systemic toxic alterations interfere in the results of studies concerning the reproductive toxicology. Usually the evaluation of the animal`s general health condition is investigated by means of food consumption, body mass and external clinical signs such as piloerection, chromodacryorrhea, increased diuresis, diarrhea and stereotypical movements. In this study, no alterations in those parameters were observed in the groups treated with valerian (Figure 2). In addition, no significant hematological and biochemical alterations (Table 1) as well as alterations in the weight of the kidneys, liver and spleen (Table 2) were detected. These data suggest that valerian extract is not systemically toxic corroborating the findings of Olaya et al. (2008) who investigated the oral systemic toxicity of Valeriana pavonii L. extract up to the dose level of 2000 mg/kg.
The weight of the reproductive organs is a useful tool for the investigation of the reproductive toxicity of a substance or a group of substances (ICH, 2005) and is one of the most sensitive parameters for detection of a substance influence on the male reproductive system (Mangelsdorf et al., 2003). According to Lanning et al. (2002), weight loss or weight gain of sex organs and glands can be relevant indicators of toxicity, although toxicity may occur without the occurrence of such changes. In spite of alterations in the testicular weight can indicate modifications in the seminiferous tubules or interstitial edema and consequently in the sperm production (Sellers et al., 2007), no alterations were found in the weight of the organs of the reproductive system from valerian-treated and control animals. The detailed histological analysis of the testicle and epididymis is another parameter also considered to be relevant for the assessment of male reproductive toxicity (Parker, 2005). The treatment with valerian did not significantly alter the epithelial and luminal diameter of the seminiferous tubules nor did it produce losses of cell lines that could result in the tubule atrophy and sperm production. In addition, no significant difference on sperm concentration was found between the treated and the control animals.
The valerian extract contains certain substances that can potentially interfere with spermatogenesis. For example, the estrogen-like flavonoid hesperedin can bind to estrogen receptors present in the testicle (Das et al., 2004; Guo et al., 2012), and the valepotriates can exert cytotoxic and genotoxic actions and display deleterious effects on the abundantly occurring cell division processes often found in this organ (Hui-Lian et al., 2003; Lin et al., 2015). In this study, however, no evidence of these effects was found in the testicle of the treated animals, suggesting that the valerian extract and its constituents did not interfere with the morphology of the testicle and sperm production.
The height of the tubular epididymal epithelium has been used in many studies as an investigatory method of deleterious effects in reproduction (Manonayagi et al., 1989; Vawda and Mandlwana, 1990; Del Rio et al., 2003; Chandra et al., 2010). It is known that after orchiectomy, there is a diminished synthesis of RNA and proteins, reduction in the relationship nucleus/cytoplasm in the epithelial cells followed by endoplasmic reticulum and Golgi complex regression as well as regression of the organelles involved in the endocytic pathway, thus causing the reduction in the height of the tubular epididymal epithelium (Robaire et al., 2006; Cornwall, 2009; Chandra, 2010). A significant reduction in the height of the epididymal epithelium was observed in the groups treated with valerian at doses of 1000 and 2000 mg/kg, indicating that there was an anti-androgenic effect on this organ, similar as related by Robaire et al. (2006) and Cornwall (2009). However, neither the serum testosterone concentration of the treated animals nor the epidydimal and male accessory glands (prostate and seminal vesicle) weights showed significant differences when compared to the control group (Figure 5). Therefore, the atrophy observed in the epididymal epithelium does not seem to be caused by a hormonal deficiency, but rather by some other direct toxic effect or alteration on the epididymis. For instance, such effect could be due to the presence of valepotriates in the valerian suspension whose cytotoxic and genotoxic actions have been shown by Lin et al. (2015).
Another hypothesis that could explain the height reduction of the epididymal epithelium refers to the prolonged exposure of the epididymis to substances present in the valerian extract that have potential anti-androgenic action. The flavonoids linarin, hesperedin and 6-methylapegenin, found in the dry extract, are known to induce sleep (Marder et al., 2003; Fernandez et al., 2004), but they can also bind to estrogenic receptors owing to the structural similarity between this class of substances and estradiol (Bhargava, 1989; Hiremath and Hanumantharao, 1990; Hiremath et al., 1997; Das et al., 2004; Guo et al., 2012). Thus, these compounds could be interfering with the fine adjustment between androgens and estrogens in the reproductive system, which could then affect the normal morphological structure of the epididymis.
