ABSTRACT
The descriptive epidemiology of orofacial clefts (OFC) is an essential prerequisite towards improved care, investigations into the etiology, and eventually prevention. In the present study the distribution of OFC in sub-Saharan Africa using post-surgical data from the Smile Train organization, was examined. Data from 46,502 individuals from Ethiopia (16,049), Nigeria (8,209), Uganda (5,138), Kenya (4,084), Tanzania (2,750), Congo DR (1,371), Zambia (1,319), Somalia (1,039), and a total of 6,543 individuals from another 26 African countries were available for analysis. Individuals without a cleft diagnosis and those who indicated non-black African as their racial group were excluded, and a total of 46,502 individuals were available for analysis. There was a significant difference in frequency between unilateral cleft lip and palate (70.24%) versus bilateral cleft lip and palate (29.76%; p < 0.0001), and these were also significant within each sex (p < 0.0001). In the database, there were more females (53.50%) with cleft palate only than males (46.50%) (p = 0.0002). Data reported here did not take into account infant mortality during the perinatal period. Nonetheless, this study provides estimates from the largest recorded body of data for clefts in the continent, therefore providing valuable information on the need for comprehensive cleft registries in Africa.
Key words: Clefts, Africa, epidemiology.
Africa is the second most populous continent in the world, with a population of over 1 billion people as of 2012 and estimated by the United Nations to reach 2.4 billion by 2050 (United Nations report World population prospects, 2012). Broadly, Africa can be divided into North Africa and sub-Saharan Africa. There are 54 countries in Africa according to the United Nations, and most of these countries are classified as developing (International Monetary Fund, 2014). Sub-Saharan Africa has a population of over 900 million people, with a growth rate of 2.7% per annum (David and Murray, 2013). The gender distribution in sub-Saharan Africa is equal (50% male and 50% female), and over 65% of the entire population is rural. The rural population correlates with over 50% of births delivered outside the hospital (Ahmed et al., 2004; Bukar and Jauro, 2013), as most hospitals and delivery centers are located in urban centers.
Infant mortality rates in African countries are among the highest in the world as a result of health, political, and economic challenges (United Nations Inter-agency Group for Child Mortality Estimation, 2013). The health challenges include infectious diseases like HIV/AIDS, tuberculosis, and malaria. However, infant mortality due to infectious diseases is reducing and being controlled successfully worldwide. Nonetheless, infant mortality is increasing globally due to the rising tide of birth defects such as orofacial clefts (OFCs) (WHO, 2005). Infant mortality as a result of birth defects in Africa could be due lack of infrastructure, limited number of trained personnel and cultural beliefs. Cultural beliefs such as witchcrafts, evil spirits and the devil have been reported to contribute to infanticides. In some instances, children have been deliberately ignored to starve to death or allowed to aspirate during breast feeding (Akinmoladun et al., 2007; Oginni et al., 2010). Therefore, it is a matter of life and death for children with birth defects in a resource low setting in Africa. This is because majority of the population have limited knowledge on the causes of the defects and availability of support for individuals and families (Awoyale et al., 2016).
OFCs are the most visible and most common congenital birth defects in the head and neck region. OFC affects 1 in 700 live births worldwide (Mossey and Modell, 2012). However, there are variations in reported prevalence across geographical and ethnic regions. In Asia, the rates are as high as 1.4/1000 (Dai et al., 2010); in Europe around 0.7/1000 (Calzolari et al., 2004), and in Africa 0.5/1000 (Butali et al., 2014). The difference in prevalence across the world provides support for the role of multiple genetics and environmental factors that increases the risk for OFCs. OFCs occur following disturbances of the normal genomic architecture during palatal development in the embryonic (primary palate) and fetal (secondary palate) phases of development. An environmental insult around this time increases the risk for OFCs.
