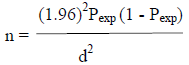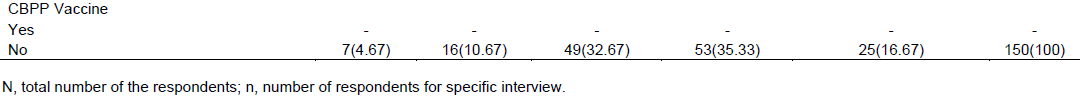ABSTRACT
Contagious bovine pleuropneumonia (CBPP) is a highly infectious cattle disease, which is widespread in pastoral areas of Africa and it is a major problem for Ethiopian livestock. A cross-sectional study on sero-epidemiology and risk factors for contagious bovine pleuropneumonia infection of cattle in Dello Mena and Sawena districts of Bale Zone was conducted from February, 2016 to May, 2016. Competitive Enzyme Linked Immunosorbent Assay test was used to analyze 384 cattle sera for contagious bovine pleuropneumonia antibodies and the overall sero-prevalence rate was determined to be 6.51%. A higher sero-prevalence rate of 8.26% was observed in Dello Mena district when compared to Sawena (3.89%) district. The prevalence in each kebele ranged from 0 to 15.91% and the highest prevalence (15.91%) was found at Hi-Oda, located in Dello Mena district. A Chi-square computed statistical analysis indicated that age (χ2=5.738; p<0.017), sex (χ2=11.105; p<0.001), breed (χ2=13.29; p<0.014), body condition score (χ2=6.063; p<0.048) and purpose of rearing cattle (χ2=14.877; p<0.001) were the major putative risk factors associated with the occurrence of contagious bovine pleuropneumonia in the study districts. The difference, however, was not statistically significant (p>0.05) for origin (Dello Mena/Sawena), contact with other herds (yes/no) and herd size (small/medium/large). Multivariable logistic regression statistical analysis revealed that age (adult/young), body condition score (good/medium/poor) and purpose of rearing cattle (beef/draft power/milk) were significantly associated with contagious bovine pleuropneumonia sero-positivity (p<0.05). Consequently, cattle rearing purpose (for beef/draft power/milk) was statistically identified as the major risk factor for contagious bovine pleuropneumonia occurrence in relation to other factors and cattle(oxen/bull) used for the purpose of draft power were more likely to be infected by CBPP(Adjusted Odds Ratio=49.052). This study showed that the overall sero-epidemiology of contagious bovine pleuropneumonia in Bale Zone of Oromia Regional State was relatively high. This warrants the implementation of appropriate preventive and control measures to minimize the economic losses arising from the disease.
Key words: Cattle, contagious bovine pleuropneumonia, Dello Mena, risk factors, Sawena, seroepidemiology
Contagious bovine pleuropneumonia (CBPP) is a highly contagious disease of cattle that is caused by Mycoplasma mycoides subsp.mycoides (Mmm) (Yaya et al., 2008; Billy et al., 2105).The disease is endemic in many African countries, and the Sahara region is under constant threat due to the carrier status of its host. The disease spread alarmingly during the 1990s, infecting several countries previously free from the disease, causing greater losses in cattle than any other diseases, including rinderpest (OIE, 2001). Due to high economic losses caused by CBPP in endemic regions, OIE declared CBPP as one of the most serious contagious animal disease and listed it in the group of notifiable animal diseases of high socio-economic impact and is regarded as one of the major transboundary animal diseases (TADs) (FAO, 2002; Wade et al., 2015).
M. mycoides is mainly transmitted from animal to animal in aerosols. This organism also occurs in saliva, urine, fetal membranes and uterine discharges. Carrier animals, including sub-clinically infected cattle that can retain viable organisms in encapsulated lung lesions (sequestra) for up to two years. These animals may shed organisms, particularly when stressed (Rovid, 2008). The disease is manifested by anorexia, fever and respiratory signs such as dyspnoea, polypnoea, cough and nasal discharges in cattle (OIE, 2014; Wade et al., 2015).
