ABSTRACT
Datura stramonium more commonly known as Jimson weed or thorn apple is a wide growing flowering plant which has been employed by the local community to treat several ailments in Ethiopia. The purpose of the present work was to assess the antibacterial activity of ethanol, methanol, acetone, chloroform and water extracts of Datura stramonium leaf extracts using broth dilution and agar well diffusion methods against human pathogenic bacteria. Chloroform extracts showed the highest zone of inhibition against most of the tested bacterial strains at the concentration of 50 mg/ml. Water extracts are not able to show any zone of inhibition against all tested bacterial strains as compared to other four solvents used for extraction. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by standard methods. The MIC and MBC results range from 6.25 to 12.5 mg/ml. The present work shows that D. stramonium has maximum antibacterial activity against Staphylococcus aureus (ATCC 25923; 18.2±2.1 mm) in the chloroform extract, while the minimum antibacterial activity was recorded against Escherichia coli clinical isolate (8.2±1.8 mm) (acetone extract). Chloroform extracts showed the highest zone of inhibition against most of the pathogenic bacterial strains tested as compared to the other solvents used for the extraction. In this study, D. stramonium leaf extracts showed considerable antibacterial activity that sustains the local residential area which uses this plant to treat bacterial and fungal infections, hence leading to the conclusion that, this plant would serve as sources of antimicrobial agents to obtain the best treatment alternatives for the infective disease. Further investigation of this potential antibacterial agent is required, especially using chloroform as an extraction solvent to precisely demonstrate the antimicrobial effects of the plant.
Key words: Antibacterial activity, Datura stramonium, minimum bactericidal concentration (MBC), minimum inhibitory concentration (MIC), zone of inhibition.
Plants are rich in a broad diversity of secondary metabolites such as tannins, alkaloids, terpenoids and flavonoids that have been set up to exhibit antimicrobial, antioxidant, anti-infectious and antitumor activities (Kirtikar and Basu, 1994; Gupta et al., 2010). The demand for the medicinal plant is increasing in both developed and producing rural areas due to the affordability, reliability, accessibility and low side effects in therapeutic use have made their recognition worldwide.
During the past ten years, infectious diseases have been listed as the cause of destruction of millions of lives throughout the globe, particularly in the developing countries (Sharma et al., 2013). To treat these diseases, the modern treatment mechanisms have continuously been facing a problem due to associated side effects. Several pathogens have evolved immunity to multiple antibiotics as a result of the mutagenic characteristics of the bacterial genome, rapid multiplication, and transformation of bacterial cells. Consequently, numerous surveys have been carried on in search of medicinal plants having better antibacterial effects against pathogens (Kumar et al., 2007).
In Ethiopia, there are nearly 1000 medicinal plants that constitute about 10% of the entire flora available in the state (Abreham et al., 2015). For several years, the bulk of these plant materials has been employed by the local community as an alternative medicine to treat many diseases, even though most of them are not well characterized scientifically (Geleta et al., 2015).
Datura stramonium is mostly available in tropical and temperate regions and belongs to the family, Solanaceae (Tariq et al., 1989). D. stramonium contains tropane alkaloids such as scopolamine, atropine and hyoscyamine. As a result of the presence of these significant biomedical components, D. stramonium is considered to be important in treating heart disease, dental and skin infections, ulcer, asthma (Boumba et al., 2005), bronchitis, Leucoderma, fever and piles, sinus infections; it has antimicrobial, anticholinergic (Taha and Mahdi, 1984; Diker et al., 2007; Sharma and Sharma, 2010), anti-inflammatory, anti-fungal (Akinyemi et al., 2005; Giadado et al., 2007; Bouzidi et al., 2011), antioxidant, hypolipidemic, anti-inflammatory, anti-rheumatoid and hypoglycemic properties, (Tariq et al., 1989; Gharaibeh et al., 1988; Rasekh et al., 2001; Couladis et al., 2003).
The present work aimed to test the antibacterial activity of D. stramonium leaf extracts against the standard and clinical isolate pathogenic microorganisms.
