Full Length Research Paper
ABSTRACT
Nigeria accounts for a considerable proportion of maternal deaths that occur annually worldwide. The study investigated the incidence of pre-pregnancy and pregnancy-related illnesses in women accessing antenatal care services at health facilities in Awka South Local Government Area, Anambra State. The study adopted cross-sectional research design. The population comprised 3,207 registered pregnant women from January to September 2012. The sample for the study consisted of 650 pregnant women. A pre-tested questionnaire was administered by the interviewers to women who had attended antenatal care services within six months prior to the date of data collection. Malaria (66.6%), morning sickness (58.0%), hyperemesis gravidarum (39.7%), sexually transmitted infections (28.6%), gestational diabetes (23.8%), pre-eclampsia and eclampsia (23.0%) and anaemia (15.8%) were the common illnesses in women. There were statistically significant differences in the women’s pre-pregnancy illnesses according to the level of education (p = 0.032) and pregnancy-related illnesses according to age (p = 0.023) and level of education (p = 0.045). It was concluded that the interplay of several factors is responsible for the incidence of pre-pregnancy and pregnancy-related illnesses in women. Identification of these factors is expedient while scaling up of maternal health interventions; improved access and uptake of facility-based care hopefully, will drastically reduce morbid conditions in women and improve maternal and newborn outcomes.
Key words: Prevalence, Pregnancy-related illnesses, antenatal care, maternal health.
INTRODUCTION
Illnesses during pregnancy portend severe threats to maternal and fetal health with adverse maternal, fetal and newborn health outcomes. The status of maternal health in poor countries is often described in terms of maternal mortality alone, despite the evidence that far more women suffer from morbidities/disabilities relating to pregnancy and childbirth (Filippi et al., 2007). About 289,000 maternal deaths occur annually worldwide, and Nigeria accounts for about 14% of these preventable deaths (United Nations, 2010). About 20 million women suffer from acute severe obstetric complications, including haemorrhage, obstructed/prolonged labour, preeclampsia/ eclampsia, puerperal sepsis, and septic abortion (Glasier et al., 2006; Hogan et al., 2010). The burden of maternal ill-health extends beyond these complications and includes different short- and long-term morbid conditions that can result from acute obstetric complications or poor management at delivery (Gulmezoglu et al., 2004; Zwart et al., 2008; Lindquist et al., 2013). In Nigeria, the estimated maternal mortality ratio (MMR) of 576 deaths per 100 000 live births reported in 2013 was higher than the 545 deaths per 100,000 live births recorded in 2008 (National Population Commission-NPC and ICF International 2013) and fell far short of the Millennium Development Goal of 250 deaths per 100,000.
Hospital records according to Federal Ministry of Health (FMoH, 2013) and National AIDS and Reproductive Household Survey (NARHS) in 2012 have shown that for many years, illnesses are the major causes of maternal mortality with variations between women in urban and rural communities (Doctor et al., 2011; Ajayi and Osakinle, 2013). Vanderkruik et al. (2013) asserted that complications of pregnancy, childbirth, and the postpartum period may lead to death or cause a continuum of morbidities that affect a woman’s health for short or long-term periods during and after pregnancy, and even throughout her life. Community-based studies conducted in various countries have reported that women suffer significant morbidity both during pregnancy and postnatal period (Wall, 1998; Zishiri et al., 1999; Walraven et al., 2001; Bang et al., Filippi, 2004).
Such morbidities are also associated with poor fetal and newborn outcomes. Defining maternal morbidity poses serious challenges because there are diverse definitions, identification and classifications of maternal morbidity in literature. Currently, there is a lack of an agreed-upon definition for maternal morbidity (Vanderkruik et al., 2013). Existing work on maternal morbidity includes an array of conditions, both short- and long-term in varying combinations (Waterstone et al., 2003; Chersich et al., 2009; Ukachukwu et al., 2009). Three major issues have limited valid, routine, and comparable measurements of maternal morbidity to date. These include lack of a common definition and identification criteria for maternal morbidity, lack of standardized assessment tools especially at community or primary health care level, and lack of common indicators to measure morbidity (Vanderkruik et al., 2013). To guide this study, the authors used a well-defined term-illness.