In Africa, there have been various attempts to examine the incidence, prevalence, and distribution of OFCs in different countries (Butali et al., 2014; Spritz et al., 2007;Carneiro and Massawe, 2009; Agbenorku et al., 2011; Eshete et al., 2011; Manyama et al., 2011). Here, a descriptive report of 46,502 cleft cases in the Smile Train database from all countries in sub-Saharan Africa was presented. To our knowledge, this is the largest descriptive study for clefts in the African continent. This study provides valuable information on the need for holistic care. It also renders an opportunity for proper surveillance and for the establishment of registries in order to provide accurate estimates of prevalence rates. Finally, it is hoped that this report will attract the attention of governmental and non-governmental organizations in Africa and other parts of the world to the plight of individuals with this condition and the need for team care.
Data
An application form to the Smile Train Organization requesting for data from sub-Saharan Africa was completed. This form was submitted in May 2013 and approval was received in June 2013. The data requested did not contain identifiable data and qualifies as a non-human subject research according to the University of Iowa IRB. The data was stratified into countries. All the cleft types based on the clinical information provided in the database were also classified.
Statistical analysis
Study design
This is a retrospective study using the Smile Train data for clefts treated in Africa.
Study population
All cleft cases treated at Smile Train centers in Africa. Only Africans with racial indication as “Black” and individuals with a cleft diagnosis in the database in the final analysis (N = 46,502) were included.
Data collection and classification
Data collected from June, 2007 to December, 2013 was used for analyses. The cleft types was classified into the various broad categories: Bilateral cleft lip and palate (BCLP), left cleft lip and palate (LCLP), right cleft lip and palate (RCLP), bilateral cleft lip only (BCLO), left cleft lip only (LCLO), right cleft lip only (RCLO), and cleft palate only (CPO). Each of these cleft types was divided into males and females.
Data analysis
Frequency data was generated and a frequency distribution table with the observed and expected data was constructed. Estimated prevalence for each country was not conducted due to the lack of live births data (denominator data) in the current data set. Test for differences in proportions were performed to see if there was a significant difference in the proportion of BCLP and unilateral cleft lip and palate (UCLP; LCLP and RCLP was combined). The test was done for all individuals first and then for each gender. Cleft lip only categories (BCLO, LCLO, and RCLO) were combined under
unilateral and bilateral in a similar way and the analogous tests were performed (Table 2). The types of clefts alone was also compared, that is, RCLO versus LCLO, BCLO versus LCLO, BCLO versus RCLO, RCLP versus LCLP, BCLP versus LCLP, and BCLP versus RCLP. All these categories were stratified by gender, and laterality tests were performed within each gender. Chi square statistics was used to compare the equality of frequencies of laterality, and p < 0.002 was considered significant (Bonferroni corrected p-value for 25 different tests where 0.05 is significant for each test). A distribution of clefts in first degree and second degree relatives was also analyzed. A breakdown of the frequency of clefts and distribution of cleft types in eight of the countries with highest number of treated cases and 26 others was also conducted.
Table 1 shows the distribution of clefts into the different types observed in the database and an estimate of the expected frequency for males and female under the null hypothesis.
Overall test for equality
Unilateral vs bilateral
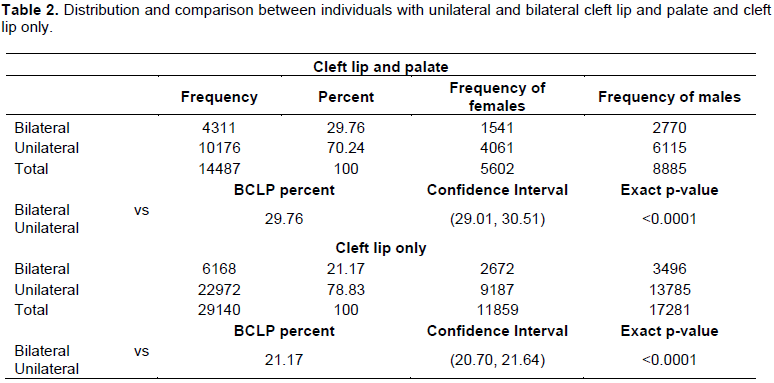
Table 2 presents the frequencies for the unilateral and bilateral cleft lip and palate (CLP), and cleft lip only (CLO), followed by the test results. The binomial test showed that the proportion of BCLP cases was not the same as the proportion of UCLP (p < 0.0001). In fact, the proportion of unilateral cases was greater than the proportion of bilateral cases. A similar result was found for the cleft lip (CL) cases (p < 0.0001).
