ABSTRACT
The purpose of this research was to investigate the effect of crude extracts from the root of Stemona tuberosa Lour. on the replication of Autographa californica multiple nucleopolyhedrovirus (AcMNPV). Cytotoxicity of crude hexane, dichloromethane and ethanol extracts from the root of S. tuberosa was tested against Spodoptera frugiperda cell line (Sf9) using MTT assay. The cytotoxic effect, represented as CC50 (µg/ml) was observed after 48 and 96 h. It was shown that dichloromethane extract was more toxic than hexane and ethanol extracts and 96-h CC50 for the dichloromethane extract was 1,708.98 µg/ml. Crude dichloromethane extract at the concentration of 31.25 µg/ml was then tested on AcMNPV. The extract was added after 1 h post-infection of AcMNPV at the multiplicity of infection (MOI) of 2, in Sf9 cell line cultivated in vitro. No significant difference between the percentage of infected cells in the control and the test sample with crude dichloromethane extract was found. The average number of polyhedra (OBs/ml) in the control (5.11×106±0.63 OBs/ml) was not significantly different from the test sample (4.19×106±0.31 OBs/ml). However, there was significant difference between the average virus titer (budded virus, BV) in the control (2.06×108±0.71 PFU/ml) and the test samples (2.65×108±0.79 PFU/ml). Crude dichloromethane extract (31.25 µg/ml) did not toxic to Sf9 cells but it could enhance AcMNPV replication in Sf9 cell line cultivated in vitro. It could be concluded that S. tuberosa can be a good candidate for developing the insect virus production in vitro for controlling insect pests.
Key words: AcMNPV, crude extract, cytotoxicity test, by 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay.
In Thailand, Stemona species are known as Non Tai Yak. Stemona spp. can be used as insect repellent and insecticides such as scabicide and pediculocide (Greger, 2006; Chanmahasathien et al., 2011). The total alkaloid profiles could be grouped into four types represented as the major component by stenine-type Stemona alkaloids such as tuberostemonine and neotuberostemonine or by non-stenine types such as croomine and stemoninine. The biological activities of Stemona alkaloids were insecticidal and larvicidal activities (Brem et al., 2002; Kaltenegger et al., 2003; Xu et al., 2006).
For the replication of baculovirus in insect cell culture, there are three phases in the infection cycle: early (reprogramming the cell for virus replication), late (producing BV) and very late (producing ODV) (O’Rielly et al., 1992). The transition from the early phase to the late phase of infection is dependent upon viral DNA replication and occurs between 6 and 12 h after the initiation. During the late phase of infection, newly replicated viral DNA is condensed and packaged within the nucleus, in association with the virogenic stroma, into capsid structures to form nucleocapsids. From about 12 to 20 h, these nucleocapsids leave the nucleus, travel through the cytoplasm, and bud through a modified plasma membrane to acquire a loosely fitting envelope important for BV infectivity. Beginning at about 20 h, there is a transition from the late phase to very late phase of infection, nucleocapsids remain within the nucleus, become bundled together, and are enveloped by a membrane elaborated within the nucleus (Olszewski and Miller, 1997). The BVs enter insect cells by endocytosis that include: (1) virion binding to a host cell receptor, (2) invagination of the host plasma membrane, (3) formation of endocytic vesicle containing the enveloped virion, (4) acidification of the endosome, (5) activation of the viral envelope fusion protein, (6) fusion of the viral and endosomal membranes, and (7) release of the viral nucleocapsid into the cytoplasm (Blissard, 1996). The Autographa californica multiple nucleopolyhedrovirus (AcMNPV), which is the type species and the most widely studied of the Baculoviridae (Wu et al., 2006). It has a wide host range, replicates well in the commonly used insect cell culture systems (Spodoptera frugiperda) and there is a wide range of commercially available transfer vectors (Hitchman et al., 2009). The size of AcMNPV is 25×250 nm, and contains approximately 128 kbp double-stranded DNA (Arif, 1986). The replication of AcMNPV occurs in the nuclei of infected cells and takes place in two phases. In the first phase, nucleocapsids are formed in the nucleus. These nucleocapsids reach the cytoplasm by passing through nuclei pores. The nucleocapsids gain envelope during the budding through the plasma membrane, and the particles released from the cell (Fraser, 1986). In the second phase, after nuclocapsids acquire envelope (apparently de novo) within the nucleus, viral occlusion bodies of NPV are known as polyhedral inclusion bodies (PIBs), and there are infective particles among insect in nature (Bilimoria et al., 1992).
