There is a growing need for research of photoprotective molecules from natural sources. Flavonoids have shown significant absorption in ultraviolet A (UVA), ultraviolet B (UVB) region, due to their chemical structure with conjugated double bonds, and may be used as ingredients in cosmetics formulations for skin protection. This systematic review reports what has been researched over the past decade on flavonoid and photoprotective activity, as well as some mechanisms of action by which these metabolites act. The search was conducted in three databases (Science Direct, PubMed and Scopus) using the descriptors “flavonoid”, “photoprotection” and “sunscreen” combined, published between January 2006 and January 2016. Twenty-two articles were selected and 17 flavonoids were cited. The data reviewed here indicate that flavonoids are potential in the fight against UVA and UVB radiation and which may be used as adjuvants in photoprotective formulations.
Sunscreens are mainly used to prevent the formation of erythema arising from exposure to sunlight. Reducing the amount of ultraviolet (UV) radiation reaching the skin by sunscreens may also reduce the risk of skin cancer induced by sun. The UV spectrum is divided into three groups based on wavelength: Ultraviolet C (UVC) that ranges 100 to 290 nm, ultraviolet B (UVB), 290 to 320 nm, and ultraviolet A (UVA), 320 to 400 nm. UVA is subdivided into UVA2 (320 to 340 nm) and UVA1 (340 to 400 nm). The solar UV radiation on Earth's surface is approximately 90 to 99% UVA and 1 to 10% UVB (Gardiner et al., 2006; Verschooten et al., 2006; Faurschou and Wulf, 2007).
UVB radiation causes both erythema as photocarcinogenesis. Photocarcinogenesis occurs mainly due to their direct interaction with the cell DNA and subsequent formation of dimers cyclobutane pyrimidine and glycols thymine. However, there are several reasons why the investigation of UVA role is also relevant. The major consequence of UVA radiation buildup is the generation of reactive oxygen species (ROS) that can also cause cancer due to generation of derivatives of nitrogenous bases of oxidized DNA such as 8-hidroxidesoxiguanosina as well as tumor suppressor genes modified as p53 (Seité et al., 2000; Heinrich et al., 2004; Vielhaber et al., 2006).
In aerobic organisms, oxidation processes occur as a result of natural oxygen consumption. The oxygen is typically found in its most stable form, although in certain situations such as the use of tobacco or alcohol, inflammatory processes, ionizing radiation and in particular, exposure to UV radiation can produce these reactive species, which are highly unstable and can do skin damage through oxidation. Free radicals are particularly harmful, because the presence of an unpaired electron makes them highly unstable and gives them a tendency to react with other molecules, making it stable (Gálvez, 2010).
Over the past three decades, with the depletion of the ozone layer, which has become a major environmental problem, the special role of flavonoids in the short wavelength absorption of UVB solar radiation has been strongly emphasized (Rozema et al., 1997; Burchard et al., 2000).
The use of botanical agents to reduce the occurrence of skin cancer by photochemoprotection has increasingly gained attention. Photochemoprotection is the term that defines the use of agents capable of ameliorating the adverse effects of ultraviolet radiation on the skin, and much has been appreciated as a viable way of reducing the occurrence of skin cancer (F'guyer et al., 2003). Studies show that this type of prevention is required in daily life to avoid serious damage (Wu et al., 2011). In recent years the use of agents, especially botanical antioxidants, present in the common diet and in beverages consumed by the population has received considerable attention as photochemoprotection agents for human use. Many of these agents have found a place in skin care products (Afaq et al., 2005).
One of the trends of the market and of Cosmetic Science is the development of products with a greater number of components of natural origin, especially those of vegetal origin, rationally exploiting biodiversity (Biavatti et al., 2007). This is seen with great interest by the international market, especially if the material presents scientific studies proving the safety and efficacy, besides the commitment with the sustainable development (Franquilino, 2006).
There is a structural analogy between the synthetic sunscreens and the active ingredients of natural products that present a protective action in the skin, since the ultraviolet absorption has been verified when using vegetal extract in pharmaceuticals and cosmetics (Ramos et al., 1996). In this way, sunscreens are substances capable of absorbing, reflecting or refracting ultraviolet radiation and thus protect the skin from direct exposure to sunlight (Giokas et al., 2005).
Flavonoids have shown several functions in photoprotection routes (Agati and Tattini, 2010). This is consistent with the flavonoid location in a wide range of plant organs, and different cells and cell compartments (Agati et al., 2012). These molecules have been recognized to perform various functions in higher plants responses in several environmental constraints (Winkel-Shirley, 2002; Roberts and Paul, 2006).
Promising photoprotective activity of plant extracts from Brazilian Caatinga Biome has been reported, such as Neoglaziovia variegata (Oliveira-Junior et al., 2015), Triplaris gardneriana (Macedo et al., 2015) and Alternanthera brasiliana (Silva et al., 2014), and its activity was associated to the phenolic compounds as flavonoids. Thus, it was decided to carry out this systematic review, where articles that related flavonoid with photoprotective activity were researched and analyzed.a
Selection of articles
The primary search identified 446 articles, with 83 from PubMed, 67 from Scopus and 296 from Science Direct. However, out of this total, 42 were indexed in two or more databases and were considered only once, resulting in 404 articles. After initial screening of the titles, abstracts or keywords, 22 articles were selected as the others did not meet the inclusion criteria (n= 382) or referred to studies with flavonoids and photoprotection. A flowchart illustrating the progressive study selection is shown in Figure 1.