Binomial test comparing pairs of classifications based on gender and laterality
For each pair of categories based upon laterality (among bilateral, left or right) the exact binomial test was used to test the hypothesis that the proportions in each pair of categories are 0.5. That is, considering individuals with CLP, with frequencies given below, a test was carried out to determine significant differences in the proportion of CLP based on the location (bilateral, left, and right). Then, these evaluations were repeated among patients with CL.
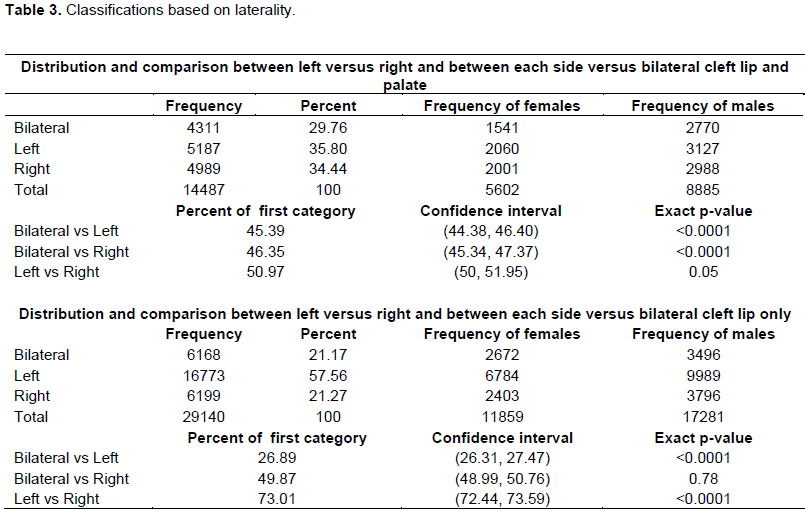
The test for differences in proportions showed that there were more LCLP than BCLP (p < 0.0001; Table 3). There was also more RCLP than BCLP (p-value <0.0001; Table 3). However, proportion of left vs right CLP was not too different from 0.5, there was no significant difference (p = 0.05; Table 3). The test for differences in proportions showed that there was more LCL than BCL (p < 0.0001; Table 3). There was more LCL than RCL (p < 0.0001; Table 3). However, the proportion of BCL was not different from the proportion of RCL (p = 0.78; Table 3).
By gender
The test for differences in proportions was used to determine if the proportion of cleft was greater in one category than in another category. Among females, the proportion of BCLP was lesser than the proportion of LCLP and RCLP (p < 0.0001 in both cases; Table 4). The proportions of CLP among males were similar to those for females (Table 4).
Among females, the proportion of BCL was less than the proportion of UCL (p < 0.0001; Table 4). However, the proportion of BCL was slightly greater than the proportion of RCL (p = 0.0002). Also, the proportion of LCL was greater than the proportion of RCL (p < 0.0001; Table 4).
In males, the proportion of BCL was smaller than the proportion of LCL (p-value <0.0001; Table 4). The proportion of BCL was lesser than the proportion of RCL (p-value 0.0005; Table 4). The proportion of LCL was greater than the proportion of RCL (p-value <0.0001; Table 4). There was a significant difference between males and females with CPO (p-value 0.0002).
Affected relatives
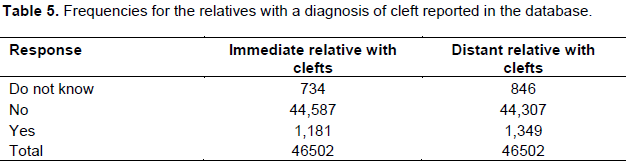
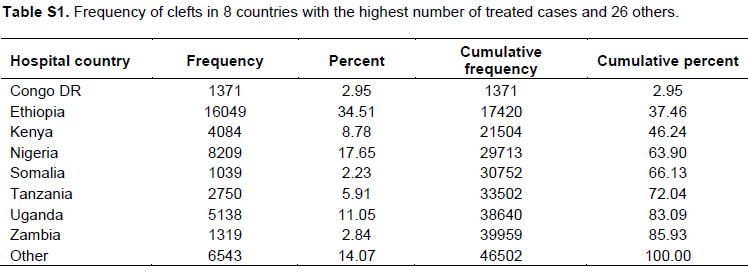
Table 5 shows the distribution and percentages of first and second degree relatives with a cleft diagnosis recorded in the database. Frequency of clefts and distribution of cleft types in eight of the countries with highest number of treated cases and 26 others are presented in supplementary Table 1.