The extract of Stemona tuberosa roots was found to have antibacterial, antifungal and insecticidal activities. Thus, the objective of this study was to evaluate the cytotoxicity of crude hexane, dichloromethane and ethanol extracts of S. tuberosa roots against insect cell line (Sf9) using MTT assay. The crude extract that showed the highest cytoxicity to Sf9 cells was to investigate the efficiency of the extract on the replication of insect virus (AcMNPV) using the endpoint dilution assay.
Preparation of S. tuberosa extracts
Fresh S. tuberosa roots were purchased from Chatuchak market in Bangkok, Thailand. Fresh roots were dried at 45°C until they were quite dried and were ground to powder using a milling machine. The powdered plant material (30 g) was macerated for 7 days sequentially with 450 ml of the following solvents: hexane, dichloromethane and ethanol. The hexane, dichloromethane and ethanol extracts were carefully filtered through Whatman® No. 1 filter paper and were evaporated to dryness under reduced pressure using rotary evaporator to give 0.155, 0.256 and 0.596 g, respectively.
Cell line and virus
The S. frugiperda cell line (Sf9) was used as the host cell for virus infection. Cells were cultivated in TNM-FH medium supplemented with 5% fetal bovine serum (FBS), 100 units/ml penicillin G, 100 µg/ml streptomycin in 25 cm2 culture flasks at 28°C (Petcharawan et al., 2009; Petcharawan et al., 2012). The AcMNPV stock was prepared by inoculating in Sf9 cells and incubated at 28°C. The virus suspension was removed seven days post infection and the virus titer of stock was determined as TCID50/ml (tissue culture infectious dose per ml) and converted to pfu/ml (plaque forming unit per ml) (Reed and Muench, 1992), virus stock was stored at -20°C until needed.
Cytotoxicity assay
The extracts of S. tuberosa root were tested for in vitro cytotoxicity, using Sf9 cell line by 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Mosmann, 1983). Each crude extract was separately dissolved in 1 ml of dimethyl sulfoxide (DMSO) and volume was made up to 10 ml with maintained medium to obtained a stock solution of 5 mg/ml concentration, sterilized by filtration to make stock solution, then diluted to various concentrations (2,000, 1,000, 500, 250, 125, 62.5, and 31.25 µg/ml) in TNM-FH medium. The cells were seeded in a 96-well plate with 2×104 cells/well and incubated at 28°C for 24 h. After 24 h of seeding, the culture medium was replaced with fresh medium containing the different concentrations of the extracts. Each concentration was applied in 4 wells and within 3 independent experiments. Control cells were incubated without the extracts. After incubation at 28°C for 48 and 96 h, the MTT (10 µl of 5 mg/ml in PBS) was added in each well and the cells were incubated at 28°C for 4 h. Thereafter, the medium with MTT was removed from the wells and formazan crystal formed was dissolved by adding 150 µl/well a mixture of 10% SDS:DMSO (1:9). The plates were then shaked for 5 min and the absorbance of the solution in each well was measured at 570 nm on a microtiter plate reader (Siddiqui et al., 2010).
The percentage of cytotoxicity was calculated as {(A-B)/A}×100, where A and B are the absorbance of the control cells and treated cells, respectively. All absorbance values were corrected against blank wells which contained only growth medium. For the evaluation of the concentration that induced 50% cytotoxicity relative to controls (CC50 values) was calculated in each assay using GraphPad Prism software program (Chiba et al., 1998; Krzyminska et al., 2010; Duman, 2012; Petcharawan et al., 2012).