Flavonoids with photoprotective activity
Table 1 shows the chemical structures, SPF-UVB, PF-UVA, UVA/UVB ratio, λc and mentioned studies with polyphenols.
Rutin
Rutin, also called rutoside and quercetin-3-O-β-rutinoside, is a glycoside flavonoid of quercetin and disaccharide rutinose (α-L-rhamnopyranosyl-(1→6))-β-D-glucopyranose) (Lucci et al., 2009).
The sun protection factor UVB (SPF-UVB) and protection factor UVA (PF-UVA) were verified by several authors, both in pharmaceutical formulations containing only rutin as principle active ingredient as in association with other photoprotective agents using a transmittance method proposed by Diffey et al. (1989). Rutin formulations presented SPF-UVB 4.72 ± 0.20 and FP-UVA 4.92 ± 0.20. When associated to physical filters such as titanium dioxide (TiO2) and zinc oxide (ZnO), rutin showed an increase in the protective factor. Formulations in the presence of rutin with TiO2, the SPF-UVB were 34.29 ± 8.31, and PF-UVA 16.25 ± 2.71. For formulations in the presence of rutin with ZnO, SPF-UVB was 11.25 ± 3.31, and the PF-UVA 9.75 ± 2.81. The authors considered that rutin act synergistically with TiO2 because there was a significant increase in the SPF-UVB and PF-UVA values when associated. However, the authors have considered the effect of rutin with ZnO only an additive effect, because the increase was not considered as significant. Also, it was found that formulations containing rutin were photostable, varying less than 10% the value of SPF-UVB and PF-UVA after 2 h of irradiation under controlled conditions (Choquenet et al., 2008).
In another study, researchers found that rutin incorporated in cosmetic photoprotective formulations have presented biocompatibility with human skin. Rutin formulations showed high antioxidant potential, with increase of 75% to radical scavenging activity compared to formulations containing only synthetic filters used (ethylhexyl methoxycinnamate 3.75 and 7.5%, ethylhexyl salicylate 2.5 and 5.0%, ethylhexyl dimethyl PABA 4.0 and 8.0%, octocrylene 5.0 and 10.0%). Rutin was incorporated in a fixed concentration of 0.1%. Furthermore, the synthetic filters and rutin association significantlyincreasedcriticalwavelengthofthe formulations, by a spectral transmittance method, showing improved photoprotection, especially in UVA region, which supports the use of this flavonoid as adjuvant formulations (Peres et al., 2015).
Rutin association with different other filters such as benzophenone-3 (BF-3) and butyl methoxydibenzoylmethane (BMDBM), resulted in different profiles interaction. In general, rutin provides antioxidant properties to the samples, and all the formulations were considered safe for human use, with good skin compatibility. Surprisingly, the combination of rutin 0.1% and BF-3 6.0% caused a SPF increase from 24.3 ± 1.53 to 33.3 ± 2.89, showing synergistic effect of the flavonoid in this association, by Colipa Guidelines method (2011). However, the same result were not observed for the combination of rutin and BMDBM, because there was a decrease in the SPF value. However, the critical wavelength values obtained were consistent with the BBM profile, indicating that rutin was not effective when coupled with this UVA filter, but did not affect the filter absorption (Oliveira et al., 2015).
The encapsulation of natural products such as rutin can offer improvements in the effectiveness of sunscreen. This approach can provide enhanced flavonoid content and a bioactive compound produces improved with new functional and physical characteristics (Oliveira et al., 2016). Studies aimed at creating formulations with nanostructured lipid carriers were performed with rutin. A lipid based formulation Apifil® and rutin (2%) reached the highest occlusive effect, achieved higher encapsulation efficiency (96.96% ± 6.58) and release of flavonoid (82.23% ± 5.48) in addition to the higher sun protection factor (2.72 ± 0.20). Furthermore, different TiO2 concentrations were added to this formulation to increase the SPF-UVB. The formulation containing 5% TiO2 reached the highest value of SPF-UVB (5.33 ± 0.20), larger area on the UV absorption curve and critical wavelength above 370 nm, which demonstrated its high efficiency as a sunscreen. The critical wavelength (λc) is the wavelength below which lies 90% of the area under the absorbance curve. A higher critical wavelength guarantees more UV protection, especially protection from the rays with longer wavelengths (UVA) (Kamel et al., 2015).
As an alternative to common synthetic sunscreens, gelatin nanoparticles with rutin encapsulated were formulated and associated with ethylhexyl dimethyl PABA (EHDP), ethylhexyl Methoxycinnamate (EHMC) and butyl methoxydibenzoylmethane (BMDBM) in sunscreen formulations. Gelatin nanoparticles with rutin promoted an increasing of antioxidant activity by 74% in relation to the free rutin. Furthermore, there was an increase in SPF-UVB of 48%, by Colipa Guidelines method (2011), indicating again the potential of natural sunscreens (Oliveira et al., 2016).