Collection
D. stramonium leaves used in this study were gathered from different regions of Gondar town. The plant was authenticated by the Department of Biotechnology, University of Gondar, Gondar, Ethiopia. It was rinsed with tap water and dried at room temperature.
Preparation of plant extracts
D. stramonium leaves were ground to a fine powder using an electronic grinder. The solvents used for extraction were ethanol, methanol, acetone, chloroform and distilled water. Approximately, 100 g powder was blended with 300 ml of each solvent. Orbital shaker was used for the extraction purpose in which the sample was subjected to continuous shaking for 3 successive days. The sample was then filtered out using Whatman No. 1 filter paper, then the filtrate was evaporated using a rotary evaporator under reduced pressure at 4°C. The extract was pooled and dried in vacuo and stored at 4°C in a refrigerator until screened for antibacterial activity. The stock solution was prepared by taking 100 mg/ml in 50% dimethyl sulfoxide (DMSO), mixed with vortex and stored at 4°C until use in the refrigerator.
Preparation of test microorganisms
The bacterial strains, Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Streptococcus pneumoniae (ATCC 63), E. coli (clinical isolate), Klebsiella pneumoniae (clinical isolate) and S. pneumoniae (clinical isolate) were used. Permission and approval to use clinical isolates was obtained from the ethical clearance board of the University of Gondar Hospital. Microorganisms were grown on nutrient agar at 37°C for 24 h. Standards 0.5 McFarland was prepared by acquiring up to 4 colonies in the normal saline solution according to standard operations.
Antibacterial activity assay
Agar well diffusion method was employed to assess the antibacterial activity of D. stramonium ethanol, methanol, acetone, chloroform and water extracts against the human pathogenic bacteria (Taye et al., 2011). The overnight bacterial culture was taken to prepare the inoculums and adjust to 0.5 McFarland standard in 0.9% autoclaved normal saline. The Muller Hinton agar medium was prepared and autoclaved at the 121°C for 15 min. The media were poured into each Petri dish and set aside to solidify under the laminar hood. After solidifying the media, the sterile cotton swab was used to spread the inoculums throughout the medium uniformly. Wells were made using a 6 mm diameter cork borer. Then, 100 µl of each extract adjusted to the same concentration (50 mg/ml) was totaled to a respective well. Vancomycin (30 µg/disc) and chloramphenicol (30 µ g/disc) were practiced as a positive control, while DMSO 50% was applied as a negative control. The agar plate was allowed to rest for 1 h under the laminar hood and incubated later at 37°C for one daytime. The sensitivity of the test microorganisms was found by assessing the diameter of the zone of inhibition in which significant susceptibility was taken as ≥ 7 mm in diameter. All experiments were executed in duplicate and repeated three times.
Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) was determined for the extracts that inhibited the growth of testing bacteria in the concentration of 50 mg/ml and for the extracts which showed a zone of inhibition ≥ 7 mm in diameter. Agar well diffusion and microtube dilution methods were used to perform MIC test. Inagar well diffusion method, double serial dilution was applied from 50 mg/ml to get 1:2, 1:4, 1:8, 1:16, 1:32 and 1:64 in order to get 25, 12.5, 6.25, 3.125,1.56 and 0.78 mg/ml concentration of extracts, respectively, using 50% DMSO. Next, 100 µl of the extract was added to the wells on Muller Hinton agar and then MIC concentration was identified. In microtube dilution method, a similar principle was applied except dilution performed in 1 ml of nutrient broth. To each labeled concentration, a 30 µl of a standard suspension of test microorganisms was added. The controls were developed without any test microorganisms inoculated. The microtube was incubated at 37°C for 24 h. The existence of growth was examined by checking the turbidity of bacteria on each tube before and after inoculation and the results were compared with the control tube.
Minimum bactericidal concentration (MBC)
Dilutions having no visually detectable growth were taken and subcultured on Muller Hinton agar to perform MBC and then incubated for 24 h at 37°C. Ultimately, the outcomes with no visible growth were taken as MBC values.