Parsons (1951) viewed illness as a human response to the disease process or to the perception by an individual that he has some form of impairment. It is an abnormal process in which the person’s level of functioning is changed. Illness is described as feelings experienced by an unwell person. A person may feel ‘ill’ without a disease being evident or diagnosed (Alpert et al., 1967). Illness has been conceptualized as the patient’s experience of ill-health and comprises his/her impaired sense of well-being, the perception that something is wrong with the body and various symptoms of pain, distress, and disablement (Abanobi, 2003). Existing literature includes an array of methods for identifying and classifying maternal illness. Maternal morbidities or illnesses have been grouped under various categories such as direct obstetric morbidity, indirect obstetric morbidity and psychological obstetric morbidity (Reed et al., 2000) or obstetric complications, pre-existing medical conditions (Danel et al., 2003; Berg et al., 2009) or categories of obstetric morbidity that occur during pregnancy, during delivery, or after delivery (Zurayk et al., 1993). In addition to the wide range of how maternal morbidity conditions are categorized, the methodology for detecting a maternal morbidity varied across studies as well, including interview-based diagnosis and hospital records (for example birth/hospital discharge data) (Stewart et al., 1996; Roberts et al., 2009). This study explored a few typologies of maternal morbidity/illness. Two typologies of illnesses associated with pregnancy have been identified in the literature (Arkutu, 1995). These are illnesses caused by pregnancy such as ectopic pregnancy, hyperemesis gravidarum, miscarriage, pre-eclampsia, eclampsia, sepsis and haemorrhage and pre-existing illnesses or medical conditions which anybody can suffer but which are made worse in pregnancy. These include malaria, anaemia, diabetes, essential hypertension, backache and sexually transmitted infections (STIs). Another category of illnesses in pregnancy as identified in literature (Elaine, 2000; Nursing and Midwifery Council of Nigeria, 2006) include: malaria, anaemia, diabetes, essential hypertension, pulmonary tuberculosis, sickle cell disease, urinary tract infection, cardiac disease, asthma, sexually transmitted infections (STIs), renal problems as well as backache and headache. This study investigated typologies of illnesses in pregnancy based on literature, which include: illnesses that are caused by pregnancy such as backache, pre-eclampsia, hyperemesis gravidarum, gestational diabetes (GDM); hypertensive disorders (pre-eclampsia and eclampsia) and pre-pregnancy illnesses which are made worse in pregnancy such as malaria, anemia, diabetes, hypertension, STIs, tuberculosis and depression. Studies have indicated associations between severe maternal morbidity and factors such as advanced maternal age, pre-existing medical conditions and obesity (Bouvier-Colle et al., 1997; Zhang et al., 2005). However, these factors are unable to explain entirely the differences in maternal morbidity found between different populations of women both within and between countries (Lindquist, 2013). Nevertheless, this study explored differences in pre-pregnancy and pregnancy-induced illnesses in women according to maternal factors (maternal age and education). Primary health centres (PHCs) in the study area offer ANC services amidst challenges such as shortage of vaccines and essential drugs, logistic problems, skilled health personnel, and unstable power supply, which hinder effective service delivery. The interplay of all or some of these variables may influence pregnant women’s access to ANC services with attendant consequences for maternal and neonatal outcomes. This situation accentuates the need for the choice of the study area. In addition, studies have indicated that severe maternal morbidity may be a valid indicator of the quality and effectiveness of obstetric care than mortality alone (Mantel et al., 1998; Koeberle et al., 2000). There is paucity of data on incidence of pre-pregnancy and pregnancy-related illnesses among pregnant women accessing ANC at PHCs in Awka South LGA, Anambra State, Nigeria. In view of high maternal mortality rates in Nigeria, and potential complications inherent sub-standard care for pre- and pregnancy-related illnesses among women in Nigeria, this study was carried out.
