ABSTRACT
Vitamin D (VD) deficiency and insufficiency have been linked to poor quality of life (QoL); general weakness, fatigue, and nonspecific pain. Despite abundance of sun light, there is a wide spread VD deficiency in the Arabian Gulf countries. However, the impact of VD deficiency on the quality of life has not been well documented in the Arabian Gulf countries, including the Kingdom of Saudi Arabia (KSA). This study aims to analyze the effect of crude vitamin D levels on quality of life in none pregnant Arab women in the Kingdom of Saudi Arabia (KSA). The study was a prospective cross sectional study, using a sample of 152 subjects of patients aged 13 years and above attending the outpatients’ clinic at King Faisal Hospital, Kingdom of Saudi Arabia (KSA). The study collected anthropometric and demographic variables, in addition to a small plasma sample from all subjects to measure VD levels, calcium (Ca), phosphorus (P), parathyroid hormone (PTH) and lipid profile by standard methods. Subjects were categorized according to VD levels as normal (>30 ng/ml), insufficiency (21 to 29 ng/ml), or deficient (≤ 20 ng/ml). The RAND Short Form -36 (SF-36), which consists of 36 questionnaire items was used to measure (QoL) across eight domains for both physically and emotional wellbeing: physical functioning; role limitations due to physical health; role limitations due to emotional problems; energy/fatigue; emotional well-being; social functioning; pain and general health. Logistic regression analysis was used to investigate the associations between VD levels and QoL. The mean scores in the total sample were 65.5 (SD ±14.6) for the physical component summary (PCS) score and 58.2 (SD ±15.2) for the mental component summary score (MCS). Vitamin D deficiency was overwhelmingly high among subjects across age and sex. The PCS and MCS QoL scores were also lower than the average. However, the study found no association between VD levels and QoL, presumably due to sample size and con-founders. Further studies are needed to identify the impact of VD levels on perceived QoL.
Key words: Vitamin D, poor quality of life (QoL), kingdom of Saudi Arabia (KSA), physical component summary (PCS).
Vitamin D refers to a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, iron, magnesium, phosphate, and zinc. The most important compounds in this group are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol) (Holick, 2006). The term 'vitamin D' specifically refers to two very similar vitamin forms. The first form, known as Vitamin D2 is obtained from the ultraviolet (UV) irradiation of the yeast sterol ergosterol and is found naturally in some sun-exposed mushrooms. The second form, vitamin D3, also known as cholecalciferol, is created by skin cells in response to the sun light or ultraviolet-B (UVB) light. UVB strikes the skin, and humans synthesize vitamin D3 which is considered as the most “natural” form. Unlike vitamin D3, human beings do not make vitamin D2, but most oil-rich fish such as salmon, mackerel, and herring contain vitamin D3. Both forms of vitamin D ingested, is combined into chylomicrons, which are absorbed into the lymphatic system and enter the venous blood. Evidence shows that vitamin D, whether from the skin or diet, is biologically inactive and requires its first hydroxylation in the liver by the vitamin D-25-hydroxylase (25-OHase) to 25(OH) D (Ecemis and Atmaca, 2013; Holick, 2011).
However, 25(OH) D requires a further hydroxylation in the kidneys by 25(OH)D-1-OHase (CYP27B1), to form the biologically active form of vitamin D 1,25(OH)2D (Ecemis and Atmaca, 2013; Holick, 2007). The latter, 1,25(OH)2D, is known to stimulate intestinal calcium absorption (Mithal and Kaur, 2012). Accumulated evidence shows that without vitamin D, only 10 to 15% of dietary calcium and about 60% of phosphorus are absorbed (Mithal and Kaur, 2012). The same evidence shows that vitamin D sufficiency enhances calcium and phosphorus absorption by 30 to 40% and 80%, respectively (Li et al., 2004; Ecemis and Atmaca, 2013). It is thus clear that, the major role of vitamin D is to maintain normal blood levels of calcium and phosphorus. Sufficient levels of vitamin D are essential to help the body to absorb calcium, which forms and maintains strong bones. It is used alone or together with calcium to improve bone health and decrease fractures. Vitamin D may also protect against osteoporosis, high blood pressure, cancer, and other diseases. 1,25(OH)2D is known to have many protective features, such as the inhibition of cellular proliferation and inducing terminal differentiation, inhibiting angiogenesis, stimulating insulin production, inhibiting renin production, and stimulating macrophage cathelicidin production (Zittermann, 2006; Dawodu et al., 1998; Elshafie et al ., 2012; Ardawi et al., 2012).