Data analysis
All data were analyzed using SPSS version 20 and the results are presented as means±standard deviations. Analysis of variation (ANOVA) was employed to define the significant differences between test microorganisms. The statistical significance was determined when P values are ≤ 0.05.
Antibacterial activity of D. stramonium using 50 mg/ml concentration of five extracts was measured. The results are presented in Table 1. There was no antibacterial activity observed against all tested microorganisms using water as the extraction solvent. Ethanol extract did not inhibit the growth of E. coli (ATCC 25922) and S. pneumoniae clinical isolate. However, ethanol extract inhibited the rest tested bacteria with inhibition zone ranging from 9.1 to 16.7 mm. Methanol extract inhibited all tested bacteria strains with the exception of E. coli (ATCC 25922) and K. pneumoniae clinical isolate. On the other hand, acetone extract inhibited tested pathogenic microorganisms with the exception of K. pneumoniae clinical isolate. The chloroform extract inhibited all pathogenic microorganisms investigated with the highest inhibition zone for most tested microorganisms. As compared to methanol and acetone extracts, ethanol extract showed higher (P <0.05) inhibition zone against bacterial strains investigated in this study.
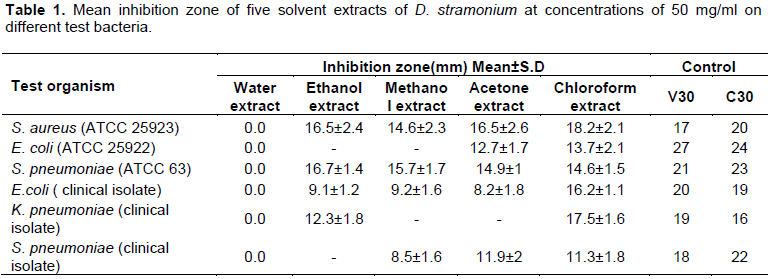
The highest inhibition zone of ethanol extract (16.7 mm) was recorded for S. pneumoniae (ATCC 63) and S. aureus (ATCC 25923) (16.5 mm). While the maximum zone of inhibition of methanol extract was received from S. pneumoniae (ATCC 63) (15.7 mm), S. pneumoniae clinical isolate showed the lowest zone of inhibition (8.5 mm). On the other hand, the highest zone of inhibition using acetone extract was recorded for S. aureus (16.5 mm), while E. coli clinical isolate showed the lowest zone of inhibition which was 8.2 mm. Using chloroform extract, the maximum zone of inhibition was recorded for S. aureus (ATCC 25923) (18.2 mm), while the minimum zone of inhibition was obtained for S. pneumoniae clinical isolate (11.3 mm).
Vancomycin (30 µg/disc) and chloramphenicol (30 µg/disc) standard antibiotics were applied as positive control while 50% DMSO was applied as a negative control. Chloroform extract showed highest inhibition zone (18.2 mm) for S. aureus (ATCC 25923) as compared to Vancomycin (17 mm). In the same extract, K. pneumoniae clinical isolate showed better zone of inhibition (17.5 mm) than chloramphenicol (16 mm).
The MIC of D. stramonium extracts was determined by agar dilution method. The ethanol extract showed MIC of 6.25% with the exception of E. coli clinical isolate and S. pneumoniae clinical isolate that showed 12.5%. Methanol extract gave 12.5% MIC for E. coli and K. pneumoniae clinical isolates, while the rest tested microorganisms showed 6.25%. In the case of acetone extract, S. aureus (ATCC 25923), S. pneumoniae (ATCC 63) and S. pneumoniae clinical isolates showed 6.25% MIC, while the rest showed 12.5% MIC. Chloroform extracts gave 6.25% MIC for all microorganisms tested with the exception of E. coli clinical isolate that showed 12.5% MIC (Table 2).