During epididymal transit, sperm travels through different microenvironments that show specific features such as pH, gene expression and subsequent luminal protein profiles. The regulation of the luminal fluid is important for the complete and efficient maturation of sperm, which may vary between the microenvironments. Therefore, it is noteworthy the role displayed by epithelial cells such as the principal and clear cells, which are responsible for the maintenance and control of the luminal fluid. In particular, the principal cells are responsible for secreting proteins important for the constitution of the microenvironments, while the clear cells display great endocytic action and have vacuolar H+-ATPase enzymes for maintaining the luminal pH. As the epididymis is androgenic-dependent, a normal hormone balance is essential for the normal functioning of this organ as well as for specific cellular functions and microenvironment distinction (Cornwall, 2009). Hence, it is possible that non androgen-dependent specific alterations may have contributed to the reduction of the epididymal epithelium height of the valerian-treated rats, leading to increased rates of abnormal sperm morphology in the treated groups as a result of deficient phagocytosis of abnormal sperm.
Signs of reproductive toxicity in Swiss mice exposed to valerian extract for two weeks were reported by Al-Majed et al. (2006). They included reduced concentration of proteins and nucleic acids in the testis, increased concentration of reactive oxygen species (ROS), and increased number of abnormal gametes. The latter corroborates our findings regarding the high incidence of abnormal sperm, indicating a possible toxic effect on the gametes as a consequence of the direct action of the valerian extract and its cytotoxic and genotoxic constituents in the germ cell, and also the functional alteration of the epididymal cells that can interfere in the normal process of sperm maturation in the epididymis. In both hypothesis, there were no alterations in the number of sperm produced.
It is well known that once exposed to cyto or genotoxic agents the gametes may undergo chromosomal damage that results in function loss, that is loss of capacity to fecundate the oocyte or loss of mobility. However, chromosomal lesion in the gamete does not necessarily mean that it cannot fecundate an oocyte and generate a conceptus, but when this happens, pre- or post-implantation losses or malformations are commonly observed (Yakubu and Afolayan, 2009). The functional evaluation of the gametes can be assessed by the mating of treated males with untreated females with proven fertility, as was done in the present study. In the treated males, valerian did not alter the functional viability of the gametes, despite the increase in the number of abnormal spermatozoa and the reduced thickness of the epididymis epithelium. Besides, considering the fact that rodents exhibit a high concentration of spermatozoa in the semen, these findings indicate that either the deleterious effects observed did not happen at the chromossomal level or they were not sufficiently strong to impair the reproductive parameters assessed in the lethal dominant test.
Considering the consumption of valerian by humans, specifically by men in reproductive age undergoing treatment for anxiety and sleep disorders, the lower concentration of human sperm compared to rodents, and the influence of defects of sperm maturation on men infertility rates (Cornwall, 2009), further studies about the defects of valerian on epididymal physiology are necessary to better elucidate the adverse effects observed on the male reproductive system.
The aqueous valerian extract reduced the epididymis wall height and increased the concentration of abnormal spermatozoa in Wistar rats treated during one complete spermatogenic cycle, indicating potential reproductive toxicity of V. officinalis.
The authors have not declared any conflict of interest
The authors are grateful to FAPEMIG for financial support (projects 173/08 and 172/08, corresponding to TOXIFAR and Animal Laboratory Tests research groups, respectively). The authors also thank Dr. Roberto Sotto-Maior for helping with the software; MSc student Marcella Martins Terra for the histological advices, and Dr. Luis Cláudio Ribeiro for contributing to the statistical analysis.
REFERENCES
|
Al-Majed AA, Al-Yahya AA, Al-Bekairi AM, Al-Shabanah OA, Qureshi S (2006). Studies on the cytological and biochemical effects of valerian in somatic and germ cells of Swiss albino mice. Food Chem. Toxicol. 44(11):1830-1837.
Crossref
|
|
|
|
Al-Majed AA (2007). Effect of Valerian on spermatogenic. Genotoxic. Reproductive and Biochemical Changes in Sex Cells after Chronic Treatment in Male Swiss Albino Mice. J. Med. Sci. 7(2):276-283.
Crossref
|
|
|
|
|
Aslam H, Rosiepen G, Krishnamurthy H, Arslan M, Clemen G, Nieschlag E, Weinbauer GF (1999). The cycle duration of the seminiferous epithelium remains unaltered during GnRH antagonist-induced testicular involution in rats and monkeys. J. Endocrinol. 161(2):281-288.
Crossref
|
|
|
|
|
Becker A, Felgentreff F, Schröder H, Meier B, Brattström A (2014). The anxiolytic effects of a Valerian extract is based on valerenic acid. BMC Complement. Altern. Med. 14(267):1-5.