MATERIALS AND METHODS
Study design, location and period
This cross-sectional study was undertaken from January to September, 2012 at the selected health facilities in Awka South LGA, Anambra State. Awka South, the area of the study is one of the 21 LGAs in Anambra State. It is located in the rainforest belt southeast, Nigeria. It shares boundaries with Awka North LGA to the North, Oji-River LGA, Enugu State to the East, Aniocha LGA to the south and Njikoka LGA to the west. Awka South comprises nine communities. The population for the study consisted of all pregnant women that registered and accessed antenatal care services in public, private and mission health facilities in Awka South LGA, Anambra State. Women in the area have limited access to quality maternal health services. Awka South LGA also has many rural communities. Thus, the study population consisted of rural pregnant women.
Study population and sample estimation
There were 3,207 registered pregnant women in Awka South LGA at the time of the study (Record and Statistics, General Hospital, Awka and Health Department, Awka South LGA, 2012). We used the following formula for sample size calculation:
Where, Z is a standard normal variant (Z = 1.96 when the confidence interval is 95%), p is the expected proportion of the outcome in population based or other studies, and d is the absolute accuracy or precision (Charan and Biswas, 2013). Since there was no previous study in the study area, we assumed that 50% of the population had suffered from at least one pre-pregnancy or pregnancy-related illness in the last 12 months. We calculated a minimal sample size of 384 that would be required to give a 95% probability of measuring the prevalence of pre-pregnancy and pregnancy-related illnesses with 5% accuracy. To minimize the effect of non-response rate and errors that may ensue to due small sample size, we increased the study sample to 650. Multi-stage sampling procedure was employed to select the pregnant women who attended health facility ANC services in the six months preceding the study. All the PHCs located in the study area were involved in the study. In each community, a health facility was randomly selected. In each of the selected health facilities, women were conveniently selected to make the sample for the study. The inclusion criteria were women aged 15-49 years and women who had attended antenatal clinics at least twice in the six months prior to the study.
Instruments for data collection
Two instruments were used for data collection-a checklist and structured questionnaire. The checklist was designed to collect data on reported pregnancy-related illnesses among pregnant women from hospital/health facility records three months (May, 2012) prior to the data collection period (data collection period began in August and ended in September, 2012). The most common pregnancy-induced illnesses were identified and integrated in the 19-item structured paper-based questionnaire titled Pre-Pregnancy and Pregnancy-Related Illnesses Questionnaire (PPRIQ), which was used for data collection. The PPRIQ was subdivided into five sections to elicit the following information:
1) Introduction: Contains information of what the study was all about;
2) Informed consent;
3) Respondent’s sociodemographic data, which were made up of two items;
4) Pre-pregnancy Illnesses in Pregnant women: this consists of nine items to obtain information on illnesses prior to pregnancy;
5) Pregnancy-related illnesses: This is a 10-item section to elicit information on illness induced by pregnancy.
The authors used a self-reported instrument to collected data from the participants. The PPRIQ was administered by trained interviewers (research assistants). After the women have responded to questionnaire items, researchers and research assistants retrieved copies of the questionnaire from the women. Nine pre-existing illnesses identified via literature (Zurayk et al., 1993; Danel et al., 2003; Zhang et al., 2005) were included in the questionnaire. The women were asked to indicate if they had suffered from any of the outlined illnesses six months prior to pregnancy. These included: malaria, anaemia, diabetes, hypertension, STIs, TB, sickle cell anaemia, urinary tract infections (UTIs) and asthma. The items were assigned dichotomous response of ‘Yes’ or ‘No’. The participants were asked to tick (Ö) as many as applied to them. A “Yes” response implied “experience of illness” while a “No” response implied “absence of illness”. Ten pregnancy-related illnesses of pregnant women were identified and included in the structured questionnaire based on the checklist. These included high blood pressure (pre-eclampsia and eclampsia), sexually transmitted infection (STIs), HIV/AIDS, severe vomiting (Hyperemesis gravidarum), diabetes due to pregnancy (gestational diabetes), anaemia, malaria, ectopic pregnancy, threatened abortion and morning sickness. The items were also assigned dichotomous response of ‘Yes’ or ‘No’. The participants were asked to tick (Ö) as many as applied to them. A “Yes” response implied “experience of illness” while a “No” response implied “absence of illness”.