Effect of crude extracts on the replication of AcMNPV
The effect of S. tuberosa crude extract on the replication of AcMNPV was conducted using cell monolayers in 35-mm tissue culture dishes. Exponential phase Sf9 cells in TNM-FH medium were seeded into a 35-mm tissue culture dish at a density of 4×105 cells/dish and incubated at 28°C overnight. The medium in each dish was aspirated and carefully replaced with 1 ml of AcMNPV at a multiplicity of infection (MOI) of 2. After inoculation of virus, the dishes were placed on a rocker platform and rocked for 1 h. The inoculum was removed and washed with PBS, then fresh medium was added to each dish. At this time, it was considered as 0 h post infection (p.i.). The dishes were incubated at 28°C for 4 days. At the end of the incubation period, the cells were scraped and counted, and then centrifuged at 3,500 rpm for 15 min to separate the supernatant and the pellet. The supernatant that contained budded virion was kept at -20°C until needed. The pellets were resuspended with 0.1% sodium dodecyl sulfate (SDS) and incubated at room temperature overnight, then suspended cells were centrifuged and the SDS was discarded. The pellets were resuspended in 2 ml of sterile distilled water and counted the number of polyhedra that liberated from the infected cells in the control and the test samples. Each panel included toxicity control (cells incubated with the extract), uninfected cell control (cells incubated with medium) and infected cell control (cells incubated with virus) and the corrected % mortality was calculated using Abbot’s formula, corrected % mortality = 100×{(T% - C%)/(100% - C%)}, where T% = the percentage of dead test cells and C% = the percentage of dead control cells (Abbot, 1925; Undeen and Vávra, 1997).
Finally, the virus concentration was determined as TCID50/ml and converted to pfu/ml (Reed and Muench, 1992). The percentage of infected cells, the number of occlusion bodies (OBs)/ml in the control and the test samples were determined. The percent of reduction in the pfu/ml produced by virus in the presence of extracts was calculated as % reduction = 100 - [(pfu at given extract dose/pfu in control) × 100] (Sökmen, 2001).
Statistical analysis
The program GraphPad Prism 5.0 was used for the calculation of cytotoxicity curves and CC50 (Motulsky, 2007). All the data were statistically evaluated with SPSS statistics 17.0 software. Hypothesis testing methods include one way analysis of variance (ANOVA) followed by Duncan’s new multiple range test, a post hoc or multiple comparison test. A value of p<0.05 was regarded as statistically significant differences (Custódio et al., 2011).
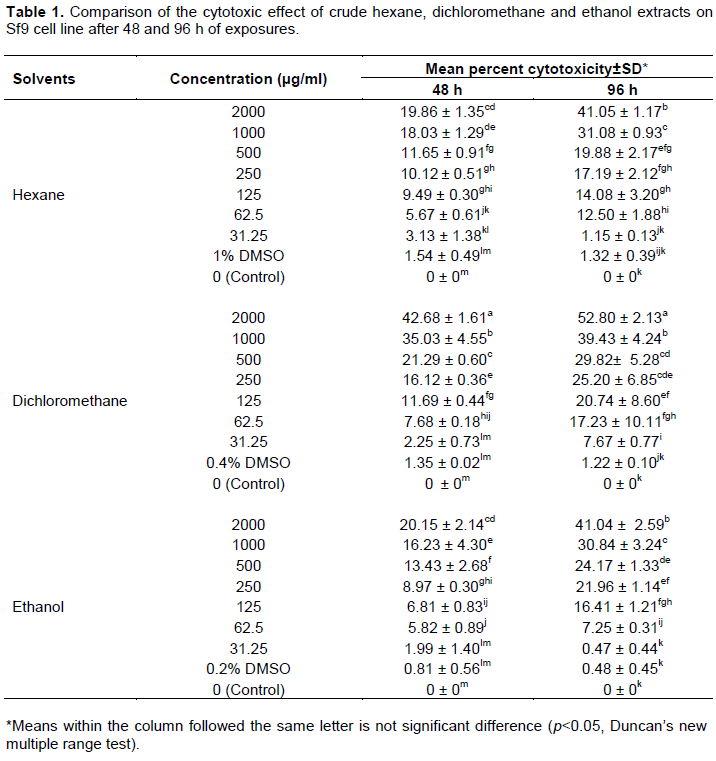
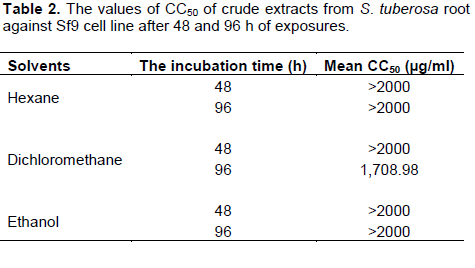
The effect of crude hexane, dichloromethane and ethanol extracts from S. tuberosa against Sf9 cell line was evaluated using MTT assay. Results of the cytotoxicity evaluation of these extracts against Sf9 cell line are shown in Table 1. The dichloromethane extract was more toxic than the others. The values of CC50 of these extracts against Sf9 cell line after 48 and 96 h of exposures are shown in Table 2. Based on 96 h exposure results obtained from hexane, dichloromethane and ethanol extracts from S. tuberosa against Sf9 cell line, the CC50 values were >2,000, 1,708.98, and >2,000 µg/ml, respectively. Among the crude extracts tested, the dichloromethane extract gave the highest toxic to Sf9 cells. However, the cytotoxicity of these extracts can be categorized using the classification of the cytotoxicity of natural ingredients reported by Gad Shayne (1999) and Shirazi et al. (2004), we can conclude that these extracts had partially nontoxic to Sf9 cell line, because of the CC50 were more than 1,000 µg/ml (Table 2).