Crossref
|
|
|
|
|
Bhargava SK (1989). Antiandrogenic effects of a flavonoid rich fraction of Vitex negundo seeds: a histological and biochemical study in dogs. J. Ethnopharmacol. 27(3):327-339.
Crossref
|
|
|
|
|
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992). Evidence of decreasing quality of semen during past 50 years. BMJ 305(6854):609-13.
Crossref
|
|
|
|
|
Chandra AK, Chatterjee A, Ghosh R, Sarkar M (2010). Vitamin E-supplementation protect chromium (VI)-induced spermatogenic and steroidogenic disorders in testicular tissues of rats. Food Chem. Toxicol. 48(3):972-979.
Crossref
|
|
|
|
|
Creasy DM (2003). Evaluation of testicular toxicology: a synopsis and discussion of the recommendations proposed by the society of toxicologic pathology. Birth Defects Res. B Dev. Reprod. Toxicol. 68(5):408-415.
Crossref
|
|
|
|
|
Cornwall GA (2009). New insights into epididymal biology and function. Hum. Reprod. Update 15(2):213-227.
Crossref
|
|
|
|
|
Das S, Parveen S, Kundra CP, Pereira BM (2004). Reproduction in male rats is vulnerable to a treatment with the flavonoid-rich seed extracts of Vitex negundo. Phytother. Res. 18(1):8-13.
Crossref
|
|
|
|
|
Del Rio AG, Palaoro LA, Canessa OE, Blanco AM (2003). Epididymal cytology changes in hypothyroid rats. Arch. Androl. 49(4):247-255.
Crossref
|
|
|
|
|
Fernandez S, Wasowski C, Paladini AC, Marder M (2004). Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 77(2):399-404.
Crossref
|
|
|
|
|
Gharib M, Samani LN, Panah ZE, Naseri M, Bahrani N, Kiani K (2015). The effect of valeric on anxiety severity in women undergoing hysterosalpingography. Glob. J. Health Sci. 7(3):358-63.
Crossref
|
|
|
|
|
Gooneratne NS (2008). Complementary and Alternative Medicine for Sleep Disturbances in Older Adults. Clin. Geriatr. Med. 24(1):121-138.
Crossref
|
|
|
|
|
Gromball J, Beschorner F, Wantzen C, Paulsen U, Burkart M (2014). Hyperactivity, concentration difficulties and impulsiveness improve during seven weeks' treatment with valerian root and lemon balm extracts in primary school children. Phytomedicine 21(8-9):1098-103.
Crossref
|
|
|
|
|
Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang ZT, Vollmer G, Lau DT, Tsim KWK (2012). Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin. Med. 7:1-7.
Crossref
|
|
|
|
|
Hess RA, Franca LR (2008). Spermatogenesis and the cycle of seminiferous epithelium. Adv. Exp. Med. Biol. 636:1-15.
Crossref
|
|
|
|
|
Hiremath SP, Hanumantharao S (1990). Antifertility efficacy of the plant Striga Lutea on rats. Contraception 42(4):467-477.
Crossref
|
|
|
|
|
Hiremath SP, Badami S, Swamy HK, Patil SB, Londonkar RL (1997). Antiandrogenic effect of Striga orobanchioides. J. Ethnopharmacol. 56(1):55-60.
Crossref
|
|
|
|
|
Houghton PJ (1999). The Scientific Basis for the Reputed Activity of Valerian. J. Pharm. Pharmacol. 51(5):505-512.
Crossref
|
|
|
|
|
Hui-Lian W, Dong-Fang Z, Zhao-Feng L, Yang L, Qian-Rong L, Yu-Zhen W (2003). In vitro study on the genotoxicity of dichloromethane extracts of valerian (DEV) in human endothelial ECV304 cells and the effect of vitamins E and C in attenuating the DEV-induced DNA damages. Toxicol. Appl. Pharmacol. 188(1):36-41.
Crossref
|
|
|
|
|
International Conference on Harmonisation of Techinical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2005). Detection of toxicity to reproduction for medicinal products & toxicity to male fertility S5 (R2). pp. 1- 21.
|
|
|
|
|
Kimmel GL, Clegg ED, Crisp TM (1995). Reproductive toxicity testing: A risk assessment perspective. In: Witorsch RJ (Ed). Reproductive Toxicology. Raven Press New York. pp. 75-98.
|
|
|
|
|
Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, Goodman DG (2002). Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol. Pathol. 30(4):507-520.
