Full Length Research Paper
ABSTRACT
Ebola virus Disease EVD is deleterious to the health system, food security and social activities. However, here we highlight the risk and fiscal impact an outbreak can exert - economic cost (direct cost of clinical treatment, contact tracing and surveillance system) and repugnant cost on the citizens and foreign business partners. This paper reviews the indicator parameters (risk factors) that can lead to an EVD index case in Nigeria using probabilistic risk model, exponential and Beta-Poisson distribution model. It examines the negative impact of EVD in hardest hit countries during the 2014 West African outbreak, and the need for preemptive attention in Nigeria. Although this risk assessment process has limitation of exposure data with many assumptions, precautionary lessons are drawn from ecological, sociological and environmental drivers that lead to Ebola virus spill over and/or emergence in previously known outbreaks since 1976.
Key words: Ebola virus, risk, probability, infection.
INTRODUCTION
Once an epidemic is over, it is important to reassess the risk of reoccurrence. Risk assessment systematically determines the likelihood of negative consequences resulting from exposure to biological hazards. In epidemiology, risk assessment is used for disease surveillance and a basis for evaluating the potential future consequences of exposure to any hazardous biological agent. Comprehensively assessing the risk factors of Ebola re-emergence and the sustained human-to-human transmission is important as precautionary measures for importation or emergence of the disease. Three components in risk assessment are hazard assessment (identification of the etiology-Ebola virus), exposure assessment (evaluation of the population exposed to the etiology, that is dose-response), and context assessments (evaluation of the physical and socio-economic environment the event is taking place) (WHO, 2012).
Several studies have determined the risks of EVD outbreak based on these components. United Nation Development Group (UNDG) simulated the probability of Ebola prevalence in 15 West African countries, and grouped countries into high or low Ebola scenario. From the model, the probability of having Ebola index in Nigeria is between 0.1 and 0.2 which places the country in the medium –risk group (UNDG, 2015). Another study uses the Susceptible-Exposed-Infectious-Removed model (SEIR) and estimated the reproductive number of EVD and risk of an outbreak from an undetected case at 9.0 and 89% respectively (Althaus et al., 2014). Although these estimates provide an immediate insight into the likelihood of outbreak in Nigeria, they do not elucidate several specific risk factors capable of driving Ebola epidemic in the country. What are these risk factors in Nigeria that leads to EVD index and sustained human transmission, and what impact can an outbreak wield on the Economy?
Brief overview and epidemiology of ebola virus
Ebola virus disease EVD or “Ebola” is a severe human and non-human primate disease caused by Ebola viruses. These viruses belongs to the family Filoviridae, Genus Ebola virus, which include the species Tai forest ebolavirus (TAFV), Reston ebolavirus (RESTV), Sudan ebolavirus (SUDV), Zaire ebolavirus (EBOV) and Bundibugyo ebolavirus (BOBV) (Bukreyev et al, 2014). Zaire ebolavirus (ZEBOV) is the most virulent of the genus and accounts for the highest numbers of outbreaks. The virus spreads by direct contact with body fluids or secretions of infected animals. Although the natural reservoir of the virus is yet to be ascertained, several scientific studies has proven that fruit bats is the natural host (Olival et al., 2013; Calisher et al., 2006; Swanepoel et al., 1996; Biek et al., 2006). Humans get infected through direct physical contact with infected bats or other animals (duikers and non-human primates) that serve as intermediate hosts. Ebola virus then spreads among humans through contact with symptomatic person or contaminated fomite. There have been various speculations with regards to the pathogenesis of EBOV. Generally, studies have shown that on a successful entry into body tissues, the virus invade the immune system suppressing the production and response of interferon proteins using its VP24 and VP35 (Hoenen et al., 2006). It infects monocytes, macrophages and dendritic cells resulting in rapid viral replication, hemorrhage and hypovolemic shock and edema, fever and gastrointestinal dysfunction (Lai et al., 2014).The incubation period is usually 2 to 21 days, as disease symptoms is usually characterize by fever, pains in joints and muscles, headache, bloody vomit and diarrhea, abdominal pains and internal or external bleeding. Diagnostic technique include detecting viral
The first outbreaks of EVD occurred on August 1976 in Nzara, South Sudan and Yambuko, Zaire (Democratic Republic of Congo, DRC). Prior to the 2014 West Africa outbreak, about 24 episodes of EVD have been reported in 7 African countries-Sudan, DRC, Uganda, Gabon, Congo republic of, Ivory Coast and South Africa- with 2387 reported cases, (case fatality rate 50-90%) (WHO, 2015). The West African ZEBOV epidemic began in December 2013 in Guinea and had spread to other West African countries- Liberia, Sierra Leone, Nigeria, Senegal and Mali, and outside West Africa- Spain and USA. As of August 2015, World Health Organization (WHO) has reported 28639 cases including 11316 deaths have been reported worldwide, the most occurred in Liberia, Guinea and Sierra Leone (WHO, 2016). The index case for the 2013 outbreak which began in Guèckedou region of Guinea is believed to be through contact with infected bat (Saéz et al., 2015) . The index case in Nigeria was imported by a Liberian-American, resulting in 20 confirmed cases with 8 deaths. On 20th October 2014, Nigeria was declared Ebola free by WHO after successfully containing the virus.