Quercetin
In the study with quercetin (3,3',4',5,7-pentahidroxyflavone), it was observed that this flavonoid has absorption in both UVA and UVB regions, and its further absorption into the UVA region. The SPF-UVB checked was 10.3, by Diffey et al. (1989) method. The UVA/UVB ratio verified was 0.93, and λc 385 nm. Because there is not agreement upon a method to measure the protection against UVA, the UVA/UVB ratio (Boots The Chemist Ltd, 1991) and the critical wavelength (λc) (Diffey, 1994) are very used to verify predictive values of absorption in the UV region. The closer to 1 the value of the UVA/UVB ratio, the greater the predictive value for absorption in UVA region, in the same way, the higher the λc, the greater the absorption spectrum in the UVA region (Stevanato et al., 2014).
The SPF-UVB and PF-UVA of quercetin were verified in both formulations containing only the flavonoid as an active principle ingredient as in association with other filters using a transmittance method proposed by Diffey et al. (1989). Quercetin presented SPF-UVB formulations of 4.52 ± 0.38 and FP-UVA 5.77 ± 0.55. Furthermore, the prepared formulations were photostable, with modification least 10% of SPF-UVB and PF-UVA after 2 h of controlled irradiation. In the same study, quercetin showed an increase in protection when associated with physical filters TiO2 and ZnO. For ZnO, the displayed SPF-UVB was 29.70 ± 4.96, and PF-UVA 16.42 ± 1.67, which configures a synergistic effect compared with the effect of quercetin alone. For the zinc oxide SPF-UVB was 9.97 ± 1.61, and PF-UVA 10.28 ± 1.63 showing a purely additive effect compared to the effect of quercetin alone (Choquenet et al., 2008).
Evans-Johnson et al. (2013) found that topical application of a mixture of polyphenols derived from almonds (isorhamnetin, catechin, epicatechin, kaempferol and quercetin) in which quercetin was in higher concentration than the others, significantly attenuates apoptosis induced by UVA radiation in dermal fibroblasts, keeping the skin morphology healthy as well as cell differentiation, and tends to decrease the reducing keratinocyte proliferation induced by UVA. The mechanisms of action was not elucidated in this study, however, the authors suggest that potential mechanisms may include absorbance of UV radiation, elimination of free radicals generated from this radiation, and modulation of cell signaling and endogenous antioxidant defenses.
Inflammatory cytokines, such as IL-1β, IL-6, IL-8 and TNF-α, play a very important role in UV irradiation-induced inflammation and skin damage (Cho et al., 2003). Thus, the effects of quercetin on expression of inflammatory cytokines induced by UV irradiation in primary human keratinocytes were investigated. The pretreatment of keratinocytes in culture with different concentrations of quercetin (2, 6 and 20 µg/ml) resulted in a concentration-dependent inhibition of UV (50 mJ/cm2) irradiation-induced IL-1β mRNA expression. Maximum inhibition (80%) was observed in 20 µg/ml of quercetin. Therefore, in subsequent experiments, 20 µg/ml of quercetin was used to pretreat keratinocytes before they were subjected to UV irradiation. UV irradiation induced marked increase of IL-1 β, IL-6, IL-8 and TNF-α mRNA expression in a time-dependent manner. Pretreatment with quercetin suppressed the induction of all four cytokines: IL-1 β (60%), IL-6 (80%), IL-8 (76%) and TNF-α (69%) (Vicentini et al., 2011).
Since nuclear factor kappa B (NF-κB) pathway plays an essential role in induction of inflammatory cytokines expression by UV irradiation, quercetin ability to inhibit the activation of NF-κB was analyzed by UV irradiation. UV irradiation of primary human keratinocytes enhanced NF-κB activation by approximately 5-fold. Quercetin pretreatment inhibited UV irradiation-induced NF-κB DNA binding activity by approximately 80%. In addition, protein levels of p65, NF-κB and p50 were not affected by treatment with quercetin. Thus, it is believed that the ability of quercetin to reduce the inflammatory action induced by UV irradiation is mediated, at least in part, by their inhibitory effects on the activation of NF-κB and inflammatory cytokine production (Vicentini et al., 2011).
The in vitro results cannot always be correlated with in vivo results, and generally high SPF values are required in vitro to achieve median or lower values in vivo. The biological medium is too complex for the flavonoid easily achieve high activity, because several factors may be related to this reduction in efficacy, such as the area of application, the amount of formulation used, the individual's skin and its integrity or even the components of the formulation.
Chrysin
Chrysin (5,7-dihidroxiflavon), a natural flavonoid occurring in various plants and foods such as honey and propolis, reportedly opposes inflammation and carcinogenesis, but has rarely been applied in skin care products (Wu et al., 2011). In a study with isolated chrysin, it was found that this flavonoid presents absorption spectrum in both regions (UVA and UVB). The checked SPF-UVB by Diffey et al. (1989) method was 18.6. The UVA/UVB ratio was found to be 0.6, and λc 380 nm (Stevanato et al., 2014). To achieve SPF-UVB of 20, it was found that the concentration of chrysin (w/v) in solution should be about 7.5% (Gregoris et al., 2011).
Studies with animals have shown that topical application of chrysin was very efficient in percutaneous absorption, with no skin irritation. The same study found the effect of chrysin protect against damage to keratinocytes induced by UV radiation. The results showed that chrysin was able to reduce apoptosis, as well as the production of reactive oxygen species and the expression of the enzyme cyclooxygenase-2 (COX-2) induced by UVA and UVB radiation. COX-2 is an enzyme that participates of inflammatory processes which cleaves membrane lipids in the presence of oxygen and produces chemical mediators of inflammation such as prostanoids (Wu et al., 2011).