Although CBPP was once found worldwide, it was eradicated from most continents, by the mid-20th century. Its incidence also began to decline in Africa by the 1970s. However, because of the economic and financial difficulties that affected the ability of governments to adequately fund Veterinary Services, the disease came back in the late 1980s and early 1990s (Tambi et al., 2006; Rovid, 2008). Major CBPP epidemics have been experienced in Eastern, Southern, and West Africa over the last few years. It currently affects 27 countries in Africa at an estimated annual cost of US $ 2 billion (Otte et al., 2004). A total of 2,719 outbreaks were reported in Africa between 1995 and 2002. Countries in East Africa reported 66% of the total outbreaks (58% in Ethiopia and Tanzania, and 8% in other countries in the region) (Tambi et al., 2006; Alemayehu et al., 2014).
The livestock subsector currently supports and sustains livelihoods for 80% of all rural population in Ethiopia. However, transboundary animal diseases such as CBPP constrain the livestock sector of the country and affect livelihoods via their impact on animal health, animal food production, availability and quality. Furthermore, CBPP is one of the major diseases in Ethiopia that hampers the export of livestock and livestock products to the international markets (Gedlu, 2004; Alemayehu et al., 2014). Pastoral areas output underpins almost all of Ethiopia’s live animal and meat exports. Generally, CBPP is a threat for Ethiopian livestock export market and a well-established disease in Oromia Regional State especially in pastoral areas of Borana and Bale where the animals originated (Kassaye and Molla, 2013).
Contagious bovine pleuropneumonia (CBPP) is both an epidemic and endemic disease of cattle that affects production through mortality and reduction in productivity. It also retards genetic improvement and limits working ability of cattle. The economic impacts of CBPP in a number of African countries, including Ethiopia were studied (Tambi and Maina, 2004). Accordingly, cattle in CBPP-infected areas (epidemic and endemic) are divided into three classes namely, calves and yearlings below 3 years, adult males, and reproductive females. The losses due to CBPP (epidemic and endemic) are measured as the number of deaths that occur per class of animal, the quantity of beef lost for each class of animal, the quantity of milk lost from reproductive females and the loss in draft power from oxen (Gedlu, 2004).
In Ethiopia, the average physical losses from CBPP in terms of cattle deaths are 25,115 heads (8,372 in endemic and 16,743 in epidemic), 1,852 and 13,396 metric tons of beef and milk, respectively. In terms of animal power, averages of 3,135,000 ox days are lost. The country experiences the largest number of cattle deaths, and reduction in cattle products under both endemic and epidemic conditions compared to the other African countries, due probably to its large cattle population (Tambi and Maina, 2004). Although vaccination has been considered as a strategy for the control of CBPP in Ethiopia for the last 30 years, the disease still persists in several regions of the country, with its incidence increasing from year to year (Gedlu, 2004). This is, mainly due to lack of effective vaccine, irregular and low rate/coverage of vaccination, lack of livestock movement control, and absence of systematic disease surveillance and reliable data. For the time being, therefore, mass vaccination and where possible control of animal movement remains the most practical option in sub-Saharan Africa (Litamoi, 2000).
The vaccines are exclusively monovalent live attenuated freeze-dried products derived from broth culture of T1SR (streptomycin resistant variant) or T1/44 seed strains of M. mycoides subspecies mycoides that gives protection for 6 months up to one year. The other vaccines are V5, KH3J that gives protection for 2 and 6 months (Bamhare, 2000).
Various studies have been conducted to determine the sero epidemiology of CBPP in various regions of Ethiopia. Studies conducted by Beyene (1997) in Western Ethiopia, Takele (1998) in North West Ethiopia, Wondimu (1996) in Southern Ethiopia, NAHRC (2000) in different regions of the country, Issa (2004) in Borena zone, Gedlu (2004) in Somali Region and Regassa (2001) in West Wollega zone revealed that the disease was prevalent in different region of the country for over a decade. Recently, study conducted by Kassaye and Molla (2013), and Alemayehu et al. (2014) at export quarantine centers in and around Adama and bulls’ originated from Borena pastoral area, respectively, revealed that CBPP is posing a major threat to cattle in many parts of the country thereby causing considerable economic losses through morbidity and mortality and warranting serious attention.