The MBC of ethanol extract against all tested micro organisms was 6.25% except E.coli and S. pneumoniae clinical isolates that showed 12.5% MBC. On the other hand, methanol extract showed MBC of 6.25% against E. coli (ATCC 25922), K. pneumoniae clinical isolate and E. coli clinical isolate, 12.5% MBC was observed for S. aureus (ATCC 25923), S. pneumoniae (ATCC 63) and S. pneumoniae clinical isolate microorganisms. However, acetone extracts showed 6.25% against S. aureus (ATCC 25923), S. pneumoniae (ATCC 63) and S. pneumoniae (clinical isolate). The rest tested microorganisms showed 12.5% MBC. Moreover, chloroform extracts exhibited 6.25% minimum bactericidal concentration against in all the tested microorganisms with the exception of E. coli clinical isolate that showed 12.5% MBC (Figure 1).
The purpose of traditional medicine by local communities is attributed to its accessibility and price effectiveness and thus, the use of herbal medicine is nowadays becoming increasingly more popular in the globe. Medicinal plants constitute an efficient beginning for both traditional and advanced medical specialty. Approximately, 80% of people from developing countries use traditional medicine as primary health care (Eloff, 1998). Consequently, such plants should be investigated in a broad range to better see their properties, safety and efficacy. Over the years, the World Health Organization (WHO) recommended that countries should cooperate with traditional medicine with a survey to identify and exploit aspects that provide secure and efficient remedies for ills of both microbial and non-microbial origins (WHO, 1978).
The significance of plant extracts and phytochemicals, both with known antimicrobial properties can be of great significance in therapeutic treatments. A routine of surveys has been previously conveyed in different states to prove such efficiency (Ikram and Inamul, 1984; Almagboul et al., 1985; Artizzu et al., 1995). Various plants have been employed as a result of their antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the flora.
In the present study, antibacterial activities of D. stramonium leaf extracts using 5 extracts as extraction solvents have been conducted against the standard and clinical isolate human pathogenic microorganisms.
As per the research, there was no previous work conducted to validate D. stramonium leaf extracts using the patterns used in this research in Ethiopia. In the data, water extracts did not show any antibacterial activity against tested pathogenic microorganisms which are supported by previous findings that used water as an extraction solvent for finding active antibacterial components (El safey and Salah, 2011).
The current study shows that leaf extracts of D. stramonium inhibited the growth of human pathogenic bacteria S. aureus (ATCC 25923) E. coli (ATCC 25922), S. pneumoniae (ATCC 63), E. coli (clinical isolate), S. pneumoniae (clinical isolate) and K. pneumoniae (clinical isolate) which is in line with the outcomes obtained by Obi et al. (2002).
The leaf extracts of D. stramonium showed antibacterial activity against E. coli and K. pneumoniae which is compatible to Adebayo et al. (1989) who found high antimicrobial activity against those microorganisms. In addition, higher antibacterial activity was obtained against S. pneumoniae (ATCC 63) and S. aureus (ATCC 25923) and lower antibacterial activity against E. coli clinical isolate which is partially in line with the results obtained by Benito et al. (2011) who found higher antibacterial activity against S. aureus (ATCC 25923) and E. coli (ATCC 25922) and E. coli clinical isolate. Moreover, D. stramonium extracts showed lower antibacterial activity against E. coli clinical isolates which is supported by the results of Eftekhar et al. (2005).
Antibacterial activity of D. stramonium leaf extracts is due to the presence of phytochemicals that includes, flavonoids, phenols, tannins, saponins, sterols and alkaloids. Because of the presence of these fundamental biomedicals, D. stramonium is considered as treasured medicine and useful in the treatment of many diseases. Phytochemical constituents in the plant sample are known to be biologically active compounds and they are responsible for different activities such as, antimicrobial, antioxidant, antifungal and anticancer (Hossain and Nagooru, 2011; Powar and Powar, 2016).