Epidemiology
In the absence of established population based birth defects registries in most countries in sub-Saharan Africa, hospital based registries supported by non-governmental organizations provide a reliable estimate of birth defects including OFCs. In this study, post-surgical data from the Smile Train organization was obtained and these data by cleft type, gender and countries in SSA was analyzed. In the overall distribution of OFCs, it was observed that in major cleft types such as CLP, CLO and CPO is similar to what has been reported in some populations around the world (Butali et al., 2014; Eshete et al., 2011; Doray et al., 2012; Yáñez-Vico et al., 2012; Bell et al., 2013; McDonnell et al., 2014). Our observation for UCLP versus BCLP is not different from other studies (Yáñez-Vico et al., 2012; Lithovius et al., 2014). Nonetheless, there was no significant difference between RCLP and LCLP.
Gender difference for CPO has been consistently reported in the literature (Butali et al., 2014; Lithovius et al., 2014; Matthews et al., 2014). A statistically significant difference for CPO in our cohort even after applying the Bonferroni correction was observed. An interesting finding was the gender difference in CL. It was observed that more females had BCL than RCL. The opposite was observed in males.
In the present study, 2.5% reported clefting in first degree relatives (that is, siblings and 2.9% in second degree relatives (uncles, cousins and aunties). These rates are lower than reported rates for positive family history of 10.4% reported in Nigeria (Butali et al., 2014), and in other population groups where rates as high as 17 to 35% have been reported (Peterka et al., 1996; Jaruratanasirikul et al., 2008; Martelli et al., 2010).
Furthermore, data for cleft types in affected relatives were not available for analysis. This is a limitation because it is now known that recurrent risk varies in families according to cleft types. There is a genetic risk for having another child with cleft in a family with a positive history of clefting. A family with a particular cleft phenotype will likely give birth to offspring with the same phenotype compared to a family with no history of clefting in the general population, BCLP to BCLP: Recurrent risk is 4.6% (95% CI: 3.2-6.1%); CP to CP: recurrent risk is 3.9% (95% CI: 2.5-5.6%) (Grosen et al., 2010). This information will be valuable during genetic counselling for affected families. Recurrent risks also provide evidence supporting the role of genetic underpinnings for the different type of phenotypes. Therefore, future genetic studies investigating these phenotypes in separate cohorts will provide opportunities for novel and sophisticated strategies for prevention.
Limitations
The data is very limited and the study was unable to estimate the prevalence for each country since the birth rates for these countries from Smile Train were not available. A survival curve which will provide information on the survival of individuals with clefts in this population could not be plotted. This is a limitation since the data was cross-sectional. The estimates provided in this study are not exact prevalence data for the cleft types, as they were obtained in only hospitals supported by Smile Train. In addition, they are prone to bias and may not represent the true rates. Furthermore, the data do not include infant mortality data, and it is difficult to estimate the number of stillbirths with clefts or infants with clefts who died during the perinatal period prior to surgery. Nonetheless, this database can be developed further into a hospital birth defect registry for records of all births (live and stillbirths). Population-based surveillance and record of all births should then be linked to the hospital registry. It is only when this is done that accurate estimates of birth defects rates including OFCs can be estimated.
Data reported here provides estimates from the largest recorded body of data for clefts in the African continent, therefore providing a rationale for the establishment of a population based registry. These registries when established will support studies on cleft treatment outcomes, etiology and prevention.
The authors have not declared any conflict of interests.
REFERENCES
|
Agbenorku P, Ansah S, Acheampong A, Sabbah D, Bankas D, Adu E, Plange-Rhule G, Adiyiah Y, Donkor P (2011). Komfo Anokye Teaching Hospital Multidisciplinary Cleft Clinic. J. Craniofac Surg. 22(2):532-536.