Ethical consideration
Permission to conduct the study was obtained from Primary Health Care coordinator of Awka South LGA, and Heads of health facilities. Informed consent was obtained from pregnant women who participated in the study after a detailed explanation of the purpose of the study and the required assistance. The pregnant women were free to withdraw at any time or to refuse to answer any question. Confidentiality was maintained during the course of the study by ensuring face to face interviews by each interviewer without a third party and information obtained during the study was kept confidential.
Data analysis
The SPSS version 18 (SPSS, Inc., Chicago) was used for data entry, cleaning, and analysis. We used descriptive statistics to describe the distribution of socio-demographic characteristics of participants accessing ANC at health facilities in Awka South LGA. The Kuder-Richardson-20 reliability test was used to determine the correlation coefficient index of sections B and C. This procedure yielded coefficient values of 0.88 and 0.75 respectively. The Chi-square test was used to examine differences in the pregnancy-related illnesses suffered by women. Educational level of women was treated as a categorical variable (no formal education, primary education, secondary education and tertiary education). The ages of the respondents were coded into four categories viz < 20, 20-29 years, 30-39 years, and > 40 years. Due to incomplete responses in few copies of questionnaire administered, data from 647 properly completed questionnaire copies were used for analysis. All statistical tests were performed at p < 0.05.
RESULTS
Demographic variables
Among the participants, age ranged from 15 to 49 years, with a mean of 24.7 (SD = 2.7). About 6.18% were less than 20 years, 47.5% were within 21 to 29 years, 36.4% were within 30 to 39 years and 9.09% of women were above 40 years. In addition, about 10.2% of pregnant women had no formal education, 12.8% had primary education, 41.9% had secondary education and 35.1% had tertiary education (Table 1).
Reported pre-pregnancy illnesses by pregnant women
The findings are presented in three sections, which include: pre-pregnancy illnesses/medical conditions of pregnant women, reported pregnancy-related illnesses of pregnant women, and comparison of pregnancy-related illnesses based on maternal characteristics. Table 2 shows that the most common pre-pregnancy morbidity was malaria (66.6%). This was followed by STIs in 185 (28.6%) of the women. Anaemia occurred in 102 (15.8%) of pregnant women.
Reported pregnancy-related illnesses by pregnant women
Table 3 describes the occurrence of maternal illnesses diagnosed during pregnancy by health professionals among women. About 61.0% of pregnant women suffered from malaria. Other pregnancy-related illnesses suffered by women include morning sickness (58%), severe vomiting (39.7%), anaemia (34.6%), gestational diabetes (23.8%), preeclampsia and eclampsia (23%) and threatened abortion (22.1%).
Pre-pregnancy and pregnancy-related illnesses according to maternal characteristics
Data in Table 4 indicates that group-wise, about 22.5 and 34.79 % of women in age categories < 20 years and > 40 years respectively had suffered from at least one of the illnesses before pregnancy while 19.8 and 18.7% of women in age categories 30 to 39 years and 21-29 years respectively had suffered at least one illness before pregnancy. Table 5 indicates that group-wise, about 30.6 and 30% of women in age categories 31-39 years and > 40 years respectively had suffered from at least one of the pregnancy-related illnesses while 29.5% and 28.7% of women in age categories <20 years and 21-29 years respectively had suffered from at least one of the pregnancy-related morbidities/illnesses.