Crossref
|
|
|
|
|
Lin S, Chen T, Fu P, Ye J, Yang XW, Shan L, Li HL, Liu RH, Shen YH, Xu XK, Zhang WD (2015). Three decomposition products of valepotriates from Valeriana jatamansi and their cytotoxic activity. J. Asian Nat. Prod. Res. 17(5):455-61.
Crossref
|
|
|
|
|
Mahmoudian A, Rajaei Z, Haghir H, Banihashemian S, Hami J (2012). Effects of valerian consumption during pregnancy on cortical volume and the levels of zinc and copper in the brain tissue of mouse fetus. Zhong Xi Yi Jie He Xue Bao. 10(4):424-429.
Crossref
|
|
|
|
|
Mangelsdorf I, Buschmann J, Orthen B (2003). Some aspects relating to the evaluation of the effects of chemicals on male fertility. Regul. Toxicol. Pharmacol. 37:356-369.
Crossref
|
|
|
|
|
Manonayagi S, Vanithakumari G, Padma S, Malini T (1989). Effects of bamboo buds: structural and functional changes in the epididymis of rats. J. Ethnopharmacol. 25(2):201-212.
Crossref
|
|
|
|
|
National Research Council (NRC) (2003). Manual sobre cuidados e usos de animais de laboratório. Goiânia: Academy Press. 162p.
|
|
|
|
|
Marder M, Viola H, Wasowski C, Fernández S, Medina JH, Paladini AC (2003). 6-Methylapigenin and hesperidin: new Valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 75(3):537-545.
Crossref
|
|
|
|
|
Ontario College of Family Physicians (OCFP) (2012). Systematic review of pesticide health effects. OCFP Pesticides Review. 112p.
|
|
|
|
|
Olaya MP, Lozano MC, Rincón J, Guerrero MF (2008). Evaluación de la toxicidad oral aguda del extracto etanolico de Valeriana pavonii en ratas. Trabajos Presentados em el XI Congresso de Farmacologia y Terapeutica. Rev. Saude UIS 40(2):131-133.
|
|
|
|
|
Organización Mundial de La Salud (OMS) (2002). Pautas generales para las metodologias de investigación y evaluación de la medicina tradicional. Genebra. Available at: View
|
|
|
|
|
Parker MR (2005). Testing for reproductive toxicology. In: Hood RD. Developmental and reproductive toxicology: A practical approach. 2nd ed. Taylor and Francis, New York. pp. 439-523.
Crossref
|
|
|
|
|
Pflieger-Bruss S, Schuppe HC, Schill WB (2004). The male reproductive system and its susceptibility to endocrine disrupting chemicals. Andrologia 36(6):337-345.
Crossref
|
|
|
|
|
Robaire B, Hinton BT, Orgebin-Crist MC (2006). The Epididymis. In: Neill JD (Ed.), Knobil e and Neill's physiology of reproduction. Elsevier, New York pp. 1072-1148.
Crossref
|
|
|
|
|
Sellers RS, Morton D, Michael B, Roore N, Johnson JK, Yano BL, Perry R, Schaffer K (2007). Society of Toxicologic Pathology Position Paper: Organ Weight Recommendations for Toxicology Studies. Toxicol. Pathol. 35:751-755.
Crossref
|
|
|
|
|
Skakkebæk NE, Jørgensen N, Main KM, Meyts ERD, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK, Toppari J (2006). Is human fecundity declining? Int. J. Androl. 29(1):2-11.
Crossref
|
|
|
|
|
Sokal RR, Rohlf FJ (1995). Biometry: the principles and practice of statistics in biological research. 3rd ed. WH Freeman and Co. New York.
|
|
|
|
|
Turolla MSR, Nascimento ES (2006). Informações toxicológicas de alguns fitoterápicos utilizados no Brasil. Braz. J. Pharm. Sci. 42(2):289-305.
Crossref
|
|
|
|
|
US National Library of Medicine of National Institute of Health (NIH).
|
|
|
|
|
Vawda AI, Mandlwana JG (1990). The effects of dietary protein deficiency on rat testicular function. Andrologia 22(6):575-583.
Crossref
|
|
|
|
|
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DRJ, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012). Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 33(3):378-455.
Crossref
|
|
|
|
|
Yakubu MT, Afolayan AJ (2009). Reproductive toxicologic evaluations of Bulbine natalensis Baker stem extract in albino rats. Theriogenology 72(3):322-332.
Crossref
|
|
|
|
|
Yao M, Ritchie HE, Brown-Woodman PD (2007). A developmental toxicity-screening of valerian. J. Ethnopharmacol. 113(2):204-209.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2010). WHO laboratory manual for the examination and processing of human semen. Available at: View
|
|