ABSTRACT
Malaria is a disease which is prevalent in tropical regions; however, it is preventable and curable with the use of certain micronutrients thereby reducing the life threatening disease. The effect of micronutrients (vitamin C, calcium, iron and magnesium) on mice infected with Plasmodium berghei NK-65 species was evaluated using standard methods. The percentage suppression and the parasitemia levels were counted daily to evaluate the effect of the micronutrients given intraperitoneally to the infected mice. The results show that vitamin C and calcium caused a significant decrease in the parasitemia count from 529 to 425/field and from 533 to 441/field respectively, while iron and magnesium caused significant increase in parasitemia level from 516 to 592/field and 528 to 709/field respectively. The packed cell volume increased in the mice treated with calcium, iron and vitamin C while there was significant decrease in the mice treated with magnesium. Red blood cell increased in the mice treated with iron. The white blood cell was reduced in the mice treated with iron and vitamin C but no significant change in the white blood cell of the mice treated with calcium but there was increase in the white blood cell of the mice treated with magnesium. The biochemical components of the blood from treated mice revealed that sodium significantly increased in the mice treated with the micronutrients with the highest sodium recorded in the mice treated with Iron. Therefore, foods or drugs that are rich in iron and magnesium should not be taken when treating malaria as they will aid parasite multiplication.
Key words: Micronutrients, Plasmodium, parasitemia, mice, blood.
Malaria is a preventable and curable disease, yet it remains a devastating tropical disease, with high infection and mortality statistics. Malaria, a life threatening disease is caused by a parasitic infection of the red blood cells by Plasmodium parasites transmitted through a bite of the female Anopheles mosquitoes. This disease is prevalent in tropical and subtropical regions and is mostly associated with poverty. Clinical symptoms of malaria include headache, fever, chills and vomiting which are usually mild but if not treated immediately could lead to delirium, metabolic acidosis, cerebral malaria and multi-organ system failure (Iyiola et al., 2011). World Health Organization (WHO) estimates that each year, more than 200 million people are infected with malaria worldwide (WHO, 2016). There were 214 million cases of malaria worldwide in 2015; 90% of which occurred in Sub Saharan Africa. Out of these, 438,000 resulted in death globally of which 78% were children under the age of five (WHO, 2015).
Plasmodium berghei infection of laboratory mouse strains is frequently used in research as a model for human malaria because of its similarity to the Plasmodium species which cause human malaria. P. berghei has a very similar life-cycle to the species that infect humans, and it causes disease in mice which have signs similar to those seen in human malaria. More importantly, P. berghei can be genetically manipulated more easily than the species which infect humans, making it a useful model for research into Plasmodium genetics (David et al., 2004). Micronutrients are vitamins and minerals needed by man in small quantities for good health. They include vitamin C (ascorbic acid), iron, magnesium, calcium, etc (Sies et al., 2015).
Iron is involved in numerous biological processes. It is the most important transition metal in all living organisms (Conti et al
., 2010). Vitamin C is a
cofactor in many
enzymatic reactions, including several
collagen synthesis reactions that, when dysfunctional, cause the most severe symptoms of
scurvy. In animals, these reactions are especially important in wound-healing and in preventing bleeding from capillaries (Murray et al
., 2013). Magnesium (Mg) is the second-most abundant cation in cellular systems. It exerts a large variety of biological functions, ranging from structural roles by forming complex with negatively charged groups such as phosphates in nucleic acids, control role in enzyme activation or inhibition, and regulatory roles by modulating cell proliferation, cell cycle progression and differentiation (Tam et al
., 2003).
Parasites strain
In vivo antimalarial testing in mice was done using chloroquine sensitive strain of P. berghei (donated by the Animal Unit of the Institute for Advanced Medical Research and Training, College of Medicine, UCH, Ibadan). The parasites stock was maintained by continuous re-infection in the mice.