Epigallocatechin-3-gallate
Green tea is produced from fresh leaves of Camellia sinensis plant species (Yang and Wang, 1993). The leaves undergo fermentation process, preventing oxidation and polymerization of polyphenols contained in the plant. This tea contains four main polyphenols: (–)-epicatechin (EC), (–)-epicatechin-3-gallate (ECG), (–)-epigallocatechin (EGC) and (–)-epigallocatechin-3-gallate (EGCG), the latter being in a higher concentration, reaching 50% of total catechins (Yusuf et al., 2007).
The first evidence that green tea polyphenols may have a protective role in UV-induced skin cancer was suggested by a study conducted by Wang et al. (1991) who showed that green tea served in water (orally) to mice, exhibited a prolonged dose-dependently in tumor development time when they were subjected to a photo-carcinogenesis protocol. Similar observations were noted in relation to topical application of green tea polyphenols (Wang et al., 1991).
EGCG prevent UVB-induced immunosuppression by inducing interleukin-12 (IL-12). Because the immunosuppression is a risk factor for photocarcinogenesis, the possibility that EGCG also prevents UVB-induced photocarcinogenesis through a DNA repair mechanism, dependent on IL-12 was investigated. To investigate this possibility, it was determined the effects of EGCG on photocarcinogenesis in IL-12 KO mice using the formation of cyclobutane pyrimidine dimers (CPDs) as an extension indicator of DNA damage induced by UVB. Topical application of EGCG (1 mg/cm2 skin) prevented photocarcinogenesis in wild-type (C3H/HeN) mice in terms of tumor incidence and tumor multiplicity did but not prevent photocarcinogenesis IL-12 KO mice. DNA damage, as determined by the formation of CPDs and the number of sunburn cells, were reduced faster in wild-type mice skin treated with EGCG than the untreated control mice. In contrast, the extent of UVB-induced DNA damage and the numbers of sunburn cells were not significantly different in the EGCG-treated IL-12 KO mice and untreated control group (Meeran et al., 2006).
In addition, treatment of XPA-proficient human fibroblast cells with EGCG promoted repair of UVB-induced CPDs in a dose-dependent manner but not in an XPA-deficient cells, indicating that the nucleotide excision repair mechanism is involved in EGCG-mediated DNA repair. Taken together, these results indicate EGCG can prevent photocarcinogenesis through an EGCG-induced IL-12–dependent DNA repair mechanism (Meeran et al., 2006).
EGCG has a high sunscreen activity; however, this catechin is highly unstable under sunlight. Thus, it was found EGCG behavior in formulations with various co-antioxidants such as vitamin E, butylated hydroxytoluene (BHT), vitamin C and α-lipoic acid for their potential to protect the EGCG from photochemical degradation. The addition of vitamin C and α-lipoic acid in the formulation significantly reduced the decomposition of EGCG light-induced from 76.9 ± 4.6 to 20.4 ± 2.7%, and from 12.6 ± 1.6%, respectively. Moreover, BHT had no effect, showing loss of 78.1 ± 4.6% EGCG, and vitamin E increased the EGCG photolysis to 84.5 ± 3.4%. The functional stability of EGCG in the formulations exposed to the solar simulator was evaluated by measuring the antioxidant activity in vitro. After irradiation, the reduction in the antioxidant capacity of the formulation with EGCG was lower (21.8%) than degradation of extension (76.9%), suggesting the formation of photoproducts with antioxidant properties. Among the evaluated antioxidants, α-lipoic acid showed the most positive results, proving to be an effective antioxidant co-agent for stabilization of the (–)-epigallocatechin-3-gallate in dermatological products for photoprotection of the skin because it reduced most significantly the degradation of EGCG and stabilized better antioxidant activity of the formulation, down only 1.4% of the activity (Scalia et al., 2013).
Another attempt to stabilize EGCG formulations was performed, this time with commercial filters BMDBM, ethylhexyl methoxycinnamate (EMC), benzophenone-4 (BP-4) and Tinosorb M results compared with the control formulation (without adjuvants filters) showed a small (11.5%) but significant reduction in the photodegradation of EGCG, which was achieved by UVB-filter EMC while the UVA-filter BMDBM was ineffective. In the presence of BP-4, a more marked decrease in the light-induced decomposition of EGCG was obtained (51.6 ± 2.7%) compared to EMC. Tinosorb M produced stabilizing effect comparable to that of BP-4, indicating that the photosensitivity of EGCG is mainly caused by wavelengths in the UVB region (Bianchi et al., 2011).