Crossref
|
|
|
|
Ahmed O, Odunukwe N, Raheem Y, Efienemokwu C, Junaid M, Adesesan S, Ogedengbe O, Harry T, Salako L (2004). Knowledge, attitudes and perceptions of HIV/AIDS among traditional birth attendants and herbal practitioners in Lagos State, Nigeria. Afr. J. AIDS Res. 3(2):191-196.
Crossref
|
|
|
|
|
Akinmoladun VI, Owotade FJ, Afolabi AO (2007). Bilateral transverse facial cleft as an isolated deformity: Case report. Ann. Afr. Med. 6(1):39-40.
Crossref
|
|
|
|
|
Awoyale T, Onajole AT, Ogunnowo BE, Adeyemo WL, Wanyonyi KL, Butali A (2016). Quality of Life of Family Caregivers of Children with Orofacial Clefts in Nigeria: A mixed methods study. Oral Dis. 22(2):116-122.
Crossref
|
|
|
|
|
Bell JC, Raynes-Greenow C, Bower C, Turner RM, Roberts CL, Nassar N (2013). Descriptive epidemiology of cleft lip and cleft palate in Western Australia. Birth Defects Res. A Clin. Mol. Teratol. 97(2):101-108.
Crossref
|
|
|
|
|
Bukar M, Jauro YS (2013). Home births and postnatal practices in Madagali, North-Eastern Nigeria. Niger J Clin. Pract. 16(2):232-237.
Crossref
|
|
|
|
|
Butali A, Adeyemo WL, Mossey PA, Olasoji HO, Onah II, Adebola A, Efunkoya, Akintububo A, James O, Adeosun OO, Ogunlewe MO, Ladeinde AL, Mofikoya BO, Adeyemi MO, Ekhaguere OA, Emeka C, Awoyale TA, The Nigeriacran Collaboration (2014). Prevalence of orofacial clefts in Nigeria. Cleft Palate Craniofac J. 51(3):320-325.
Crossref
|
|
|
|
|
Calzolari E, Bianchi F, Rubini M, Ritvanen A, Neville AJ (2004). Epidemiology of cleft palate in Europe: implications for genetic research. Cleft Palate Craniofac J. 41(3):244-249.
Crossref
|
|
|
|
|
Carneiro PM, Massawe ER (2009). Pattern of cleft lip and palate in Dar-es-Salaam. Cent. Afr. J. Med. 55(1-4):10-14.
|
|
|
|
|
Dai L, Zhu J, Mao M, Li Y, Deng Y, Wang Y, Liang J, Tang L, Wang H, Kilfoy BA, Zheng T, Zhang Y (2010). Time trends in oral clefts in Chinese newborns: data from the Chinese National Birth Defects Monitoring Network. Birth Defects Res. A Clin. Mol. Teratol. 88(1):41-47.
|
|
|
|
|
David L, Murray L (2013). Global Demographic Trends and Their Implications for Employment. Post-2015 UN MDG Development Agenda Employment and Economic Growth. 2013.
View
|
|
|
|
|
Doray B, Badila-Timbolschi D, Schaefer E, Fattori D, Monga B, Dott B, Favre R, Kohler M, Nisand I, Viville B, Kauffmann I, Bruant-Rodier C, Grollemund B, Rinkenbach R, Astruc D, Gasser B, Lindner V, Marcellin L, Flori E, Girard-Lemaire F, Dollfus H (2012). Epidemiology of orofacial clefts (1995-2006) in France (Congenital Malformations of Alsace Registry). Arch. Pediatr. 19(10):1021-1029.
Crossref
|
|
|
|
|
Eshete M, Gravenm PE, Topstad T, Befikadu S (2011). The incidence of cleft lip and palate in Addis Ababa, Ethiopia. Ethiop. Med. J. 49(1):1-5.
|
|
|
|
|
Grosen D, Chevrier C, Skytthe A, Bille C, Mølsted K, Sivertsen A, Murray JC, Christensen K (2010). A cohort study of recurrence patterns among more than 54,000 relatives of oral cleft cases in Denmark: support for the multifactorial threshold model of inheritance. J. Med. Genet. 47(3):162-168.