Dosage calculations
According to the Organization of Economic Corporation and Development’s (OECD) guidelines, dosage of drug (mg) should be constituted in an appropriate volume not usually exceeding 10 ml/kg (1 ml/100 g) body weight of experimental animals (mice and rats) for non-aqueous solvent in oral route of administration (OECD, 2001). Mice collected were between 20 to 25 g, hence the dosage of drug according to standard should not exceed 0.2 ml
Dosage (mg) = [Average weight of mice (g) × Dose (mg)] / 1000 g
Infection of mice
The parasitized erythrocytes for each test were collected from an infected donor mouse with rising parasitemia of 20 to 30%. The mice were sacrificed by head blow, and blood was collected in a Petri dish with an anticoagulant (0.5% trisodium citrate) by severing the jugular vein. The blood was then diluted with physiological saline (0.9%) in proportion of 1:4. Each mouse in every group was then inoculated with 0.2 mL of blood containing about 107 P. berghei infected erythrocytes on day 0 through intra peritoneal route. This was prepared by calculating the percentage of parasitemia of donor mouse and diluting the blood with physiological saline so that 0.2 ml of diluted blood contained 1 × 107 infected erythrocytes.
Suppression
The mice were divided into five groups in which three mice are in each group (A, B, C, D and E (control)) with groups A to D receiving 10 mg/kg of Calcium, Magnesium, Iron, and Vitamin C respectively. The animals in the control group (E) received placebo (saline water). The antimalarial activity of the micronutrients was determined using the Peter’s 4-day suppressive test (David et al., 2004).
Determination of mean weight and temperature changes of the mice
The weights of the mice were measured daily using a sensitive weighing balance to monitor the change in weight of mice. Rectal temperature was also measured with a digital thermometer before infection, and then daily. All the control groups and malaria-infected mice were observed visually throughout the experiment for behavioural changes which include diarrhoea, lethargy, reduced activities, sleeplessness and loss of appetite.
Parasitemia count
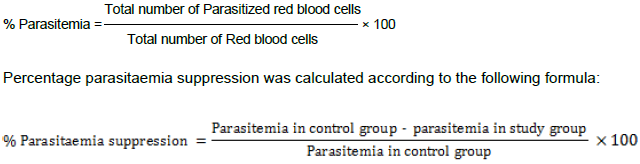
On Day 4 after infection, a thin smear of blood film was taken from the peripheral blood of the tail of each mouse in the test and control groups. The smears were fixed with methanol and then stained with Giemsa stain. Thereafter, each stained slide was microscopically examined under oil immersion of 1000 magnification power (100×) to evaluate the mean percentage (%) of parasitemia and suppression of each fraction in comparison with control group. The mean parasitaemia was calculated and expressed as follows:
Preparation of inoculum
Donor P. berghei infected mice were anesthetized and the blood was collected by heart puncture. Heparinized blood is taken from a donor mouse, diluted with 5 ml of phosphate buffer solution (PBS) and adjusted to pH 7.2 so that each 0.2 ml contained approximately 1×107 infected red cells (David et al., 2004). An aliquot of 0.2 ml of this suspension was injected intra-peritoneally into the experimental mice and treated with micronutrients orally into experimental groups.
Determination of mean survival time
Mortality was monitored daily and the number of days from the time of inoculation of the parasite up to death was recorded for each mouse in all groups throughout the follow up period.
The mean survival time (MST) for each group was calculated as follows:
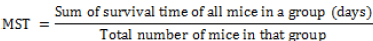
Biochemical tests
Biochemical tests such as determination of bicarbonate, creatinine, calcium, uric acid and urea level were carried out following the standard method of Baker et al. (2014).
Liver functioning tests
Liver functioning test (LFT) such as the total bilirubin, serum total protein, serum albumin, serum globulin and alkaline phosphate were done according to the method of Cheesbrough (2014) to ascertain the physiological status of the liver.
Haematological tests
Haematological tests such as packed cell volume (PCV), hemoglobin (HB), red blood cells (RBC), erythrocyte sedimentation rate (ESR) and white blood cells (WBC) differential count were done following the standard method of Cheesbrough (2014).
Statistical analysis
Statistical data are presented as mean ± SE (standard error). Significance of difference between different treatment groups was tested using one-way analysis of variance (ANOVA) and significant results were compared with Duncan's multiple range tests using SPSS window 10 version 21 software. For all the tests, the significance was determined at the level of P<0.05.
Effect of the micronutrients on the parasitemia count per field showed that magnesium has the highest increase in the number of parasites in the blood from 528 to 709/field. The administration of iron also caused an increase in the parasitemia count in the infected mice. However, administration of vitamin C and calcium caused a reduction in the parasitemia count as shown in Table 1. Figure 1 reveals the effect of micronutrients on the haematological parameters. It revealed that erythrocyte sedimentation rate significantly (p˂0.05) reduced in the mice treated with the micronutrients compared with the control. Also, the packed cell volume increased in the mice treated with calcium, iron and vitamin C while there was no significant (p˂0.05) increase in the mice treated with magnesium. Red blood cell increased in the mice treated with micronutrients and those treated with Iron. The white blood cell counts reduced in the mice treated with iron and vitamin C but no significant change in the white blood cell of the mice treated with calcium but there was increase in the white blood cell of the mice treated with magnesium. There was significant (p˂0.05) increase in the haemoglobin of all the mice treated with micronutrients compared with the control group.