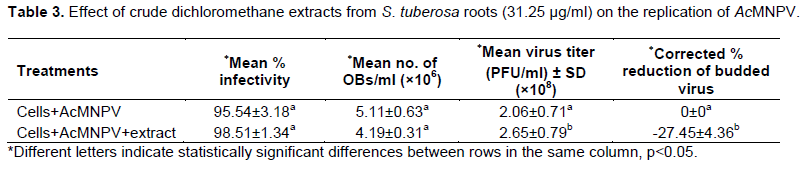
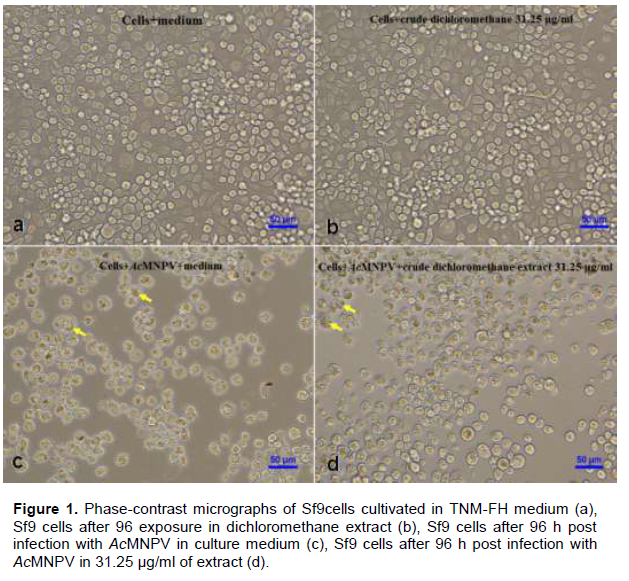
The results (Table 3 and Figure 1) showed that there was no significant difference (p>0.05) between the percentage of infected cells in the control sample (95.54%±3.18) and in the test sample with crude dichloromethane extract (98.51%±1.34). However, there was significant difference (p<0.05) between the average virus titer in the control (2.06×108±0.71 PFU/ml) and the test (2.65×108±0.79 PFU/ml) samples. The average number of polyhedra (OBs/ml) in the control (5.11×106±0.63 OBs/ml) was not significantly different (p>0.05) from the average number of polyhedra in the test sample (4.19×106±0.31 OBs/ml). However, there was significant difference (p<0.05) between the percent reduction of virus titer in the control (0%±0) and in the test samples (-27.45%±4.36). These results showed that the crude dichloromethane extract of S. tuberosa at the concentration of 31.25 µg/ml was no toxic effect to Sf9 cells and could not inhibit AcMNPV infection cultivated in vitro but it could enhance the virus titer (budded virus, BV) of AcMNPV in Sf9 cells.
According to the results of this study, the crude dichloromethane extract of S. tuberosa root was more toxic to Sf9 cells than crude hexane and ethanol extracts, with the CC50 values of 1708.98, 2,000 and 2,000 µg/ml, respectively. The result corresponded with the report of Phattharaphan et al. (2010), the highest insecticidal activity to the third instar larvae of Plutella xylostella was observed from dichloromethane extract of Stemona collinsae root with the LC50 of 0.71% and the major active compound responsible for the insecticidal activity was hydroxystemofoline (alkaloid). Brem et al. (2002) reported that tuberostemonine was dominating alkaloid in the roots of S. tuberosa Lour., showing repellency but not toxic effect to neonate larvae of Spodoptera litoralis. Lee et al. (2006) reported that three new bibenzyl glycosides were isolated from S. tuberose significantly protected human neuroblastoma SH-SY5Y cells from 6-hydroxy dopamine induce neurotoxicity.