Luteolin
The flavonoid luteolin or luteol is a polyphenol with potent antioxidant activity. The antioxidant and photoprotective properties of luteolin were investigated in human keratinocytes in vitro, ex vivo and in vivo. The spectroscopy revealed maximum absorptions of luteolin in the UVB region as both the UVA region, and less than 10% absorption was below 370 nm, showing that its λc remained above this wavelength. In human skin, luteolin effectively reduced the formation of CPDs induced by UVB. The antioxidant activity was evaluated in several trials with means and using cell-free media cells. In DPPH radical scavenge assay, the effective concentration to achieve 50% of maximal effect (EC50) of luteolin was 12 µg/ml, comparable to Trolox (25 µg/ml) and N-acetylcysteine (32 µg/ml), drugs used as positive control in the assay. In contrast, in CM-H2DCFDA assay performed with UVB-irradiated keratinocytes, luteolin was much more effective (EC50 3 µg/ml) compared to Trolox (EC50 of 12 µg/ml) and N-acetylcysteine (EC50 847 µg/ml). Luteolin also inhibit both the skin erythema induced by UVB, the upregulation of cyclooxygenase-2 and prostaglandin E2 production in human skin via interfering with the MAPK pathway. These data suggest that luteolin may protect human skin against UVB damage by a combination of UV-absorbing, DNA-protection, antioxidant activity and anti-inflammatory properties (Wölfle et al., 2011).
Another study found that the addition of ubiquinone and tocopherol increase antioxidant activity and photoprotection luteolin. The combination of luteolin with ubiquinone and tocopherol in a ratio of 4:4:1 gave the highest antioxidant efficacy than the equivalent concentration of any of isolated individual antioxidants. Luteolin greatly reduced the formation of 2',7'-dichlorofluorescein by reactive oxygen species in a concentration dependent manner, from 2 mg/ml. Ubiquinone and tocopherol were not effective at concentrations between 0 and 4 µg/ml. However, when combined antioxidants, already at 0.25 mg/ml was sufficient to completely neutralize the formation of ROS induced by irradiation. In addition, the antioxidants combined present sunscreens effects, and at concentration of 2 mg/ml the combination was able to completely protect cells against damage induced by UV. Luteolin alone showed only partial photoprotective effect at 2 mg/ml. Tocopherol and ubiquinone showed no photoprotective effect when isolated (Wölfle et al., 2013).
The combination of luteolin with tocopherol and ubiquinone is quite effective, because each antioxidant has a different activity profile. Tocopherol is the predominant antioxidant as physiological barrier to human skin, which protects cell membranes and the stratum corneum (Thiele et al., 2001). Ubiquinone is part of the electron transport chain responsible for energy production in every cell. Furthermore, the anti-apoptotic and mitochondria protective properties of ubiquinone have been described (Papucci et al., 2003). The association luteolin-tocopherol-ubiquinone combines the activities of ultraviolet absorption and DNA protection of luteolin, with stratum corneum protection of tocopherol and mitochondrial protection of ubiquinone. In summary, the findings suggest that the potent antioxidant effects and sunscreens such as flavonoids luteolin can be enhanced by addition of low concentrations of other antioxidants (Wölfle et al., 2013).
Isoflavonoids (genistein, equol, daidzein, biochanin A and formononetin)
Isoflavones are a major group of phytoestrogens from plants, with chemical characteristic of polyphenolic and non-steroidal nature, presenting biological action similar to estrogen (Lin et al., 2008).
For genistein, it was demonstrated its action in rat skin protection against the oxidative stress, photodamage and carcinogenesis induced by UVB (Wei et al., 2002; Shyong et al., 2002). The topical administration of equol effectively reduced the incidence of cancers induced by chronic exposure to UV radiation, and UVA-induced lipid peroxidation in mouse skin (Widyarini et al., 2005).
Administration of topical solutions containing 0.5% of the isoflavones genistein, daidzein, biochanin A and formononetin was able to exert photoprotective effect on pig skin, reducing the number of sunburn cells by UV radiation and/or erythema. Genistein, daidzein and biochanin A were more significant as photoprotective. The formononetin showed no effect in the prevention of burns, however, proved to be very effective in reducing erythema. Individual differences between percutaneous absorption of various isoflavones may be responsible for the difference of efficacy in protection revealed in the study. It is also likely that the pharmacodynamics of the isoflavones may be different. For example, genistein inhibits the activity of tyrosine kinase receptor, and can thereby reduce damage caused by UV radiation better than isoflavones which do not inhibit the activity of this receptor (Lin et al., 2008).
Equol, other isoflavonoid, was also able to significantly reduce pork skin erythema, however with less activity than the others isoflavones mentioned (Lin et al., 2008).
Another study showed that the photoimunoprotection of isoflavonoids can result from interaction with a natural skin antioxidant, known to modulate photodamage induced by UV, the metallothionein (MT). In rats with a mutation that inhibits the expression of two subtypes of MT (I and II), it was noticed that the immune protection of equol against simulated solar radiation of UV was revoked. Equol administered in solution topically (10 µM) did not enable the MT expression in the normal mouse skin, but increases MT expression in the epidermis of the animal after UV irradiation, demonstrating the dependence photoimmunoprotection of equol on induction of MT, as evidenced by immunohistochemistry tests performed. Interestingly, equol incorporated in lotion did not affect the MT expression in mice and also showed no immunomodulatory effect. The data for the tests with equol are consistent with the hypothesis that the photoprotection is dependent of the level of skin MT expression. This suggests that these isoflavonoid act as exogenous antioxidants interacting with endogenous cellular antioxidant activity of the skin (Widyarini et al., 2006).
It is unclear how isoflavonoids can stimulate MT when the cells are exposed to radiation. Some studies report that the regulation of MT gene may involve metals, steroid hormones, redox molecules and various cytokines (Sciavolino and Vilcek, 1995; Nishimura et al., 2000; Ablett et al., 2003).