Vitamin D deficiency (VDD), or hypovitaminosis D, can result from inadequate nutritional intake of vitamin D, inadequate sunlight exposure (in particular sunlight with adequate UVB), disorders limiting vitamin D absorption, and conditions impairing vitamin D conversion into active metabolites - including certain liver, kidney, and hereditary disorders (Holick, 2011). VDD is defined by the Institute of Medicine (IOM) as a 25(OH) D of less than 0.8 IU. Vitamin D insufficiency is defined by IOM as a 25(OH) D of 21–29 ng/mL (Holick, 2006; Ware and Sherbourne, 1992; Lee et al., 2008). Deficiency impairs bone mineralization, leading to bone softening diseases as rickets in children and osteomalacia and osteoporosis in adults (Holick, 2011). In children, vitamin D deficiency causes rickets, which is a softening or weakening of the bones. In adults, vitamin D deficiency can lead to osteomalacia, which causes weak bones and muscles. Rickets and osteomalacia are classic vitamin D deficiency diseases. People who may be at a high risk for vitamin D deficiency include those who are elderly or obese, those with limited sun exposure, and babies who are exclusively breastfed.
People who have conditions such as cystic fibrosis (mucus build-up in the lungs) or inflammatory bowel disease are also known to be at risk for vitamin D deficiency. VDD is common in Australia, the Middle East, India, Africa, and South America (Holick, 2006; Tomaschitz et al., 2010; Vaidya and Williams, 2012). Pregnant and lactating women who take a prenatal vitamin and a calcium supplement with vitamin D remain at high risk for VDD (Zittermann, 2006; Chowdhury et al., 2009; Pittas et al., 2007). In addition the deficiency of VD has been associated as a surrogate marker in the aetiology of many chronic diseases including asthma, high blood pressure, diabetes mellitus, hyperlipidemia, cardiovascular disorders (Holick, 2006), and osteoporosis (Lee et al., 2008). Evidence shows that VDD and insufficiency may be more common in women, especially, in the premenopausal state than previously thought and it may impair quality of life (QoL) (Li et al., 2004). Evidence shows that obtaining sufficient vitamin D from natural food sources alone may be difficult. The consumption of vitamin D-fortified foods and exposure to some sunlight are essential for maintaining a healthy vitamin D status.
Evidence also shows that dietary supplements might be required to meet the daily need for vitamin D in some groups of people (Vaidya and Williams 2012).Little is known regarding the effect of vitamin D supplementation on quality of life. The literature regarding the relation between VD deficiency and insufficiency and the quality-of-life or the outcomes from vitamin D supplementation in healthy and clinical populations is not conclusive. Generally, the quality of life (QoL or QOL) is defined as the perceived quality of an individual's daily life, that is, an assessment of their well-being or lack of wellbeing thereof. This includes all emotional, social, and physical aspects of the individual's life. In health care, health-related quality of life (HRQoL) is an assessment of how the individual's well-being may be affected over time by a disease, disability, or disorder, such as VD deficiency and insufficiency (Holick, 2006; Lee et al., 2008). Similar to other psychometric assessment tools, a number of health-related QoL questionnaires that meet certain quality criteria, most importantly with regard to their reliability and validity, are available for use.
The most important validated health-related quality of life questionnaires that suit the needs of various illnesses are: the RAND short form (SF-36), which include 36 questions; and, disease, disorder or condition specific instruments (e.g. the King's Health Questionnaire (KHQ) or the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF), etc. Evidence shows that QoL ratings are beneficial for both patents and healthcare practitioners, to gauge improvements in health and wellbeing, following interventions such as VD supplementation among patients. In the Arabian Gulf countries, chronic diseases are epidemic. Substantially low VD levels, much lower than those found in European, were reported among women (Tomaschitz et al., 2010), men and women (Vaidya and Williams, 2012; Zittermann, 2006) and healthy adolescents (Chowdhury et al., 2009). This is attributed, in part to ageing, obesity, sedentary lifestyle, illiteracy, poor dietary vitamin D supplementation, and despite its abundance, poor exposure to sunlight (Ardawi et al., 2012).
However, little is known about the relationship between VD deficiency and insufficiency and QoL in the Gulf Arab population. We hypothesize that adequate supplementation of VD among patients with VD deficiency and insufficiency may improve quality of life. The aim of this study was to measure and assess relationships of serum 25-hydroxyvitamin D [25(OH)D] with quality of life in children, men and non-pregnant women, using (SF-36) and a number of anthropometric measures to examine whether emotional and physical performance, are related to VD deficiency and insufficiency. The study objectives included:
1. Investigate the prevalence of VDD and VD insufficiency among the sample population, using ELISA to measure serum 25(OH) D.