Probability of EVD emergence in Nigeria
Understanding the trend that leads to outbreak of EVD throughout history will aid clarify risk factors in Nigeria. Although identifying the main reservoirs of Ebola virus and their geographic boundaries still remain limited. Human EVD cases prospectively resulted from contact with infected bats, duikers and non-human primates. (Swanepoel et al) demonstrated in laboratory conditions that bats might be the natural reservoir of Ebola virus. They are very common in sub-Sahara Africa and can migrate up to 2500 kilometers (McGuire, 2012). The 2007 outbreak in DRC is evidently linked to the annual massive migration and hunting of fruit bats in the region (Leroy et al., 2009). In 2008, Ebola virus antibodies were detected in 32 of 88 bats screened in Ghana, of these, 9 were ZEBOV positive (Hayman et al., 2012). The existence of 9 bats in Ghana infected with ZEBOV suggest that they may have migrated from Central Africa, since this viral species is believed to originate from Central Africa (Sylla et al., 2015) . ZEBOV did not spillover into human population in Ghana, probably no susceptible person had contact the infected bats. Evidently, prior to any outbreak, the virus may have being in the reservoirs host or intermediate host for unascertained period of time, for conditions for spillover (hunting) to presented itself.
Several outbreaks of EVD are associated with hunting and physical contact with zoonotic non-human primates.
Most times, the reservoir host (bats) sheds the virus to its recipient host (non-human primates and duikers) which in turn serves as intermediate host for humans (Plowright et al 2015).
The 1976 index case in DRC was thought to handle carcasses of Antelope and Monkey on his way back to Yambuko (Muyembe-Tamfum et al., 2012). A female etiologist in Cote d’Ivoire tested positive to EVD after performing necropsy on chimpanzees (Formenty et al., 1999). 1996/1997 and 2001-2003 outbreaks in Gabon and DR Congo respectively, were associated with butchering and consumption of infected non-human primates (Rouquet et al., 2005).
In Nigeria, there have been no study investigating the population of bats and its consumption rate, despite the fact that this animal is highly used for food, cultural and ritual purposes by some ethnic groups, nor it is certain whether or not there are EBOV infected Bats. However, other virulent viruses have been isolated from bats in Nigeria and studies showed that an infected Bat can circulate the viral particle among other congeners in the roost (Kia, 2014). The 2008 Bat’s screening in Ghana, the 1994 Ebola infected chimpanzees and the 2014 outbreak in Guinea strikingly demonstrate the feasibility of EBOV infected bats migrating into Nigeria. The dissemination of EBOV among bat and close human contact with this animal show the potential of spill over into human population. A survey among 50 hunters in Idanre, south west Nigeria showed an average hunts of 11.20 Bats (Eidolon heluum) per hunter, within five months (Bifarin et al., 2008), excluding hunting by other residents of the region.
In another survey, bush meat trading in Oban Hill region, South East Nigeria shows the consumption rate of Primates in this area- 35 chimpanzees, 2937 African monkeys within December and October (Eniang et al., 2008). Bush meat buyers travel thousands of kilometers to this region to purchase various species of animals. Apparently continuous consumption of Bats and non-human primates posts a public health risk of EVD index.