The classes of alkaloids are among the major poisons known. Aside from being vicious, some alkaloids have also been shown to be useful in correcting renal disorders (Fluck, 1973; Konkwara, 1976). The present work shows that D. stramonium has maximum antibacterial activity against S. aureus (ATCC 25923), 18.2 mm (chloroform extract) while the minimum antibacterial activity was recorded against E. coli clinical isolate (8.2 mm) (acetone extract).
In the present work, the antibacterial activity of D. stramonium leaf with 5 different solvents was investigated. Results of this study indicate that D. stramonium possesses considerable antibacterial activity that supports the use of the flora in the traditional scheme of medicine for the handling of several diseases. However, advance studies are required to identify and characterize the bioactive compounds responsible for the activity so that, the plant can be used as a natural antimicrobial agent. The presence of secondary metabolites in single or in combination with others could be responsible for the antibacterial activity of the plant.
Extracts obtained from D. stramonium in this study were tested only against bacteria and further investigation is necessary to validate against fungal species since the local community uses the leaf of this plant to treat bacterial and fungal infections. Further investigation of this potential antibacterial agent by other researchers is recommended with similar and different forms of pathogenic microorganisms to precisely demonstrate the antimicrobial effects of the plant.
The authors declare that there is no conflict of interest.
REFERENCES
|
Abreham B, Awokech G, Birtukan G, Rahel A, Taye M, Workabeba D (2015). Antimicrobial activity of Thymus schimperi Ronninger (Lamiaceae) against standard and clinical isolates of human pathogenic bacteria. J. Med. Plants Res. 9(11):379-384.
Crossref
|
|
|
|
Adebayo AG, Oloke JK, Aladesanmi AJ (1989). Antimicrobial activities of the leaf extract of Eugeniauniflora. Phytother. Res. 3:258-259.
Crossref
|
|
|
|
|
Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA (2005). Screening of crude extracts of six medicinal plants used in Southwest Nigerian orthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement. Altern. Med. 5:6.
Crossref
|
|
|
|
|
Almagboul AZ, Bashir AK, Farouk A, Salih AKM (1985). Antimicrobial activity of certain Sudanese plants used in folkloric medicine. Screening for antibacterial activity. Fitoterapia 56:331-337.
|
|
|
|
|
Artizzu N, Bonsignore L, Cottiglia F, Loy G (1995). Studies of the diuretic and antimicrobial activity of Cynodon dactylon essential oil. Fitoterapia 66:174-175.
|
|
|
|
|
Benito J, Shringib BN, Dinesh KP, Nehru SC, Ashok KJ (2011). Screening of antimicrobial activity of alcoholic and aqueous extract of some indigenous plants. Indo Glob. J. Pharm. Sci. 1(2):186-193.
|
|
|
|
|
Boumba A, Mitselou A, Vougiouklakis T (2005). Fatal poisoning from ingestion of Datura stramonium seeds. Vet. Hum. Toxicol. 46:81-82.
|
|
|
|
|
Bouzidi A, Mahdeb N, Kara N (2011). Toxicity studies of alkaloids of seeds of Datura stramonium and synthesis alkaloids in male rats. J. Med. Plants Res. 5(15):3421-3431.
|
|
|
|
|
Couladis M, Tzakou O, Verykokidou E, Harvala C (2003). Screening of some Greek aromatic plants for antioxidant activity. Phytother. Res. 17:194-195.
Crossref
|
|
|
|
|
Diker D, Markovitz D, Rothman M, Sendovski U (2007). Coma as a presenting sign of Datura stramonium seed tea poisoning. Eur. J. Intern. Med. 18(4):336-338.
Crossref
|
|
|
|
|
Eloff JN. (1998). Which extract should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 60:1-8.
Crossref
|
|
|
|
|
El safey M, Salah GA (2011). In vitro antibacterial activities of Rifampicin and Thyme on Methicillin Resistant Staphylococcus aureus (MRSA). Asian Trans. Basic Appl. Sci. 1:68-75.
|
|
|
|
|
Eftekhar F, Yousefzadi M, Tafakori V (2005). Antimicrobial activity of Datura innoxia and Datura stramonium. Fitoterapia 76:118-120.