Crossref
|
|
|
|
|
International Monetary Fund (2014). "Standard country or area codes for statistical use" (ST/ESA/STAT/SER.M/49/Rev. 4).
|
|
|
|
|
Jaruratanasirikul S, Chichareon V, Pattanapreechawong N, Sangsupavanich P (2008). Cleft lip and/or palate: 10 years experience at a pediatric cleft center in Southern Thailand. Cleft Palate Craniofac J. 45(6):597-602.
Crossref
|
|
|
|
|
Lithovius RH, Ylikontiola LP, Harila V, Sándor GK (2014). A descriptive epidemiology study of cleft lip and palate in Northern Finland. Acta Odontol. Scand. 72(5):372-375.
Crossref
|
|
|
|
|
Manyama M, Rolian C, Gilyoma J, Magori C.C, Mjema K, Mazyala E, Kimwaga E, Hallgrimsson B (2011). An assessment of orofacial clefts in Tanzania. BMC Oral Health 11:15.
Crossref
|
|
|
|
|
Martelli D, Bonan P, Soares M, Paranaíba L, Martelli-Júnior H (2010). Analysis of familial incidence of non-syndromic cleft lip and palate in a Brazilian population. Med. Oral Patol. Oral Cir. Bucal. 15(6):e898-901.
Crossref
|
|
|
|
|
Matthews JL, Oddone-Paolucci E, Harrop RA (2014). The Epidemiology of Cleft Lip and Palate in Canada, 1998 to 2007. Cleft Palate Craniofac J. 52(4):417-424.
Crossref
|
|
|
|
|
McDonnell R, Owens M, Delany C, Earley M, McGillivary A, Orr DJ, Duggan L (2014). Epidemiology of orofacial clefts in the East of Ireland in the 25-year period 1984-2008. Cleft Palate Craniofac J. 51(4):e63-69.
Crossref
|
|
|
|
|
Mossey PA, Modell B (2012). Epidemiology of oral clefts: an international perspective. Front Oral Biol. 16:1-18.
Crossref
|
|
|
|
|
Oginni F, Asuku M, Oladele A, Obuekwe O, Nnabuko R (2010). Knowledge and Cultural Beliefs About The Etiology And Management Of Orofacial Clefts In Nigeria's Major Ethnic Groups. Cleft Palate-Craniofacial J. 47(4):327-334.
Crossref
|
|
|
|
|
Peterka M, Peterková R, Halasková M, Tvrdek M, Fára M, Likovský Z (1996). Sex differences in the incidence of cleft and question of primary prevention in families with genetic risk. Acta Chir Plast. 38(2):57-60.
|
|
|
|
|
Spritz RA, Arnold TD, Buonocore S, Carter D, Fingerlin T, Odero WW, Wambani JO, Tenge RK, Weatherley-White RC (2007). Distribution of orofacial clefts and frequent occurrence of an unusual cleft variant in the Rift Valley of Kenya. Cleft Palate Craniofac J. 44(4):374-377.
Crossref
|
|
|
|
|
United Nations Inter-agency Group for Child Mortality Estimation (2013). Under-Five Mortality Estimates: Rates. Available at: http://www.un.org/esa/sustdev/natlinfo/indicators/methodology_sheets/health/under_five_mortality.pdf
|
|
|
|
|
United Nations report World population prospects (2012). Revision (2014). World Development Indicators. Washington, DC.
|
|
|
|
|
World Health Organization (WHO) (2005). 2nd International Conference on Birth Defects and Disabilities in the Developing World. Hospital-based registries for monitoring birth defects: The National Collaborative Perinatal Neonatal Network; 11–15 September; Beijing, China.
|
|
|
|
|
Yá-ez-Vico RM, Iglesias-Linares A, Gómez-Mendo I, Torres-Lagares D, González-Moles MÁ, Gutierrez-Pérez JL, Solano-Reina E (2012). A descriptive epidemiologic study of cleft lip and palate in Spain. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114(5 Suppl):S1-4.
Crossref
|
|