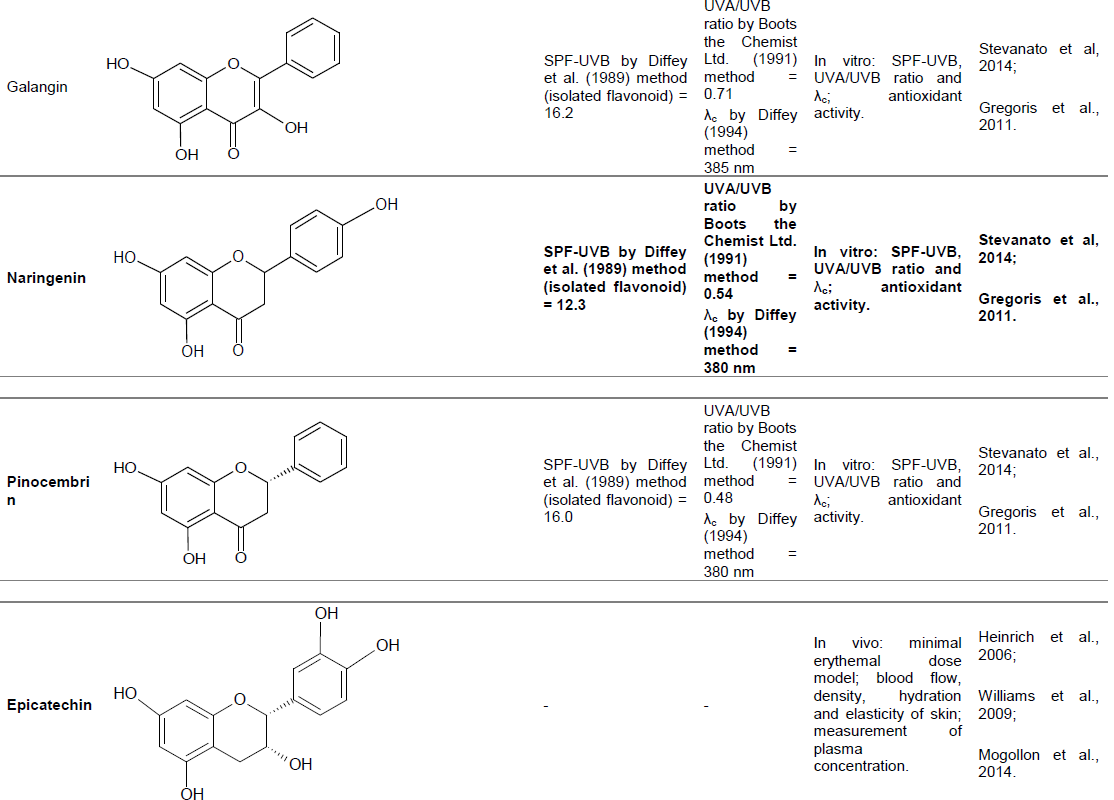
Apigenin, kaempferol, catechin, galangine, naringenin and pinocembrine
Flavonoids are important dietary components, and consequently these molecules and their metabolites rarely cause sensitization. From this, studies have verified the possibility of using these compounds in photoprotective formulations, correlating the antioxidant power with its sun protection capacity (Stevanato et al., 2014).
Among the flavonoids mentioned above (apigenin, kaempferol, catechin, galangine, naringenin and pinocembrine), UV spectra showed that kaempferol and galangine are characterized by absorptions at wavelengths relatively higher than the others. The absorbance spectra of these two flavonoids revealed absorption peak between 360 (galangine) and 365 nm (kaempferol). Apigenin obtained its maximum absorption at 330 nm, catechin at 290 nm, naringenin and pinocembrine at 290 nm. Regarding the SPF-UVB by Diffey et al. (1989) method, the UVA/UVB ratio and critical wavelength (λc), the data were: Kaempferol (24.9 SPF, UVA/UVB 0.76 and λc 385 nm); galangin (16.2 SPF, UVA/UVB 0.71 and λc 385 nm); apigenin (28.8 SPF, UVA/UVB 0.66 and λc 380 nm); catechin (7.3 SPF, UVA/UVB 0.62 and λc 385 nm); naringenin (12.3 SPF, UVA/UVB 0.54 and λc 380 nm); pinocembrin (16.0 SPF, UVA/UVB 0.48 and λc 380 nm). Associated with these photoprotection data, it was verified the antioxidant capacity against lipid peroxidation of these flavonoids. Kaempferol, catechin and galangine presented lower IC50 values (in mM), 2.0 ± 0.2, 2.0 ± 0.2 and 3.0 ± 0.3, respectively. The others showed IC50 values above 50 µM (84 ± 8 for apigenin, 56 ± 5 for naringenin and 110 ± 10 for pinocembrine (Stevanato et al., 2014; Gregoris et al., 2011). Thus, a formulation with kaempferol, apigenin and galangine could confer a broad spectrum of protection in the UVA and UVB regions and confer high levels of antioxidant activity for the skin.
Isolated flavonoids generally do not give good SPF values, therefore, studies in the cosmetic field should always be conducted in the sense of associating these polyphenols with synthetic filters to try to reduce their concentrations from different combinations among them. Even if the in vivo values of SPF are high, when added in cosmetic formulations these values tend to reduce due to dilution of the flavonoid in the formula, or loss of stability of this formulation may still occur. Using the flavonoid alone to provide photoprotection is apparently not an advantageous option.