2. Measure and investigate obesity, BP and blood insulin resistance among the sample patients population.
3. Measure and assess quality of life (QoL) among patients, using RAND Short Form (SF-36) for quality of life, across 8 domains of emotional and physical activity.
4. To quantify and analyze the effect of crude vitamin D levels on quality of life among men and none pregnant Arab women.
Ethical statement
The protocols of this study were approved by the Ethical Review Committees at King Faisal Hospital, KSA. All subjects gave an informed consent prior to their participation.
Study design
The study is a cross sectional study, conducted in the outpatients’ clinic of King Faisal Hospital, Kingdom of Saudi Arabia (KSA) during the 2013.
Subjects
Subjects were 152 male and female patients, aged 13 years and above, reporting to the outpatients’ clinics of King Faisal Hospital in
KSA during 2013. The study patient population was composed of two groups of subjects: juveniles of 13 to 18 years age and adults, 19 years and above, presented with VD deficiency and insufficiency and no pregnancy.
Inclusion criteria
Healthy individuals, juveniles of 13 to 18 years old and adults, with weakness, fatigue and nonspecific pain with 25-hydroxyvitamin D (25-OHD) levels: ≤ 20 ng/ml (VD deficient), 21 to 29 ng/ml (VD insufficient) and ≥ 30 ng/ml (VD sufficient).
Exclusion criteria
Pregnant women (before or during the study) and women with an abnormal level of serum parathyroid hormone (PTH) (>55 pg/ml) and serum calcium (> 10.4 mg/dl), known to have illnesses such as granuloma forming disorder (e.g. Tuberculosis), lymphoma, sarcoidosis or any type of cancers were excluded. Subjects on vitamin D supplementation or any forms of calcitriol (1,25 (OH)2 D3) were also excluded.
Anthropometric measurements
Qualified research nurses were trained to assist subjects in completing self-reported questionnaires. Nurses measured the height, weight, and waist circumference of participant patients by a calibrated electronic scale with mounted stadiometer from Seca (Model 769; Seca, Hamburg, Germany). Before taking the measurements, all subjects were asked to wear light clothing and no shoes. Thereafter, subjects were instructed to stand straight with their heads, backs and buttocks vertically aligned to the height gauge. Measurements of height and weight were taken and rounded to the nearest 0.5 cm and 0.5 kg, respectively. The standard tape was used to measure waist circumference (WC) at a point immediately above the iliac crest on the midaxillary line, at minimal respiration and round it to the nearest 1.0 cm. Overall, three separate measurements of height, weight, and WC were recorded for each participant and averaged for analysis to increase precision. Body mass index (BMI), the ratio of weight to height squared [Weight (kg)]/ [height (m)] 2 was calculated. Subjects with (BMI < 20 kg/m2), BMI 20 < 25 kg/m2), (BMI of 25 to 30 kg/m2) and BMI >30 kg/m2 were classified as underweight, normal weight, overweight and obese, respectively.
Blood sampling and methods of analysis
A 10 ml venous blood was collected after 12 h of an overnight fasting. The blood samples were collected by a registered staff nurse using vacutainer system from Becton Dickinson with 20 G needles (USA). The blood samples were divided into two tubes; plasma EDTA (5 ml) and Serum (5 ml) and were immediately centrifuged by 3000 g/15 min at 4°C within 2 h. Samples were separated, alliqouted, and stored in deep freezers (-80°C) for analysis. Plasma was sued to measure lipid profile, and FBS, whereas, serum was used to measure HBA1c, calcium (Ca), PTH, 25(OH) D3, and insulin. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on pairing FBS and Fasting Blood Insulin to establish a measure for insulin resistant: HOMA-IR = [{FBS (mmol/L)} * {Insulin (IU per ml)}/22.5]. ELISA was used to measure serum 25(OH) D. All tests and analysis were carried out by the Clinical Diagnostic Laboratory, at King Faisal University.