Crossref
|
|
|
|
|
Fluck H (1973). Medicinal plants and their uses. W. Feulshom and comp. Ltd, New York. pp. 7-15.
|
|
|
|
|
Geleta B, Eyasu M, Kebamo A, Mekonnen E, Abebe A (2015). In vitro vasodilatory effect of aqueous leaf extract of Thymus serrulatus on thoracic aorta of Guinea pigs. Asian Pac. J. Trop. Biomed. 5(1):15-18.
Crossref
|
|
|
|
|
Gharaibeh MN, Elayan HH, Salhab AS (1988). Hypoglycemic effects of Teucrium polium. J. Ethnopharmacol. 24:93-99.
Crossref
|
|
|
|
|
Giadado A, Zainab A, Hadiza MU, Serah DP, Anas HY, Milala MA (2007). Toxicity studies of ethanol extract of the leaves of Datura stramonium in rats. Afr. J. Biotechnol. 6(8):1012-1015.
|
|
|
|
|
Gupta S, Raghuvanshi M, Jain D (2010). Comparative Studies on Anti-Inflammatory Activity of Coriandrum Sativum, Datura Stramonium and Azadirachta Indica. Asian J. Exp. Biol. Sci. 1(1):151-154.
|
|
|
|
|
Hossain MA, Nagooru MR (2011). Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacogn. J. 3(24):25-29.
Crossref
|
|
|
|
|
Ikram M, Inamul H (1984). Screening of medicinal plants for antimicrobial activities. Fitoterapia 55:62-65.
|
|
|
|
|
Kirtikar JD, Basu BD (1994). Indian Medicinal Plants. Lalit Mohan Basu; Allahabad, India. pp 1229-1231.
|
|
|
|
|
Konkwara JO (1976). Medicinal Plants of East Africa. Literature Burea, Nairobi. pp. 3-8.
|
|
|
|
|
Kumar B, Vijaykumar M, Govindarajan R, Pushpangadan P (2007). Ethnopharmacological approaches to wound healing: Exploring medicinal plants of India. J. Ethnopharmacol. 114(2):103-113.
Crossref
|
|
|
|
|
Obi CL, Potgieter N, Randima LP (2002). Antibacterial activities of five plants against some medically significant human bacteria. S. Afr. J. Sci. 98(1-2):25-28.
|
|
|
|
|
Powar PV, Powar TA (2016). In-vitro antibacterial activity of sequential crude extracts from Datura stramonium seeds. J. Invent. Biomed. Pharm. Sci. 1(1):01-04.
|
|
|
|
|
Rasekh HR, Khoshnood MJ, Kamalinejad M (2001). Hypolipidemic effects of Teucrium polium in rats. Fitoterapia 72:937-939.
Crossref
|
|
|
|
|
Sharma MC, Sharma S (2010). Phytochemical, Preliminary Pharmacognostical and Antimicrobial Evaluation of Combined Crude Aqueous Extract. Int. J. Microbiol. Res. 1(3):166-170.
|
|
|
|
|
Sharma RA, Sharma P, Yadav A (2013). Antimicrobial screening of sequential extracts of Datura stramonium L. Int. J. Pharm. Pharm. Sci. 5(2):401-404.
|
|
|
|
|
Taha SA, Mahdi AW (1984). Datura stramonium intoxication in Riyadh. Trans. R. Soc. Trop. Med. Hyg. 78:134-135.
Crossref
|
|
|
|
|
Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS. (1989) Anti-inflammatory activity of Teucrium polium. Int. J. Tissue React. 11:185-188.
|
|
|
|
|
Taye B, Giday M, Animut A, Seid J (2011). Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac. J. Trop. Biomed. 1(15):370-375.
Crossref
|
|
|
|
|
World Health Organization (WHO) (1978). The promotion and development of traditional medicine. Technical report series. World Health Organ. Tech. Rep. Ser. 622:1-41.
|
|