Flavanol cocoa (catechin and epicatechin)
Antioxidants present in diet may contribute to the photoprotection and are important to maintain skin health. Was investigated the influence of 12 weeks of chocolate consumption with high flavanol content in skin sensitivity to UV radiation, measured by minimal erythemal dose (MED). Two groups of women have used a high dose of flavanols (HF) (326 mg) or low dose of flavanols (LF) (27 mg) in the form of cocoa powder dissolved in 100 ml of water for 12 weeks. Epicatechin and catechin were flavanols used in smaller or larger amounts. The photochemoprotection and indicators of the skin condition were analyzed before and during the intervention. After selected areas of skin exposure, the radiation MED obtained from a solar simulator was calculated. The UV-induced erythema was significantly reduced in the HF group in 15 and 25%, after 6 and 12 weeks, respectively, while no change occurred in LF group. The ingestion of high flavanol dose led to an increase in blood flow of skin and subcutaneous tissue, increased density, and skin hydration. Skin thickness was increased and the transepidermal water loss was reduced in the HF group, although neither of these variables were modified in LF group. The evaluation of the surface of the skin showed significant reduction in roughness and scaling the HF group compared with the LF group at 12 weeks. Thus, it was concluded that cocoa flavanols contribute to the endogenous photochemoprotection, improve blood circulation and affect dermal surface of the skin variables, as well as the hydration (Heinrich et al., 2006).
Another study was conducted with 30 healthy subjects to verify similar results. Two groups of fifteen persons each were randomly assigned to start HF or LF diet. Each one consumed a 20 g portion of chocolate suitable daily, with or without high dose of flavanols. The MED was assessed at baseline and after 12 weeks under standard conditions. In the HF group, MED the average more than doubled after 12 weeks of chocolate consumption, while in LF group DEM remained without significant change, demonstrating, again, that regular consumption of a rich chocolate flavonoids provides photochemoprotection significant and may be effective in protecting human skin from the harmful effects of UV radiation (Williams et al., 2009).
Another study investigated the effect of 12 weeks of chocolate consumption with high flavanol content in skin sensitivity to UV radiation, measured by MED, as well as the elasticity and skin hydration. The study only used women, aged 20 to 65 years. Two groups were formed: One received daily 30 g of chocolate with an HF and the other group received the same amount, but a chocolate with LF. DEM was assessed at baseline and at 6, 9, 12 and 15 weeks (three weeks after the daily consumption). The curious fact is that in this new study, from initial time to 12 weeks of time, the results for both groups