Quality of life
The study used the RAND Short Form-36 (SF-36), a 36 item questionnaire to measure quality of life (QoL) across eight domains of physical and emotional wellbeing: physical functioning; role limitations due to physical health; role limitations due to emotional problems; energy/fatigue; emotional well-being; social functioning; pain and general health. Data was converted in a two stage manner. First, each question response was related to the corresponding pre-coded numeric value. Secondly, scores were translated and the average for each of the eight scale components was calculated. Moreover, the study summarized the physical QoL (Physical Component Summary; PCS) from the corresponding physical activity components. Similarly, emotional QoL (Mental Component summary; MCS) was calculated as the average of the relevant mental health components. Finally, a z-score was calculated for each of these two components to transform the data into percentile.
Statistical analysis
STATA software version 13 was used for data analysis. Descriptive and inference analysis using the t-test, where applicable, were carried out. To compare categorical variables, the study used the Chai square test and the Fisher exact test, wherever appropriate, to analyze the data. Multivariate logistic regression was used to identify independent correlates between VD-levels and QoL measured by PCS and MCS scores. A p-value < 0.05 was used as the statistical significance level.
The socio-demographic characteristics of participant subjects (n=152) are summarized, according to sex in Table (1). Overall, 21% of participants were males and the majority (79%) was females (Table 1). The mean age of male patients was 38.9 years while the mean age among female patients interviewed was 33.9 years (p=0.003). The mean BMI among male patients was 27.1 compared to 26.3 among females but the difference was not significant. There were significant differences in SBP and DBP, though both were within the normal range. The mean age, height and weight of males were significantly higher than those of females (Table 1).
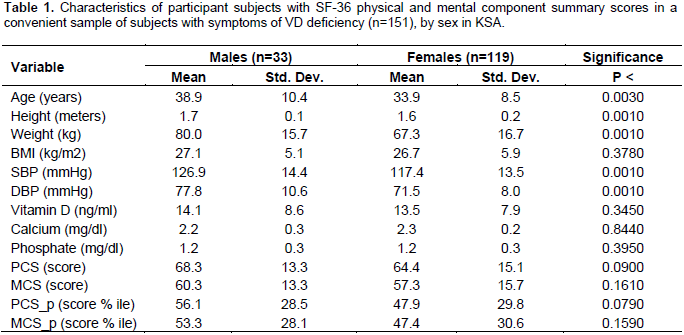
The analysis of the QoL physical component summary (PCS) from the corresponding physical activity components and the emotional QoL mental component summary (MCS) from the relevant mental health components showed that, the mean scores in the total sample were 65.5 (SD ±14.6) for PCS and 58.2 (SD ±15.2) for MCS. The linear regression analysis did not show any significant relationships between VD levels and both PCS and MCS scores (Table 2). However, when vitamin D was categorized as insufficient, deficient, or normal, it was observed that subjects with normal levels of VD have had higher mean PCS scores, but the pattern is not similar for the equivalent MCS scores (Figures 1 and 2).
As stated earlier, a sizeable evidence shows that VD deficiency and insufficiency are widely prevalent in the worldwide and are commonly thought to be responsible for a variety of chronic disease states, including diabetes, cancer, and depression. Routine vitamin D supplementation is clearly needed to meet vitamin D requirements, but little is known so far regarding the effect of vitamin D supplementation on quality of life. This study attempted to measure and assess relationships of serum 25-hydroxyvitamin D [25(OH)D] (Ecemis and Atmaca, 2013; Holick, 2007) with quality of life in children, men and non-pregnant women in KSA, using (SF-36) and a number of anthropometric measures to examine whether emotional and physical performance, are related to VD deficiency and insufficiency. The study showed that an overwhelming majority of participant focuses on those that had clear VD deficiency. Similar findings were previously reported in the Arabian Gulf countries despite the abundance of sunlight, the major source for VD.
Although plausible genetic predisposition has been suggested, there is yet no strong evidence of causal factors as such. Nonetheless, we think that lack of adequate sun exposure due to cultural reasons and lifestyle (with limited outdoor activities) may have contributed to such low levels of VD in this population (Holick, 2006). Whether the low levels of VD have led to our findings of low PCS and MCS scores is not very clear from this data. Linear regression analysis was carried out, but still, we were unable to show any association between VD levels and QoL indicators (PCS and MCS), which is possibly due to the small sample size used for the study. Interestingly, however, subjects with normal VD levels demonstrated a higher mean of PCS score but not MCS score. Although not affirmative, such finding lends weight to the possibility of association.