In order to determine the antiviral effect of crude dichloromethane extract of S. tuberosa root, the experiment was performed with the concentration of31.25 µg/ml because of this concentration did not have any effect on the growth of Sf9 cells. When the extract was added 1 h after infection with AcMNPV, the virus titer was increased and the polyhedral or occlusion bodies (OBs) could be produced in the nucleus of Sf9 cells (Table 3). The results indicated that this extract could not inhibit the viral synthesis, the viral structural and the viral occlusion protein phases. Further studies are necessary to investigate the mechanism of crude dichloromethane extract of S. tuberosa, such as the enhancement activity of AcMNPV, purification and active compounds. These results corresponded with the report of Erturk et al. (2000), the effect of Nerium oleander L., Prunus laurocerasus L., Punica granatum L., Olea europaea L. and Daphne glomerata Lam. extracts could increase the concentration of progeny virus of AcMNPV.
This study showed that S. tuberosa can be a good candidate for developing the insect virus production in vitro for controlling insect pests.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbot WS (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol.18:265-267.
Crossref
|
|
|
|
Arif MB (1986). The structure of the viral genome. The molecular biology of baculoviruses. Curr. Top. Microbiol. Immunol. 131:21-29.
Crossref
|
|
|
|
|
Bilimoria SL, Demirbag Z, Ng H, Rainisch AJ (1992). Abortive cell culture infection of nuclear polyhedrosis viruses as model system for host specificity. Pesqui. Agropecu. Bras. 27:123-141.
|
|
|
|
|
Blissard GW (1996). Baculovirus-insect cell interactions. In Insect Cell Culture: Fundamental and Applied Aspects (pp. 73-93). Springer Netherlands.
Crossref
|
|
|
|
|
Brem B, Seger C, Pacher T, Hofer O, Vajrodaya S, Greger H (2002). Feeding deterrence and contact toxicity of Stemona alkaloids-a source of potent natural insecticides. J. Agric. Food Chem. 50(22):6383-6388.
Crossref
|
|
|
|
|
Chanmahasathien W, Ampasavate C, Greger H, Limtrakul P (2011). Stemona alkaloids from traditional Thai medicine increase chemosensitivity via P- Glycoprotein-mediated multidrug resistance. Phytomedicine 18:199-204.
Crossref
|
|
|
|
|
Chiba K, Kawakami K, Tohyama K (1998). Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol. In Vitro 12:251-258.
Crossref
|
|
|
|
|
Custódio L, Escapa AL, Fajardo A, Aligue R, Neng N, Florecirc JM, Romano A (2011). In vitro cytotoxic effects and apoptosis induction by a methanol leaf extract of carob tree (Ceratonia siliqua L.). J. Med. Plants Res. 5(10):1987-1996.
|
|
|
|
|
Duman R (2012). Antiherpetic activity of some endemic Hypericum species in Turkey. Afr. J. Biotechnol. 11(5):1240-1244.
Crossref
|
|
|
|
|
Erturk O, Demirbag Z, Beldus AO (2000). Antiviral activity of some plant extracts on the replication of Autographa californica nuclear polyhedrosis virus. Turk. J. Biol. 24:833-844.
|
|
|
|
|
Fraser MJ (1986). Transposon-mediated mutagenesis of baculovirus: Transposon shutting and implication for speciation. Ann. Entomol. Soc. Am. 79:773-786.
Crossref
|
|
|
|
|
Gad Shayne C (1999). Alternatives to in vivo studies in toxicology. In: Balantyne B, Marrs T, Syversen T (Eds.). General and applied toxicology, 1. Grove's dictionaries Inc., USA. 1999. P.178.
|
|
|
|
|
Greger H (2006). Structure relationships, distribution and biological activities of Stemona alkaloids. Planta Med. 72:99-113.
Crossref
|
|
|
|
|
Hitchman RB, Possee RD, King LA (2009). Baculovirus expression systems for recombinant protein production in insect cells. Recent Pat. Biotechnol. 3(1):46-54.