Although all the above study revealed that CBPP was seriously devastating the cattle production industry in Ethiopia, there is no published data (information) about the sero epidemiology of this disease in cattle production system in Bale Zone in general and in Dello Mena and Sawena districts in particular. This scarcity of information on the presence and sero epidemiology of CBPP in livestock production may reflect a lack of resources for disease surveillance and control in the cattle production system. In addition, most livestock disease outbreaks, particularly in more remote parts of the country, remain undiagnosed and therefore, information on the sero epidemiology and significance of CBPP can only readily be obtained through serological studies in order to apply control measures based on mass vaccination or effective movement control.
Hence, this study was conducted: (i) To determine the sero-epidemiology of CBPP and putative risk factors exposing to CBPP that potentially affects the cattle production system in the selected pastoral districts of Bale Zone, and (ii) To assess knowledge, attitude and practices (KAP) of the households HHs (pastoralists/herdsmen) about the disease. Therefore, our study could be a foil for the paucity of information about the sero-epidemiology of CBPP, putative risk factors exposing to the disease, and knowledge, attitudes and practices of the pastoralists/herdsmen in the cattle production system of the two selected districts of the study areas.
Description of the study area
The study was conducted in two selected districts of Bale Zone namely, Dello Mena and Sawena districts of Bale Zone, Oromia Regional State, south east of Ethiopia. The two study districts were selected purposely to represent the cattle rearing districts of the zone based on their agricultural vocational activities and ecological conditions.
Dello Mena is located in the south western part of Bale zone and is 555 km south east of Addis Ababa. It shares borders with Madda Walabu district in the south, Goba district in the north, Harena Buluk district in the west and south west, Berbere district in north east and Guradhamole in the east. It has a total area of 1,339 km2 ranking it the 14th largest district. The mean annual temperature of the district is 29.5°C with the lowest and highest temperatures being 21 and 38°C, respectively. The mean annual rainfall is 701.5 mm; with the lowest and highest rainfall being 628mm and 775 mm, respectively. The lowland area predominates with a narrow strip of high land area in the northern part of Dello Mena district. From early days, livestock rearing has played an important role in the life of district population. In the rural and lowland areas of the district, rearing and breeding is the main stay of the people. There are about 312, 400 bovines, 5,122 sheep, 61, 626 goats, 11, 436 equines and 31, 644 camels (DMDAO, 2015).
Sawena district is located in the eastern part of Bale zone and is 623 km south east of Addis Ababa. It is bounded by Laga Hida district in the North, Ginir district and Gololcha district in the west, Rayitu district in the south and Somalia National State in the east. It has a total area of 8289 km2 ranking it the second largest among the districts. The mean annual temperature of the district is 35°C with the lowest and the highest temperatures of 30 and 40°C, respectively. The mean annual rainfall is 375 mm whereas the lowest and highest rainfall is 250 and 500 mm, respectively. Unlike Dello Mena, surface water is a serious problem in Sawena district, where only seasonal streams, ephemeral ponds and shallow temporary wells are sources of water in the rainy season and these usually dry out after a few days during the dry season. Sawena district has a very large livestock resource. It has a pastoral vocation with livestock rearing being the dominant economic activity in the district. In the rural and lowland areas of the district, rearing and breeding is the main stay of the people. There are about 52,740 bovines, 14,645 sheep, 46,450 goats, 4,603 equines and 26,586 camels (SDAO, 2015).
Study population
The target study population comprised cattle above six months of age in the two selected districts. The status of CBPP in Dello Mena and Sawena districts was unknown since no study had been conducted in the two districts before. The study animals consisted of 384 cattle above six months of age with no history of CBPP vaccination. Cattle were selected for sample collection using the simple random method for both districts.
Study design
A cross sectional study supported by questionnaire survey was conducted to determine the sero-epidemiology of CBPP and its associated risk factors in pastoral vocation with livestock rearing being the dominant economic activity of the two selected districts.
Questionnaire survey was conducted to have information on the clinical signs of cattle diseases in the afore-mentioned districts. In the two selected districts cattle owners were interviewed with semi-structured questionnaire. Emphasis was given on the frequent clinical symptoms manifested whenever outbreaks of cattle diseases occurred in the respective study sites. Tentative diagnosis was made based on the classical disease manifestation and treatment was provided for diseased animals accordingly. The questionnaires were prepared, pre-tested and adjusted by translating in to local language (Afan Oromo) and administered by the interviewer. The questionnaire focused on the potential risk factors and was conducted after carefully explaining the purpose of the work to the interviewees.