Perhaps, the small number of subjects with normal VD levels (5 subjects) have masked, otherwise plausible associations. We also cannot rule out other underlying confounders which may have been out of control. It would be logical therefore, to undertake a further interventional follow up study on the same subjects, after receiving VD supplementation, to investigate whether the changes in VD levels would improve their PCS and MCS scores significantly. We intend to follow up with this line of research and perhaps increase the sample size in the near future. In summary, although we have shown a significant higher PCS scores among subjects with normal VD levels, we were unable to present solid evidence of the impact of VD deficiency on quality of life of the sample patients. Our finding is consistent with evidence elsewhere. For example a recent systematic review of 15 research articles showed that VD supplementation was not associated with significant changes in quality of life (Li et al., 2004).
The review also showed that studies that reported changes in quality of life as a result of vitamin D supplementation were in clinical populations on short-term vitamin D. Most articles reviewed displayed poor methodological quality (e.g. no randomization/blinding, dropout description, or vitamin D assessment) (Mithal and Kaur, 2012; Muhairi et al., 2013). In conclusion the current evidence indicates that vitamin D supplementation alone may have little impact on the quality of life when used on a short-term basis in patients’ populations. It is thus clear that the evidence for the beneficial effect of a long-term vitamin D treatment supplementation on quality of life of patients remains lacking. Our data has many limitations. First, the sample size is small and convenient and as such, may have masked true statistical associations, thus limiting our ability to make adequate inference. Second, the Sf-36 instrument was meant to identify changes in QoL as opposed to a single baseline records.
Vitamin D deficiency was overwhelmingly high among the study subjects across age and sex. The PCS and MCS QoL scores were also found lower than the average levels. However, we found no association between VD levels and QoL, presumably due to our small sample size and the presence of confounders. Further studies are needed to identify the impact of VD levels on perceived QoL among patients in the Arabian Gulf countries.
The authors have not declared any conflict of interests.
REFERENCES
|
Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA (2012). High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 23(2):675-686.
Crossref
|
|
|
|
Chowdhury TA, Boucher BJ, Hitman GA (2009). Vitamin D and type 2 diabetes. Prim. Care Diabetes 3(2):115-116.
Crossref
|
|
|
|
|
Dawodu A, Absood G, Patel M, Agarwal M, Ezimokhai M, Abdulrazzaq Y, Khalayli G (1998). Biosocial factors affecting vitamin D status of women of childbearing age in the United Arab Emirates. J. Biosoc. Sci. 30(4):431-437.
Crossref
|
|
|
|
|
Ecemis GC, Atmaca A (2013). Quality of life is impaired not only in vitamin D deficient but also in vitamin D insufficient premenopausal women. J. Endocrinol. Invest. 2013; PMID: 23511484.
|
|
|
|
|
Elshafie DE, Al-Khashan HI, Mishriky AM (2012). Comparison of vitamin D deficiency in Saudi married couples. Eur. J. Clin. Nutr. 66(6):742-745.
Crossref
|
|
|
|
|
Holick MF (2006). High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 81(3):353-373.
Crossref
|
|
|
|
|
Holick MF (2007). Vitamin D Deficiency. N Eng. J. Med. 357(19):1981-1982.
|
|
|
|
|
Holick MF (2011). Vitamin D: A D-lightful solution for health. J. Invest Med. 59(6):9.
Crossref
|
|
|
|
|
Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF (2008). Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 52(24):1949-1956.
Crossref
|
|
|
|
|
Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J (2004). Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 89-90(1-5):387-392.
Crossref
|
|
|
|
|
Mithal A, Kaur P (2012). Osteoporosis in Asia: a call to action. Curr Osteoporos Rep. 10(4):245-247.
Crossref
|
|
|
|
|
Muhairi SJ, Mehairi AE, Khouri AA, Naqbi MM, Maskari FA, Al Kaabi J, Al Dhaheri AS, Nagelkerke N, Shah SM (2013). Vitamin D deficiency among healthy adolescents in Al Ain, United Arab Emirates. BMC Publ. Health 14:13:33.
|
|
|
|
|
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007). The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 92(6):2017-2029.
Crossref
|
|
|
|
|
Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W (2010). Independent association between 1, 25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin–angiotensin system: the ludwigshafen risk and cardiovascular health (LURIC) study. Clin. Chem. Acta. 411(17-18):1354-1360.
Crossref
|
|
|
|
|
Vaidya A, Williams JS (2012). The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 61(4):450-458.
Crossref
|
|
|
|
|
Ware Jr JE, Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30(6):473-483.
Crossref
|
|
|
|
|
Zittermann A (2006). Vitamin D and disease prevention with special reference to cardiovascular disease. Prog. Biophys. Mol. Biol. 92(1):39-48.
Crossref
|
|