In reference to maternal level of education, Table 6 shows that 23.8 and 19.7% of women in categories NFE and PRE respectively had suffered from at least one of the illnesses before pregnancy while 19.8 and 18.5% of women in categories SEE and TEE respectively had suffered from at least one of the illnesses before pregnancy. Data in Table 7 show that about 36.1 and 32.8% of women in categories NFE and SEE respectively had suffered from at least one of the pregnancy-related morbidities as diagnosed by a physician while 31.5 and 22.9% of women in categories PRE and TEE respectively had suffered from at least one of the pregnancy-induced illnesses as diagnosed by a physician.
Data in Table 8 show that there was no significant difference in the reported pre-pregnancy illnesses in women according to age (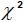 = 1.156 < 7.815, df = 3, p = 0.217). This implies that women did not differ in their experience of pre-pregnancy illnesses according to age. However, there was a significant difference in the reported pre-pregnancy illnesses in women according to level of education (
= 1.156 < 7.815, df = 3, p = 0.217). This implies that women did not differ in their experience of pre-pregnancy illnesses according to age. However, there was a significant difference in the reported pre-pregnancy illnesses in women according to level of education (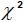 = 44.99 > 7.815, df = 3, p = 0.032). This implies that women did experience differences in illnesses according to level of education. Data in Table 9 show that there were significant differences in the reported pregnancy-related illnesses based on maternal age (
= 44.99 > 7.815, df = 3, p = 0.032). This implies that women did experience differences in illnesses according to level of education. Data in Table 9 show that there were significant differences in the reported pregnancy-related illnesses based on maternal age (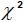 = 35.36 > 7.815, df = 3, p = 0.045) and level of education (
= 35.36 > 7.815, df = 3, p = 0.045) and level of education (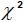 = 10.393 > 7.815, df = 3, p = 0.023) respectively. This shows that incidence of pregnancy-related illnesses in women differed according to age and educational level.
= 10.393 > 7.815, df = 3, p = 0.023) respectively. This shows that incidence of pregnancy-related illnesses in women differed according to age and educational level.
DISCUSSION
Data on obstetric morbidity in developing countries are limited and, when available, they commonly define the type of medical conditions diagnosed at the hospital level (Assarag et al., 2013). However, many challenges plagued classification of maternal morbidities in developing nations including Nigeria where accurate diagnosis of maternal morbidities is indispensable to investigations (Graham and Campbell, 1992). These problems and weakness of conceptual framework underlying study of maternal health may explain why there is dearth of studies on maternal morbidities (Graham and Campbell, 1992). This study is one of the few surveys conducted in south-east Nigeria studying incidence of pre-pregnancy illnesses and pregnancy-related morbidities in women within the population. Our results show that malaria (66.6%), STIs (28.6%), anaemia (15.8%) and pre-eclampsia (13.8%) were the most common pre-pregnancy illnesses in women as diagnosed by a physician while malaria (60.7%), morning sickness (58.0%), and nausea/severe vomiting (hyperemesis gravidarum) (39.7%) and anaemia (34.6%) were the pregnancy-related illnesses mostly experienced by women. The incidence of these illnesses is higher than the reported incidence in a study in Niamey, Niger Republic (6.45%), and higher than that of Scotland (0.38%) (Prual et al., 1998; Brace et al., 2004). The higher occurrence of pre-pregnancy and especially pregnancy-related illnesses in our study may partly be due to poor access or uptake of ANC services and data assessment identifying more cases by using standardised definition in a large population. The disparity in occurrence of maternal morbidities may also be attributed to the general level of development and that of maternal health care delivery services. The fact that pre-pregnancy and pregnancy-related illnesses cases were higher in our study is robust proof that they constitute a significant threat to pregnant women in south-east, Nigeria. Thus, pre-pregnancy and pregnancy-related illnesses deserve to be considered priority targets for public health interventions.