Crossref
|
|
|
|
|
Kaltenegger E, Brem B, Mereiter K, Kalchhauser H, Kahlig H, Hofer O, Vajrodaya S, Greger H (2003). Insecticidal pyrido [1,2-a] azepine alkaloids and related derivatives from Stemona species. Phytochemistry 63:803-816.
Crossref
|
|
|
|
|
Krzyminska S, Raczkowska M, Kaznowski A (2010). Cytotoxic activity of Serratia marcescens clinical isolates. Pol. J. Microbiol. 59(3):201-205.
|
|
|
|
|
Lee KY, Sung SH, Kim YC (2006). Neuroprotective bibenzyl glycosides of Stemona tuberose roots. J. Nat. Prod. 69:679-681.
Crossref
|
|
|
|
|
Mosmann T (1983). Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-59.
Crossref
|
|
|
|
|
Motulsky H (2007). GraphPad Prism® Version 5.0 Statistics Guide. San Diego CA, GraphPad Software, Inc. Available at: www.graphpad.com.
|
|
|
|
|
O'Rielly DR, Miller LK, Luckow VA (1992). Baculovirus Expression Vectors. A Laboratory Manual. New York: W.H. Freeman and Company.
|
|
|
|
|
Olszewski J, Miller LK (1997). Identification and characterization of a baculovirus structure protein,VP1054, required for nucleocapsid formation. J. Virol. 1(7):5040-5050.
|
|
|
|
|
Petcharawan O, Paitoon N, Sripaiboon P, Saelee S (2012). Antiviral activity of crude hexane extracts from Allamanda cathartica on the replication of Autographa californica multiple nucleopolyhedrovirus. KMITL Sci.Technol. J. 12(1):21-29.
|
|
|
|
|
Petcharawan O, Suriyasakol K, Vicharncharoensuk N (2009). Cytotoxicity of crude extracts from golden trumpet (Allamanda cartica L.) on Sf9 and KMITL- HA-E1 cell lines. In: Yang Qian (Ed.). Study on Biological Control and Biotechnology, Harbin, Heilongjiang Science and Technology Press pp. 101-107.
|
|
|
|
|
Phattharaphan N, Pitiyont B, Visetson S (2010). Potential of Stemona sp. For Plutella xylostella control. J. Biopestic 3(1 Special Issue):278-281.
|
|
|
|
|
Reed L, Muench H (1992). A simple method for estimating fifty percent endpoints. In : O'Reilly DR, Miller LK, Luckow VA (Eds.). 1992. Baculovirus Expression Vectors. A Laboratory Manual, New York, W.H. Freeman and Company, pp. 124-138.
|
|
|
|
|
Shirazi FH, Ahmadi N, Kamalinejad M (2004). Evaluation of Northern Iran Mentha Pulegium L. cytotoxicity. DARU J. Pharm. Sci. 12(3):106-110.
|
|
|
|
|
Siddiqui MJ, Ismail Z, Aisha AFA, Abdul Majid AMS (2010). Cytotoxicity activity of Catharanthus roseus (Apocynaceae) crude extracts and pure compounds against human colorectal carcinoma cell line. Int. J. Pharmacol. 6(1):43-47.
Crossref
|
|
|
|
|
Sökmen A (2001). Antiviral and cytotoxic activities of extracts from the cell culture and respective parts of some Turkish medicinal plants. Turk. J. Biol. 25:343-350.
|
|
|
|
|
Undeen AH, Vávra J (1997). Chapter IV Research Methods for Entomopathogenic Protozoa. In: Lawrence AL (Ed.).1997. Biological Techniques. Manual of Techniques in Insect Pathology. Sandiago, Academic Press pp. 117-151.
|
|
|
|
|
Wu W, Lin T, Pan L, Yu M, Li Z, Pang Y, Yang K (2006). Autographa californica multiple nucleopolyhedrovirus nucleocapsid assembly is interrupted upon deletion of the 38K gene. J. Virol. 80(23):11475-11485.
Crossref
|
|
|
|
|
Xu YT, Hon PM, Jiang RW, Cheng L, Li SH, Chan YP, Xu HX, Shaw PC, But PPH (2006). Antitussive effecs of Stemona tuberosa with different chem profiles. J. Ethnopharmacol. 108(1):46-53.
Crossref
|
|