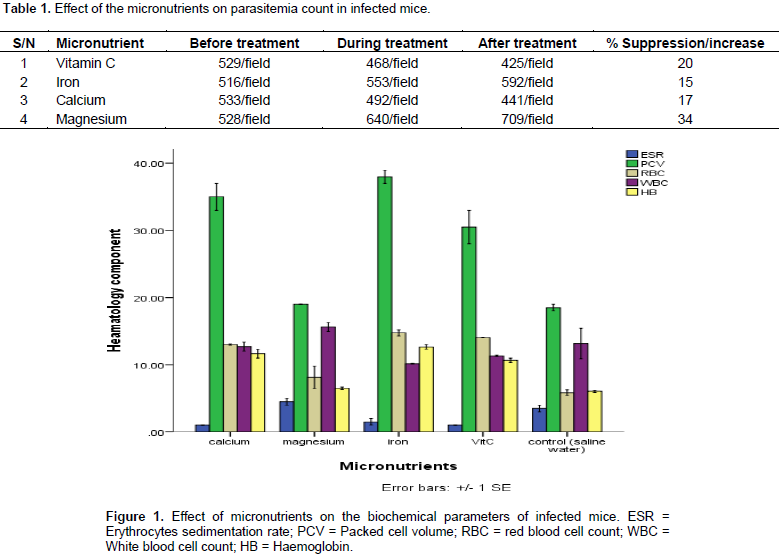
Figure 2 reveals the effect of micronutrients on white blood cell differential count as all the micronutrient used (Ca, Mg, Fe and Vitamin C) caused a decrease in the lymphocyte count in the experimental mice. While the control group recorded lymphocyte count of 75%, the group treated with iron recorded a lymphocyte count of 55%. The micronutrients also caused an increase in the neutrophils to 25% when compared to the count in the control (10.2%). The same pattern was observed for monocytes. There was however no significant (p˂0.05) increase in the eosinophils and basophils. Figure 3 shows the effects of micronutrients on the biochemical components of the blood. The result reveals that sodium significantly (p˂0.05) increased in the mice treated with micronutrients and highest sodium was observed in the mice treated with iron at a value of 150 mg. There was no significant change in the potassium of the treated mice.
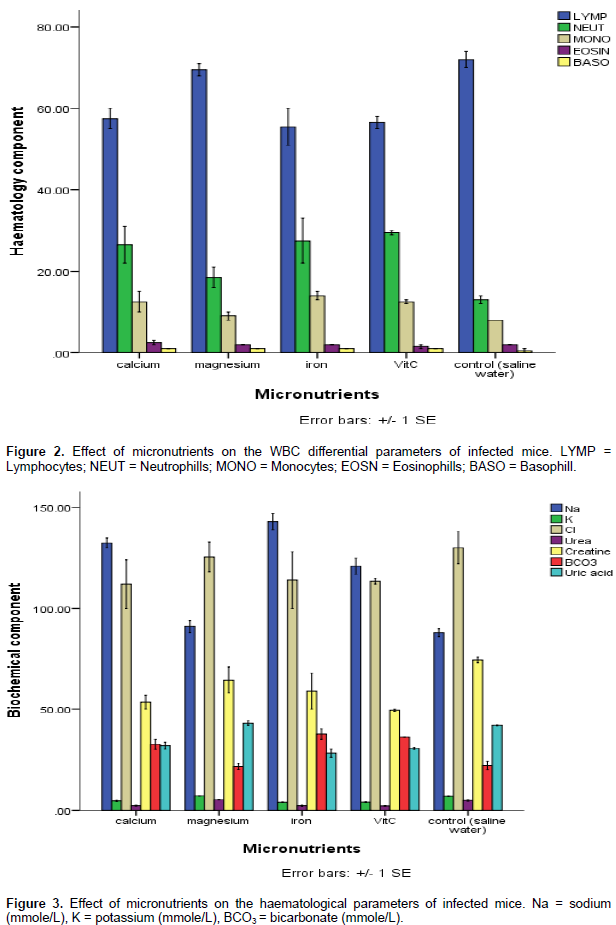
The chlorine decreased in the mice treated micronutrients with the most significant decrease recorded in the mice treated with vitamin C and iron; also, there was no significant change in the urea of the mice treated with micronutrients. The creatine in the blood component of the mice treated with micronutrients decreased, whereas the bicarbonate increased. A decrease in the uric acid was recorded in the mice treated with the micronutrients except in the mice treated with magnesium where a slight increase in the uric acids was recorded. Effects of the micronutrients on the function of liver in the mice are shown in Figure 4. The result reveals that while there was slight increase in the serum glutamic pyruvic transaminase of the mice treated with magnesium, there was significant (p˂0.05) decrease in the mice treated with calcium, iron and vitamin C.