We examined differences in pregnant women’s experience of pre-pregnancy and pregnancy-related illnesses according to maternal factors of age and level of education. We found that there was a significant difference in the reported pre-pregnancy illnesses in women according to level of education (Table 8). Furthermore, we found that there were significant differences in the reported pregnancy-related illnesses based on maternal age and level of education respectively (Table 9). These findings are in tandem with the findings of a previous study conducted in northern Nigeria that found associations between maternal socio-demographic factors and maternal deaths from pregnancy complications (Adamu et al., 2003). The authors reported that teenage mothers and mothers aged 40 years and above constituted the age groups at greatest risk. Adamu et al. (2003) further reported that the second most significant predictor of mortality was the educational level of mothers. They observed that as the level of education rose, the probability of the mother dying from delivery complications diminished in a dose-dependent fashion (p = 0.02).
CONCLUSION
The findings of this study will be useful to policy makers, healthcare professionals and public health experts. This study thus showed high prevalence of malaria, morning sickness and low prevalence of STIs, anaemia, pre-eclampsia and nausea/severe vomiting (hyperemesis gravidarum) in women. The incidence of these illnesses was significantly higher among pregnant women when compared to studies from other climes. The higher occurrence of pre-pregnancy and especially pregnancy-related illnesses in our study may partly be due to poor access or uptake of ANC services and data assessment identifying more cases by using standardised definition in a large population. The disparity in occurrence of maternal morbidities may also be attributed to the general level of development and poor maternal health care delivery services. Scaling-up of integrated maternal and child health (IMCH) services; access to high quality facility care; use of health systems approach in maternal health services provision are likely to provide a holistic improvement in the quality of care in the study area. This in turn will substantially reduce mortality and morbidity from these conditions. In addition, early identification and management of pregnancy-related complications, particularly pre-eclampsia, haemorrhage, premature rupture of membranes and STIs, identification and treatment of underlying or concurrent illnesses in pregnancy such as malaria, gestational diabetes, anaemia, STIs among others are vital to improved maternal and fetal outcomes in Nigeria.
Limitations of the study
Some inaccuracies in the classification of certain morbidities may be expected due to the use of admission diagnoses. Data collection did not extend to the medical wards as well as postpartum period. Therefore, data collected did not include postpartum obstetric conditions. Recall bias was another potential limitation because information on maternal morbidities based on recall spanned across pre-pregnancy period, pregnancy and perinatal and some women might not remember when they specifically had such morbidities. It is expected that inclusion of women around perinatal, childbirth and postpartum period may help minimize the effect of recall bias as recall period is shorter compared to using pre-pregnancy and early pregnancy periods. Furthermore, information that was obtained relied on women’s self-report and pregnant women may underreport illnesses due to social desirability bias, hence, the observed occurrence of maternal illnesses may be underestimated. The incidence of maternal morbidity in this study may depict the iceberg phenomenon. Thus, reported maternal morbidities may not be a true reflection of the burden of this problem in the sub-population as only a third of delivery takes place at the health facilities. Some women with these morbidities may not have access to PHCs or private or mission health facilities for several reasons, which include lack of transportation, ignorance, poverty, influence of traditional/religious beliefs and perceived negative attitude of health workers.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