Cross boarder risk
The index case of Ebola in Senegal was a young man in incubation period, travelled by road to Dakar, from Guinea. Mali had the first case from migration of a two-year-old child from Guinea. The disease was introduced into Sierra Leone when EVD patients from Guinea illegally cross boarder to seek for cure from a traditional healer in Kenema (WHO, 2015). A symptomatic air traveler from Liberia introduced the virus in Nigeria. Although this index in Nigeria was through an approved terminal, it is pertinent to consider the risk of illegal borders. Comptroller General of Nigeria Immigration Service revealed that there are well over 1,400 illegal unmanned routes, with only 84 approved border control posts across 4,000 sq. km (Ogundele, 2014). This creates a fertile ground to smuggle the particle into Nigeria. Illegal Importation of EVD index may likely have its first contact in Nigerian rural communities with poor health facilities.
Thus, the initiation of EVD in Nigeria can be from physical contact with an infected animal and/or initiated by infected emigrant (imported human-to-human transmission).
Risk analysis
Studies by (UNDG, 2015) estimated that the probability of EVD prevalence in Nigeria ranges between 0.1 and 0.2. The above discussed risk factors are systematically evaluated using probabilistic risk assessment (PRA) model. This method uses several specific models like Boolean Logic method or models, which includes deductive method like Fault Tree Analysis (FTA) and inductive method like Event Tree Analysis (ETA) (Stamatelatos, 2000). FTA (Figure 1) identifies single or combination of faults - (risk factors) that can leads to undesirable consequence of having EBOV infected person in Nigeria.
The above Fault Tree Analysis shows the possibility of human contact with Ebola zoonotic and/or the possibility of human importation from other infected country. Spillover of the virus depends on the distribution and population of infected Bats, shedding and viability of the virus and the immunity of recipient host. Consider human contact with EBOV zoonotic as shown in Figure 1. Though EBOV is highly virulent, many in the exposed group do not develop clinical symptoms of infection or disease. This may be rightly attributed to variations in infective dose (ID) present in the inoculum and the host immune response (Schmid-Hempel and Frank, 2007), thus the need to model the probability of infection using disease exponential model and beta-Poisson model. Now, consider a successful entry of the virus into a human host, from single-hit hypothesis, the likelihood of a single viral particle survive all immunological barriers has a non-zero value of 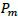 then the probability of effecting the host is
then the probability of effecting the host is 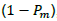 , and the probability for the second viral particle in the same inoculum to cause infection is
, and the probability for the second viral particle in the same inoculum to cause infection is  , and for n numbers of particles, the probability is
, and for n numbers of particles, the probability is 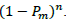 .Thus the probability of infection
.Thus the probability of infection 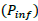 in individual who had zoonotic contact can be expressed as:
in individual who had zoonotic contact can be expressed as:
Where the viral particles in the inoculum are assumed to be at random, then using exponential model,
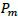 = the dose of Ebola viral particles that survives the host immune system to cause
= the dose of Ebola viral particles that survives the host immune system to cause
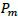 infection. n = the mean numbers of particles that had a successful entry, then
infection. n = the mean numbers of particles that had a successful entry, then
The probability of starting an infection varies with the host, following beta-Poisson distribution:
Where α and β are the parameters of beta distribution.
Transmission
With the initiation of EVD index, transmission from human-to-human could easily spread the virus away from the source. The transmission of the disease depends on an infectious individual, a susceptible individual and an effective contact. Effective contact is expressed as:
Where: T is the total contact rate (the total number of contacts, effective or not per unit time), 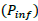 is the probability of infection.
is the probability of infection.
For EVD outbreak, Nigeria has an estimated Basic Reproductive Number of 9.01. From the estimated , the risk of an epidemic from an undetected case is 89% (Althaus et al., 2014). The 2014 EVD cases in Nigeria were in urban areas with relatively good health infrastructure and quick, effective intervention measures. However, most outbreaks of EVD among humans are often initiated in rural communities, and in most case, goes undetected for a while. From the Nigerian health care system, Primary Health Care (PHC) is responsible for disease prevention and promoting good health at the community level, however it receives the least resources. Generally, infrastructures in many Nigerian PHCs are in poor, decaying condition (World Bank, 2010), with no source of electricity, clean water onsite, frequently run out of supply of drugs and stock and low skilled personals (FMoH, 2014). In addition, referral mechanism is not effective (Abdulraheem et al., 2012). Thus on emergence of EVD, PHCs likely the first point of contact, cannot provide appropriate containment measures. Poor health system creates viable conditions for infected persons having contact with susceptible individuals. Event tree analysis inductively describes a series of possible paths (failures) that can lead to high reproductive number of EVD (Figure 2) (Clemens, 2002).