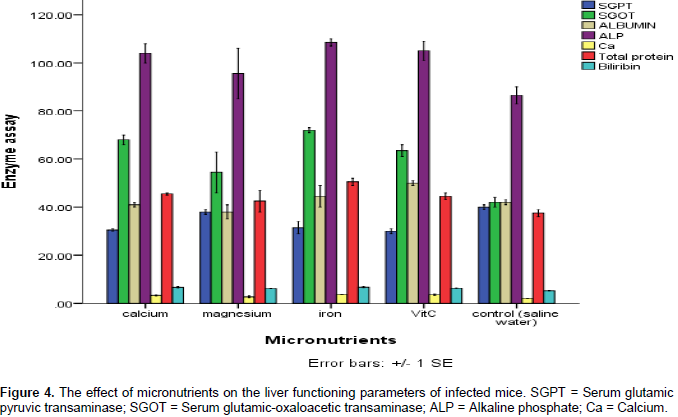
There was an increase in the serum glutamic oxaloacetic transaminase in all the mice treated with micronutrients except in the mice treated with magnesium for which was recorded a decrease in serum glutamic oxaloacetic transaminase recording a value of 55 mg/ml compared with iron which had 75 mg/ml. The albumin was increased in the mice treated with iron, calcium and vitamin C while there was a slight decrease in the albumin of the mice treated with magnesium. There was an increase in the alanine aminotransferase of the micronutrients treated mice, but the bilirubin level did not increase significantly when compared with the control.
The effect of the micronutrients on the parasitemia count per field revealed that magnesium caused the highest increase in the number of parasites in the blood which is a clear indication that magnesium containing compounds should not be taken when treating malaria. Although there is no written document on the use of magnesium containing compounds when treating malaria, compounds such as magnesium trisilicate prolong malaria infection and should not be taken along some antimalarial drugs as it may cause complications (WHO, 2016). The administration of iron also caused an increase in the parasitemia count in the infected mice. Iron (Fe) is one of the essential nutrients required by
Plasmodium for proliferation and lysing of red blood cell which often lead to anaemia (Ohnishi and Ohnishi, 2011; Sies et al
., 2015). Although the main role of iron-containing proteins are; transfer of electrons, transport and storage of oxygen, iron is a necessary
trace element found in nearly all living organisms.
Iron-containing enzymes and proteins, often containing
heme prosthetic groups, participate in many biological oxidations and in transport, hence it is advisable for everyone, most especially the pregnant women not be given forlates during the antenatal to reduce malaria burden (WHO, 2016), rather they should be encouraged to take foods containing iron such as beans, nuts, fish, liver and sea foods. The effect of micronutrients on the haematological parameters which showed the erythrocyte sedimentation rate to be significantly reduced in the mice treated with the micronutrients compared with the control has shown that the presence of calcium can increase ESR value. Also, the packed cell volume increased in the mice treated with calcium, iron and Vitamin C while there was no significant increase in the mice treated with magnesium. Red blood cell increased in the mice treated with iron than in other groups. This may be due to the fact that presence of iron normally aids the proliferation and production of red blood cells. The white blood cell was reduced in the mice treated with iron and vitamin C, two micronutrients that have been known to boost immunity of animals against parasites (Cheesbrough, 2014).