REFERENCES
|
Abanobi OC (2005). Health: Wellness and illness states. Biological, Social, Cultural, Environmental, Nutritional, Behavioural and Health System Factors. Owerri, Opinion Research Communications Inc, pp. 57-65. |
|
|
Adamu YM, Salihub HM, Sathiakumar N, Alexander GR (2003). Maternal mortality in Northern Nigeria: A population-based study. European Journal of Obstetrics and Gynecology and Reproductive Biology, 109(2):153-159. |
|
|
Ajayi IO, Osakinle DC (2013). Socio demographic factors determining the adequacy of antenatal care among pregnant women visiting Ekiti State Primary Health Centers. Online Journal Health and Allied Sciences, 12:1-6. |
|
|
Alpert JJ, Kosa J, Haggerty R (1967). A month of illness and healthcare among low-income families. Public Health Reports, 82(8):705-713. |
|
|
Arkutu AA (1995). Healthy woman, healthy mothers: An information guide. New York: Family Care international. |
|
|
Assarag B, Dubourg D, Maaroufi A, Dujardin B, De Brouwere V (2013). Maternal postpartum morbidity in Marrakech: What women feel what doctors diagnose? BMC Pregnancy and Childbirth, 13(1):225. |
|
|
Bang RA, Bang AT, Reddy MH, Deshmukh MD, Baitule SB, Filippi V (2004). Maternal morbidity during labour and the puerperium in rural homes and the need for medical attention: A prospective observational study in Gadchiroli, India. BJOG: An International Journal of Obstetrics and Gynaecology, 111(3):231-238. |
|
|
Berg CJ, Mackay AP, Qin C, Callaghan WM (2009). Overview of maternal morbidity during hospitalization for labour and delivery in the United States: 1993–1997 and 2001–2005. Obstetrics and Gynecology, 113(5):1075-1081. |
|
|
Bouvier-Colle MH, Varnoux N, Salanave B, Ancel PY, Bréart G (1997). Case-control study of risk factors for obstetric patients' admission to intensive care units. European Journal of Obstetrics and Gynecology and Reproductive Biology, 74:173-177. |
|
|
Brace V, Penny G, Hall M (2004). Quantifying severe maternal morbidity: A Scottish population study. BJOG: an International Journal of Obstetrics and Gynaecology, 111(5):481-484. |
|
|
Charan J, Biswas T (2013). How to calculate sample size for different study designs in medical research? Indian Journal of Psychological Medicine, 35:121-126. |
|
|
Chersich MF, Kley N, Luchters, SM, Njeru C, Yard E, Othigo MJ (2009). Maternal morbidity in the first year after childbirth in Mombasa Kenya: A needs assessment. BMC Pregnancy Childbirth, 9(1):51. |
|
|
Danel I, Berg C, Johnson CH, Atrash H (2003). Magnitude of maternal morbidity during labour and delivery: United States, 1993-1997. American Journal of Public Health, 93(4):631-634. |
|
|
Doctor HV, Bairagi R, Findley SE, Helleringer S (2011). Northern Nigeria maternal, newborn and child health programme: Selected analyses from population-based baseline survey. Open Demography Journal, 4:11-21. |
|
|
Elaine H (2000). Disease associated with pregnancy. In V.R. Bennett, & L.K. Brown, (eds). Myles textbook for midwives. New York: Churchill Livingstone. pp. 317-334. |
|
|
Federal Ministry of Health (FMoH) (2013). National HIV/AIDS and Reproductive Health and Serological Survey, 2012 (NARHS Plus). Abuja, Nigeria: FMoH. |
|
|
Filippi V, Ganaba R, Baggaley RF, Marshall T, Storeng KT, Sombie I (2007). Health of women after severe obstetric complications in Burkina Faso: A longitudinal study. Lancet, 370:1329-1337. |
|
|
Glasier A, Gulmezoglu AM, Schmid GP, Moreno CG, Van Look PF (2006). Sexual and reproductive health: A matter of life and death. Lancet, 368:1595-1607. |
|
|
Graham WJ, Campbell OMR (1992). Maternal health and measurement trap. Social Science and Medicine, 35(8):967-977. |
|
|
Gulmezoglu AM, Say L, Betran AP, Villar J, Piaggio G (2004). WHO systematic review of maternal mortality and morbidity: Methodological issues and challenges. BMC Medical Research and Methodology, 4(1):16. |
