Full Length Research Paper
ABSTRACT
Seasonal malaria chemoprevention is tailored for children 3 to 59 months in areas of high transmission of malaria between July and October. However, SMC was administrated to children outside the pre-defined age range. This study aims to evaluate to which extent the treatment was given to these children. The authors carried out a cross sectional survey in November 2018 in eight (8) health regions. Sampled of 944 household where mothers of children under the age of 10 were interviewed about SMC coverage. They used a structured questionnaire to mothers/tutors including child’s age, the history of SMC administration including the adherence to the three daily doses, the possession or not of SMC cards, use of bed nets, and finally episodes of illnesses during the SMC period. Proportion of treated children included proportions captured both during mother/tutor’s interview or card inspection. A total of 1191 children between the age of 5 years and over were identified. According to mother’s declaration, 19.65% (234/1191) children received at least one dose of SMC; only 150 children had SMC card, of whom 146 (97.33%) have received at least one dose of SMC. Despite the well-known age target of SMC, older children were treated; however reduced, this deserves further attention.
Key words: SMC coverage, children over 59 months, Burkina Faso.
BACKGROUND
Malaria in Sub Saharan Africa remains a major public health concern despite sustained efforts that have often led to a certain drop in the incidence of cases (WHO, 2019). According to the annual report on malaria in 2019, the Africa region of the World Health Organization (WHO) concentrated 93% of malaria cases in 2018, most of the cases occurred in six countries (Nigeria 25% of cases, the Democratic Republic of the Congo 12%, Uganda 5%, and 4% in each of the countries of Côte d'Ivoire, Mozambique, and Niger (WHO, 2019). Burkina Faso was in 2017 among the 11 countries of the world who contributes up to70% of total malaria burden (WHO, 2018). As always, children under 5 and pregnant women are the most vulnerable and effective complementar strategies are warranted. Strategies directed toward vulnerablepopulations include seasonal malaria prevention chemotherapy (SMC) which consists of giving a full dose of sulfadoxine-pyrimethamine plus amodiaquine to children 3-59 months per month for 4 months during the period of high transmission to prevent the occurrence of simple and complications forms (Wilson, 2011).
This strategy, endorsed by WHO (WHO, 2019), has been scaled up in the countries of the Sahel for the past five to six years with the support of various technical and financial partners. Burkina Faso, likewise other countries, is implementing seasonal malaria chemotherapy with consistently high coverage rates between 2015-2016 (Milligan, 2017) and 2017 (Zongo, 2018; ACCESS-SMC partnrship, 2020). During the mass administration of SMC in real life, identifying precise age limits often becomes a challenge to overcome as vital documents to confirm the ages of children are missing; older children receive the drugs improperly. This situation has consequences not only on the quantity of drugs which is abnormally increased as this was not planned well in advance but also exposes to under dosing in older children, source of possible selection of parasite strain resistant to the used molecules. In this context this study sets out to assess the extent of SMC administration in children over 59 months of age during the 2018 distribution campaign in Burkina Faso.
METHODS
Study design
This study is a cross sectional survey, carried out in November 7th to 18th, 2018. It took place in eight health regions where SMC is implemented with Malaria Consortium support. The research team was first trained in the study protocol and data collection forms. The field survey was organized around three supervisors and twelve interviewers. Interviewers administered the questionnaire in pairs to ensure better data quality. At the end of the day, the supervisors of each team were responsible for verifying the completeness of the field data before it was transferred to a server. These transferred data were taken over by the team's data manager for the correction of errors, omissions and inconsistencies that arose during the collection before depositing them again (clean database).
Population and sample size
The target population for this study is all the children aged between 5 to 10 years old living in the eight health regional directions during the SMC campaign in 2018.
Although the target population was children, mothers were interviewed about child’s history of SMC. Therefore, all children aged at least 59 months were eligible to participate owning their parents provided a signed informed consent. This study is part of a large survey intended to assess the coverage of SMC among target children in Burkina Faso in 2018. As a reminder for this study, the required number of households was 944 and all children aged 3 months to 10 years in the compound were enumerated and their mother was interviewed. The required number of compounds was determined using the following formula:
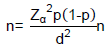 = sample size, d = degree of precision, p = expected proportion in the population, power = 80%.
= sample size, d = degree of precision, p = expected proportion in the population, power = 80%.
Operational definition of variables
Each mother was interviewed about child’s age (or date of birth), the history of SMC administration during the four rounds including the adherence to the three daily doses per round. The authors also inquired about the possession and use of bednets, the possession or not of SMC cards and finally episodes of illnesses during the SMC period. When the SMC card was available, the SMC coverage is also captured according to the informations collected on the card. Others relevant informations have been collected such as the knowledge of community health workers by the mothers and the channy used to inform mothers about the SMC campaign.
Study instruments
The authors administrated a structured questionnaire to mothers/tutors. All the data were captured using tablet PCs with Magpi data collection software. Proportion of treated children was captured through both mother/tutor’s interview and SMC card direct inspection.
Data analyses
For data analysis, Stata software version 13.0 was used. The coverage of SMC in children over 59 months was estimated as the proportion of children over 59 months who received the various monthly SMC treatments (called cycles) compared to the total population of children over 59 months.
Research ethics
Approval for the conduct of this survey was obtained from the Ethics Committee for Health Research of Burkina Faso. The confidentiality of participants was maintained throughout data collection. Informed consent was given to potential participants with a clear statement that their participation was voluntary, and that they could withdraw at any time without justification. Only children whose parents signed the informed consent actually participated in the survey. A copy of the consent was given to the parents. Access to the server and database was controlled by a password system given to a limited number of study staff.