Evaluation of the effect of the micronutrients assayed for in the white blood cell differential count revealed that all the micronutrient used (Ca, Mg, Fe and vitamin C) caused a decrease in the lymphocyte count in the experimental mice, which is an indication that these micronutrients actually boost the immunity of the mice. Equally, the complementary effects of iron on the animals can be seen in the lymphocyte count as well as the neutrophils which are well reduced when compared with the control. The effects of micronutrients on the biochemical components of the blood from treated mice have shown that micronutrients caused an increase in sodium level. This may result from the fact that the calcium, iron and vitamin C particularly increased sodium well above the values obtained in the control group. Urea, creatine and bicarbonate are end products that results from metabolized nutrients from the body of an animal (Brooks et al., 2014). Therefore, the ability of some of these micronutrients to reduce the parasitemia count in these mice might be by increased metabolic activity including generation of energy to combat the Plasmodium parasites.
There was no significant change in the urea of the micronutrient treated mice which may be because there was no damage to the kidney. The creatinine in the blood component of the mice treated with micronutrients decreased whereas the bicarbonate increased. A decrease in the uric acid was recorded in the mice treated with the micronutrients except in the mice treated with magnesium where a slight increase in the uric acids was recorded. Effects of the micronutrients on the liver functioning tests of the treated mice revealed that there was slight increase in the serum glutamic pyruvic transaminase of the mice treated with magnesium while there was significant decrease in the mice treated with calcium, iron and vitamin C. This may be because magnesium is not easily excreted from the body of animals by the liver when taken in excess, hence the need to always check their level in water and foods. There was an increase in the serum glutamic oxaloacetic transaminase in all the mice treated with micronutrients except in the mice treated with magnesium for which was recorded a decrease in serum glutamic oxalo-acetic transaminase. This result is an indication of good working condition of the liver (Momoh et al., 2013).
The results obtained in this research therefore have shown that iron and magnesium taken either as drugs or food supplement causes increase in parasitemia level thereby aggravating the malaria and complicating it. On the other-hand, vitamin C and calcium, when taken in malaria cases can help fight the malaria parasite and reduce the parasitemia level. People in malaria endemic area should be given orientation on the type of food to be consumed while treating malaria.
The authors have not declared any conflict of interests.
REFERENCES
|
Baker JF, Breach MR, Chris P (2014). Medical Laboratory Science. Chris Publisher, United Kingdom. 487p.
|
|
|
|
Brooks GF, Butel JS, Morse SA (2014). Medical Microbiology. 26th edition, Mc Graw Hill, New York, USA. P 826.
|
|
|
|
|
Cheesbrough M (2014). District Laboratory Practice, Tropical Countries; 2nd Edition, Cambridge University Press, United Kingdom. 480p.
|
|
|
|
|
Conti B, Canale A, Bertoli A, Gozzini F, Pistelli L (2010). Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 107(6):1455-1461.
Crossref
|
|
|
|
|
David AF, Philip JR, Simon IC, Reto B, Solomon N (2004). Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. 3:509-520.
|
|
|
|
|
Iyiola OA, Tijani AY, Lateef KM (2011). Antimalarial activity of ethanolic stem bark extract of Alstoniabooneiin mice. Asian J. Biol. Sci. 4:235-243.
Crossref
|
|
|
|
|
Momoh AO, Adebolu TT, Ogundare AO (2013). Evaluation of beniseed extract and fermented liquor in treatment of diarrhoea in albino rats infected with Salmonella typhi. Eur. J. Biol. Med Sci. Res. 1(2):16-23.
|
|
|
|
|
Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, Yolken RH (2013). Manual of Clinical Microbiology (8th ed.). Herdon, VA, United States of America. Am. Society Microbiol. P 405.
|
|
|
|
|
Organization for Economic Co-operation and Development (OECD) (2001). OECD Guidelines for the testing of chemicals, 2001. Revised draft guidelines. P 423.
|
|
|
|
|
Ohnishi ST, Ohnishi T (2011). In vitro effects of aged garlic extract and other nutritional supplements on sickle cell erythrocytes. J. Nutr. 131:1085S-92S.
|
|
|
|
|
Sies H, Stahl W, Sevanian (2015). Nutritional, dietary and postprandial oxidative stress. J. Nutr. 135(5):969-72.
|
|
|
|
|
Tam M, Gómez S, González-Gross M, Marcos A (2003). Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 57:1193-1197
Crossref
|
|
|
|
|
World Health Organization (WHO) (2016). Tasty Mushrooms from Dirty Diapers. Discovery Communication inc. World malaria day 2016 fact sheet, Geneva.
|
|
|
|
|
World Health Organization (WHO) (2015). Prevention of Malaria Infections: A Practical Guide. 2nd Edition. WHO/CDS/CSR/EPH/2014.12.
|
|