What is the cost to the economy?
The 2014 outbreak in the epicenter countries has enormous fiscal effect, ranging from reduction in revenue, human capital investment to increase in expenditures on treatment, contact tracing and quarantine, and community outreach. The economic impact is also measurable in terms of direct cost of behavioral change that results in restricted mobility, lower supply of labor and income, heightened poverty rate and amplified food insecurity. This behavioral effect has negatively influenced sector of the economy- Mining, Agriculture, Manufacturing and Services. Consequently, World Bank report (World Bank, 2014) show a significant reduction in Gross Domestic Product (GDP) GDP? in the hard-hit countries. GDP growth in Liberia reduced from 5.9 to 2.2%, Sierra Leone shrink in GDP growth from 11.3 to 4.0% while Guinea had a decrease from 4.5 to 0.5%. With a reduction in revenues, Liberia, Sierra Leone and Guinea have made direct public cost of containing Ebola of US$62 million, US$43 million and US$106 million respectively. This can be compared to their 2013 health expenditure of US$191 million, US$584 million and US$291 million for Liberia, Sierra Leone and Guinea respectively (WHO, 2015). This truly damaged the future growth process. As of July 2015, World Bank has mobilized US$1.62 billion to support containment measures- US$385 million for Liberia, US$318 million for Sierra Leone and US$260 million for Guinea (UNDG, 2015).
Thus, at the macroeconomic level, the friable economies of this countries now meets fiscal shortfall as a result of reduction in fiscal revenue and economic activities, and increase in health expenditure. The outbreak exerts damages to the health sector, reducing other healthcare services and depleting trained healthcare work force. In addition, it lessened the feeble minimum health care packages, education and other service sectors, resulting in low human capital development. Post-Ebola recovery plan to re-commence the economy costs US$1.3 billion for Liberia, US$1,063 million for Sierra Leone and US$2.9 billion for Guinea (World Bank, 2015).
United Nation Development Group (UNDG, 2015) modified Bloom and Mahal‘s HIV/AIDS economic model to express the economic consequences of EVD (in terms of GDP) as:
Where:
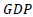 is the Gross Domestic product per capita;
is the Gross Domestic product per capita; 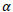 is a constant
is a constant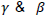 are vector of parameters;
are vector of parameters; 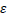 is the error each with zero mean
is the error each with zero mean 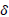 is the coefficient of
is the coefficient of 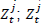 is the probability of having EVD case; J is Ebola scenario; X are variables that determines GDP and T is the time.
is the probability of having EVD case; J is Ebola scenario; X are variables that determines GDP and T is the time.
Although there was no significant reduction in GDP growth, Nigerian government spent about US$13 million for direct cost of containing EVD (World Bank, 2014). The health sector also had a prick as 4 health workers died from the disease. In addition, behavioral change in the two affected cities led to slight reduction in local business activities.
CONCLUSION AND RECOMMENDATION
Should Nigeria close its border with affected and high prone countries? On one hand, this might help to protect its citizens from exposure; on the other hand, closing approved borders can results in increased illegal emigrants with less supervision and negative impact on trade-flow. Besides, border closure does not prevent animal migration from highly prone countries. Rather, strengthening the health system to a responsive and efficient healthcare delivery system will contain Ebola spread lest an outbreak. The health system in the epicenter was not prepared for highly virulent viral disease, resulting in average reproductive number of 2.5 (UNDG, 2015).
Nigerian health system indicators are almost similar to Liberia, Sierra Leone, and Guinea (WHO,2015). An outstanding factor resulting in Nigeria’s success in containing EVD is that the index was detected in good secondary health facility in developed urban centers. It is important to consider having and index in rural communities, utilizing PHC facilities. The economic consequence of such outweighs the direct cost of prevention. “The cost of [2014/2015] Ebola response is estimated to be at least US$4.3 billion.