|
|
Hogan MC, Foreman KJ, Naghavi M, Anh SY, Wang M, Makela SM (2010). Maternal mortality for 181 countries, 1980-2008: A systematic analysis of progress towards Millennium Development Goal 5. Lancet, 375:1609-1623. |
|
|
Koeberle P, Lévy A, Surcin S, Bartholin F, Clément G, Bachour K, Boillot A, Capellier G, Riethmuller D (2000). Severe obstetric complications necessitating hospitalization and intensive care: A ten year retrospective study. In Annales francaises d'anesthesie et de reanimation, 19(6):445-451. |
|
|
Lindquist A, Knight M, Kurinczuk JJ (2013). Variation in severe maternal morbidity according to socioeconomic position: A UK national case–control study. BMJ Open, 3(6):e002742. |
|
|
Mantel GD, Buchmann E, Rees H, Pattinson RC (1998). Severe acute maternal morbidity: A pilot study of a definition for a near-miss. BJOG: An International Journal of Obstetrics and Gynaecology, 105(9):985-990. |
|
|
Nursing and Midwifery Council of Nigeria (2006). Post-basic midwifery curriculum. Abuja: City Way Printing and Paper Industry. |
|
|
Parsons T (1951). The social system. London: Routledge and Kagan Paul. |
|
|
Prual A, Huguest D, Garbin O, Rabe G (1998). Severe Obstetric morbidity of the third trimester, delivery and early puerperium in Niamey (Niger). African Journal of Reproductive Health, 2(1):10-19. |
|
|
Reed HE, Koblinsky MA, Mosley WH (2000). The consequences of maternal morbidity and maternal mortality: Report of a workshop, October 19–20 1998, NAS Board Room, Washington D.C. Washington, DC: National Academy Press. |
|
|
Roberts CL, Ford JB, Algert CS, Bell JC, Simpson JM, Morris JM (2009). Trends in adverse maternal outcomes during childbirth: A population-based study of severe maternal morbidity. BMC Pregnancy and Childbirth, 9(1):7. |
|
|
Stewart MK, Stanton CK, Festin M, Jacobson N (1996). Issues in measuring maternal morbidity: Lessons from the Philippines safe motherhood survey project. Studies in Family Planning, 27(1):29-35. |
|
|
Ukachukwu VE, Unger H, Onoka C, Nduka C, Maina S, Ngugi N. (2009). Maternal morbidity and mortality in peri-urban Kenya–assessing progress in improving maternal healthcare. East African Journal of Public Health, 6(2):112-118. |
|
|
United Nations (2010). The millennium development goals report: We Can End Poverty, 2015 MDGs. New York: United Nations. Available at: |
|
|
Vanderkruik RC, Tunçalp Ö, Chou D, Say L (2013). Framing maternal morbidity: WHO scoping exercise. BMC Pregnancy and Childbirth, 13(1):213. |
|
|
Wall LL (1998). Dead mothers and injured wives: The social context of maternal morbidity and mortality among the Hausa of northern Nigeria. Studies of Family Planning, 24(4):341-359. |
|
|
Walraven G, Scherf C, West B, Paine K, Coleman R, Bailey R, Morison L (2001). The burden of reproductive organ disease in rural women in the Gambia, West Africa. The Lancet, 357 (9263):1161-1167. |
|
|
Waterstone M, Wolfe C, Hooper R, Bewley S (2003). Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG: An International Journal of Obstetrics and Gynaecology, 110(2):128-133. |
|
|
Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane AJ (2005). Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG: An International Journal of Obstetrics and Gynaecology, 112(1):89-96. |
|
|
Zishiri C, Shodu LK, Tsimanga M, Nyirongo L (1999). Postnatal maternal morbidity patterns in mothers delivering in Gweru City (Midlands Province). The Central African Journal of Medicine, 45(9):234-239. |
|
|
Zurayk H, Khattab H, Younis N, El-Mouelhy M, Fadle M (1993). Concepts and measures of reproductive morbidity. Health Transition Review, 3(1):17-40. |
|
|
Zwart JJ, Richters JM, Ory F, De Vries JIP (2008). Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: A nationwide population-based study of 371,000 pregnancies. BJOG: An International Journal of Obstetrics and Gynaecology, 115(7):842-850. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0