RESULTS
Sample characteristics
During the survey, 1191 children aged over 59 months were counted with mean age of 7.11 years (±1.59 years) ranging from 5 to 10 years. Child’s age was only confirmed in 13.43, 95% CI [11.55-15.5%] of cases with birth certificate. The proportion of children owning a SMC card was 12.59% (150/1191).
Proportion of children who received the monthly round of SMC
The survey revealed that the proportion of children receiving monthly first doses varied from 18.81% (224/1191) in July to 19.14% (228/1191) in August according to mother’s interview, higher proportions were observed over direct SMC card inspection. Considering each monthly cycle, the proportion of children who received the intervention was comparable between sources (Table 1).
Proportion of children by number of contacts with SMC
This section displays the number of contacts of every child with the SMC intervention. At least 18.81% had four contacts over the four cycles (Table 2).
Proportion of children according to the cumulative number of cycles received
This indicator shows that the adherence to the different cycles was very high, 18.89% of the children received at least three cycles of SMC against 18.22% for all cycles according to the mothers, and this trend was also observed during the inspection of SMC cards (Table 3).
Bednet use, episodes of malaria and case management during SMC
There was widespread use of bednets and especially long-acting insecticide-treated bednets among children aged 5 years and more with 90.62% (1072/1183). Mothers reported that 18.52% (219/1182) of the children had been sick since the last cycle. Of these sick children, 80.37% (176/219) would have consulted according to the mothers of whom 96.02% (169/176) benefited from a rapid diagnostic test for malaria. The rapid diagnostic test was positive in all tested children (169/169). Parents did not consult as child’s clinical condition has improved and there was no community health workers nearby. Furthermore high cost of care and long distance to the health facilities were cited as additional reasons for not consulting the formal health system include lack of proximity to a community worker, improved clinical condition of the child, distance from the health care center and high cost of care.
DISCUSSION
This study showed that children over 59 months received of aged SMC during the 2018 distribution campaign but in relatively small proportions, around one in five children. The study also found a low proportion of card possession, just over one in ten children. Administration of SMC to children outside the target age has been recorded in Burkina Faso in previous campaigns (WHO, 2018, 2019). It is important to estimate the extent of this administration in order to assess its consequences and finally see what perspective can be considered. Although overall, few children over 59 months have been treated, it the fact remains that such an administration not only exposes to the risk of ineffective prevention in these children due to under sufficient dosinges (initially intended for children 3-59 months) but also to a possible selection of resistant parasite strains, all together that compromises the effectiveness of SMC. On a practical level, for example, the drugs have been estimated to cover a known target number, any administration outside this range could create a gap that needs to be filled. Experience from countries like Senegal has shown that SMC can be given to children over 59 months of age if this was planned at the beginning thus avoiding the consequences discussed above (Cissé et al., 2016; Thera et al., 2018).
Studies have shown that as progress is made towards malaria control (as is probably the case now in Africa), older children will carry a greater burden of malaria (Wilson, 2011; Global Malaria Programme: WHO, 2015; Milligan, 2017). Thus, evaluations of the strategy's effectiveness in children aged 5 years old and more, especially school-aged children, have shown a reduction in malaria related morbidity and mortality (Ceesay et al., 2008; Zongo et al., 2018). These arguments support the inclusion of children over 59 months of age for the benefit of SMC.
This study was somewhat limited by the low possessionof children's cards, which prevent a large cross check with mother’s interview improving the reliability of the data. Nevertheless, the study provided a better appreciation of the phenomenon and its consequences.
CONCLUSION
During the 2018 seasonal malaria chemo-prevention campaign, children over 59 months of age were treated for various reasons. It is now time to discuss the opportunity of treating these children over 59 months so that this can be planned well in advance.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors expressed gratitude to mothers / guardians / caregivers and parents for their participation in the survey. They would like to thank the regional health directors of the villages concerned, the health districts authorities, the Nurses responsable for epidemiological surveillance at the district level, the local guides, their field teams as well as the administrative staff of the Institute of Sciences and Techniques for all their respective contributions to the implémentation of this coverage survey in the eight health regions. Finally, the authors would like to thank Malaria consortium office in Burkina Faso for funding the survey.
REFERENCES
|
ACCESS-SMC Partnership (2020). Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. The Lancet 396 (10265):1829-1840 |
|
|
Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, Conway DJ (2008). Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. The Lancet 372(9649):1545-1554. |
|
|
Cissé B, Ba EH, Sokhna C, NDiaye JL, Gomis JF, Dial Y, Milligan P (2016). Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS medicine 13(11):e1002175. |
|
|
Global Malaria Programme: WHO (2015). 'Global Malaria Programme: WHO Global', WHO Library Cataloguing 2011, pp. 1-4. |
|
|
Milligan P (2017). ACCESS-SMC Evaluation: preliminary results. |
|
|
Thera MA, Kone AK, Tangara B, Diarra E, Niare S, Dembele A, Doumbo OK (2018). School-aged children based seasonal malaria chemoprevention using artesunate-amodiaquine in Mali. Parasite epidemiology and control 3(2):96-105. |
|
|
World Health Organization (WHO) (2018) 'High burden to high impact A targeted malaria response pp. 1-8. |
|
|
World Health Organization (WHO) (2019) WHO World malaria report 2019, WHO Regional Office for Africa. |
|
|
Wilson AL (2011). 'A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc)', PLoS One 6(2). |
|
|
Zongo I, Ouedraogo JB, Lal S, Scott S, Snell P, Moroso D, Miligan P (2018). Coverage of seasonal malaria chemoprevention in Burkina Faso 2017' |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0