This is nearly three times the funding gap of US$1.58 billion needed to provide the minimum package of essential health service for all in Sierra Leone, Guinea and Liberia” and 15 times their annual health budget (STCF, 2015). This demonstrates that investment in health is a direct function of economic growth, “health is wealth”, it is substantial to poverty reduction. Strengthening prevention and preparedness plan involves improving epidemiological surveillance, effective alert and referral system in rural PHCs and convalescing supply chain system. Integrating EVD prevention and preparedness courses in the training curricula during capacity building of public health workers will improve standard medical practice during care giving, regardless of patient’s presumed diagnosis. Nigeria being in medium risk of EVD outbreak indicates that proper attention should be given to this, for the economic consequences outweighs the direct cost of prevention.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdulraheem I, Olapipo A, Amodu M (2012). Primary health care services in Nigeria: Critical issues and strategies for enhancing the use by the rural communities. J. Public Health Epidemiol. 4(1):5-13. |
|
|
Bifarin J, Ajibola M, Fadiyimu A (2008). Analysis of marketing bush meat in Idanre local government area of Ondo State, Nigeria. African J. Agric. Res. 3(10):667-71. |
|
|
Clemens P (2002). Event tree analysis. JE Jacobs Sverdrup. |
|
|
Eniang EA, Eniang ME, Akpan CE (2008). Bush meat trading in the Oban Hills region of south-eastern Nigeria: implications for sustainable livelihoods and conservation. Ethiopian J. Environ. Stud. Manage. 1(1):70-83. |
|
|
Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A (1999). Human infection due to Ebola virus, subtype Cote d'Ivoire: clinical and biologic presentation. J. Infect. Dis. 179(Supplement 1):S48-S53. |
|
|
Hayman DT, Yu M, Crameri G, Wang L-F, Suu-Ire R, Wood JL (2012). Ebola virus antibodies in fruit bats, Ghana, West Africa. Emerg. Infect. Dis. 18(7):1207. |
|
|
Kia GSN (2014). Molecular studies of rabies in trade Dogs and detection of some RNA viruses in Bats in Plateau State, Nigeria. |
|
|
Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez JP, Muyembe-Tamfum JJ (2009). Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector-borne Zoon. Dis. 9(6):723-728. |
|
|
McGuire LP (2012). Physiological ecology of bat migration. pp 4-6. |
|
|
Muyembe-Tamfum JJ, Mulangu S, Masumu J, Kayembe J, Kemp A, Paweska JT (2012). Ebola virus outbreaks in Africa: past and present. Onderstepoort J. Vet. Res. 79(2):06-13. |
|
|
Ogundele K (2014). 'Nigeria has 1,400 illegal borders'. Punch. 2014 APRIL 24, 2014. |
|
|
Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL (2015). Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. London B: Biol. Sci. 282(1798):2014-2124. |
|
|
Rouquet P, Froment JM, Bermejo M, Kilbourn A, Karesh W, Reed P (2005). Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 11(2):283-90. |
|
|
Saéz AM, Weiss S, Nowak K, Lapeyre V, Zimmermann F, Düx A (2015). Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 7(1):17-23. |
|
|
Schmid-Hempel P, Frank SA (2007). Pathogenesis, virulence, and infective dose. PLoS Pathog. 3(10):1372-1373. |
|
|
Stamatelatos M (2000). Probabilistic Risk Assessment: What Is It And Why Is It Worth Performing It? NASA Office Safety Mission Assurance 4(05):00. |
|
|
STCF (2015). A Wake-up Call: Lessons from Ebola for the world's health systems. United Kingdom2015; viii]. |
|
|
Sylla M, Pourrut X, Diatta M, Diop BM, Ndiaye M, Gonzalez JP (2015). Chiropteran and Filoviruses in Africa: Unveiling an ancient history. Afr. J. Microbiol. Res. 9(22):1446-1472. |
|
|
WorldBank (2010). Improving Primary Health Care Delivery in Nigeria Evidence from Four States. Washington, D.C. 20433, U.S.A.: Department ARHD; Contract No.54037. |
|
|
WorldBank (2014). The Economic Impact of the 2014 Ebola Epidemic: Short- and Medium-Term Estimates for West Africa. Washington, DC. |
|
|
WorldBank (2015). Update on the economic impact of the 2014-2015 Ebola epidemic on Liberia, Sierra Leone and Guinea. Washington, D.C. 20433, U.S.A.: 2015 95804 Contract No.: 95804. |
|
|
WHO (2014). Global Health Observatory Data Repository. 2014 [cited 2015 22-10-2015]. View |
|
|
WHO (2015). Ebola virus disease. 2015.WHO (2015). Health System Financing Profile by country. Geneva2014 |
|
|
WHO (2015). Sierra Leone: a traditional healer and a funeral. 2015 |
|
|
WHO (2016). Ebola Situation Report - 28 February 2016. 2016 [cited 2016 07/03/2016]. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0