showed no significant differences for the MED, unlike the study cited above. Age over 50 years was associated with major changes in the MED, within the same group in 15 weeks. Both the elasticity and skin hydration increased by six weeks in the HF group compared to the LF group. Plasma concentrations of polyphenols were similar in both groups at baseline. LF group showed a small increase, but statistically significant, compared epicatechin dosage in plasma at weeks 6, 9 and 12 compared to baseline. The HF Group was marked by more considerable increases of epicatechin in plasma (Mogollon et al., 2014).
Other polyphenols with photoprotective activity
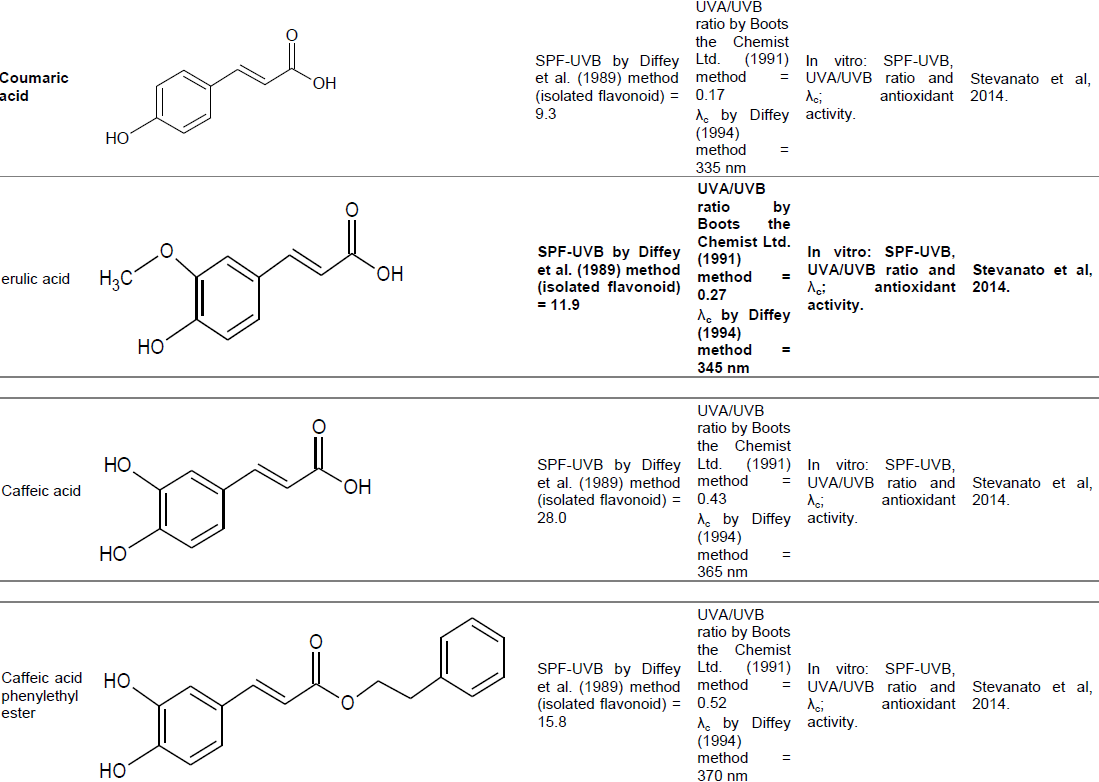
Hydroxycinnamic acid derivatives (coumaric acid, ferulic acid, caffeic acid, caffeic acid phenylethyl ester and dimethyl caffeic acid)
Compounds derived from hydroxycinnamic acid and its homologues and/or derivatives, found in fruits, vegetables, coffee and wine, are considered good candidates to provide photoprotection due to their chemical structures. SPF-UVB by Diffey et al. (1989) method, UVA/UVB ratio and critical wavelength were evaluated for each of these compounds. The results were: coumaric acid (9.3 SPF, UVA/UVB 0.17 and λc 335 nm); ferulic acid (11.9 SPF, UVA/UVB 0.27 and λc 345 nm); caffeic acid (28.0 SPF, UVA/UVB 0.43 and λc 365 nm); caffeic acid phenylethyl ester (15.8 SPF, UVA/UVB 0.52 and λc 370 nm); dimethyl caffeic acid (16.6 SPF, UVA/UVB 0.52 and λc 370 nm). Of all the compounds, caffeic acid showed better SPF-UVB (28.0), followed by dimethyl caffeic acid (16.6). Best UVA/UVB ratio and better critical wavelengths were checked for caffeic acid phenylethyl ester and dimethyl caffeic acid (0.52 and 370 nm for both) (Stevanato et al., 2014). Thus, a formulation containing a mixture of caffeic acid with caffeic acid phenylethyl ester, or dimethyl caffeic acid, ​​could provide significant protection values in both the UVA region as in UVB.
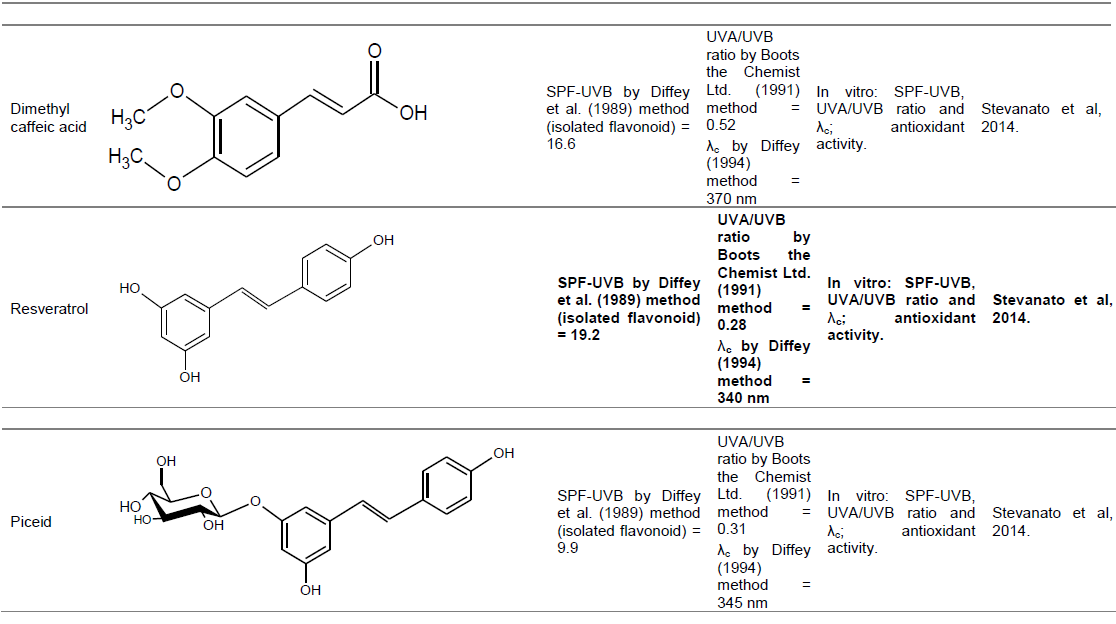
Resveratrol and piceid
Long term studies have shown that topical application of resveratrol (pre and post treatment) results in inhibition of tumor incidence induced by UVB, and delay in the onset of tumor of the skin (Baumann, 2009). However, Regev-Shoshani et al. (2003) demonstrated that resveratrol is susceptible to enzymatic oxidation while piceid, its glycosylated form, it is sturdy and retains its antioxidant capacity and beneficial biological properties. SPF-UVB by Diffey et al. (1989) method, UVA/UVB ratio and critical wavelength were evaluated for each of these stilbenes. The results follow: Resveratrol (19.2 SPF, UVA/UVB 0.28 and λc 340 nm) and piceid (9.9 SPF, UVA/UVB 0.31 and λc 345 nm) (Stevanato et al., 2014). A formulation containing the two stilbene could confer good protection in the UVB region, although not as effective in the UVA region due their low ​​UVA/UVB ratio values and low critical wavelength.