Sampling method and determination of sample size
The sample size required for the study was calculated according to the formula given by Thrusfield (2007) for simple random sampling.
Where n = required sample size, Pexp = expected prevalence, and d = desired absolute precision.
Since there has been no research conducted in this area; the sample size was calculated at 95% CI, 5% desired absolute precision and expected prevalence of 50%. Accordingly, the total numbers of sample required for this study was 384 cattle above six months of ages.
Dello Mena district contains 17 kebeles while Sawena district contains 28 kebeles. Five kebeles from each district were selected purposely by their proximity to roads, accessibility of infrastructure and cattle population holdings of each kebele. Prior to commencement of the study, list of HHs of those kebeles (sampling frame) was obtained from both district Agricultural offices. The sample size of HHs was determined using the formula recommended by Arsham (2007) for survey studies.
N=0.25/(SE)2
Where: N = sample size and SE= standard error of the proportion.
Assuming the standard error of 4.1% at a precision level of 5%, and the confidence interval of 95%, 150 HHs owning cattle were selected by a simple random sampling technique for interview. The numbers of HHs selected per kebeles were fixed based on the proportion of HHs owning cattle in each kebele.
Inclusion criteria
Cattle above six months of ages without a history of vaccination for CBPP were included.
Exclusion criteria
Apparently healthy cattle with history of CBPP vaccination were excluded (Actually no CBPP vaccination had been given in the selected districts).
Sera collection
Animals were restrained by animal handlers and 10 ml of blood sample was collected from the jugular vein using vacutainer tubes with 18 to 20 gauge hypodermic needles. Vacutainer tubes with blood samples were then labeled with tag number of animals set tilted on a table overnight at room temperature to allow clotting and kept protected from direct sun light until the blood clotted and sera were separated. Then serum was filled into serum storage vials (cryovials) with appropriate identification and stored at -20ºC until transported to National Veterinary Institute (NVI) and the C-ELISA was performed. Corresponding to each sample, age, sex, breed of each animal, body condition, geo-reference information and other risk factors contributing to the occurrence of CBPP were collected and registered on a separate case book.
Serological testing
Competitive Enzyme Linked Immunosorbent Assay (C-ELISA) test was conducted as recommended by CIRAD-UMR15 (FAO and OIE World reference centre for CBPP) based on a monoclonal anti-Mmm antibody named Mab 177/5 as described previously (LeGoff and Thiaucourt, 1998). The test was undertaken at NVI, Bishoftu, Ethiopia. Sera samples were mixed with specific monoclonal antibody (Mab 117-5) in a dilution plate and were incubated with gentle agitation at 37°C for one hour, and then it was transferred into the Mmm coated microplate. After washing, anti-mouse IgG serum conjugated-horse radish perioxidase (HRP) was added. After series of washings the HRP substrate (TMB) was added forming a blue compound that was turned yellow when the reaction stopped. The optical density (OD) was read in an ELISA reader at 450 nm and the cut off points were calculated to validate the results. All sera with Percentage Inhibition (PI) > 50% was considered as positive. Sera with PI between 40 and 50% were considered doubtful and those sera with PI less than 40% were considered negative.
Data storage and analysis
Data generated from questionnaire survey and laboratory investigations were recorded and coded using Microsoft Excel spreadsheet (Microsoft Corporation) and analyzed using STATA version 11.0 for Windows (Stata Corp. College Station, TX, USA). The sero-epidemiology of CBPP was calculated as the number of sero-positive samples divided by the total number of samples tested. Association of sero-positivity with the potential risk factors (origin, age, sex, body condition, breed type, herd size, vaccination history, contact with other herds and etc.) was computed by Chi-square test and logistic regression (both simple and multiple). Regression models are used to explore the relationships between a dependent or response variable (CBPP in this case) and one or more independent or predictor variables of interest (risk factors in this case). A P-value <0.05 was considered statistically significant.
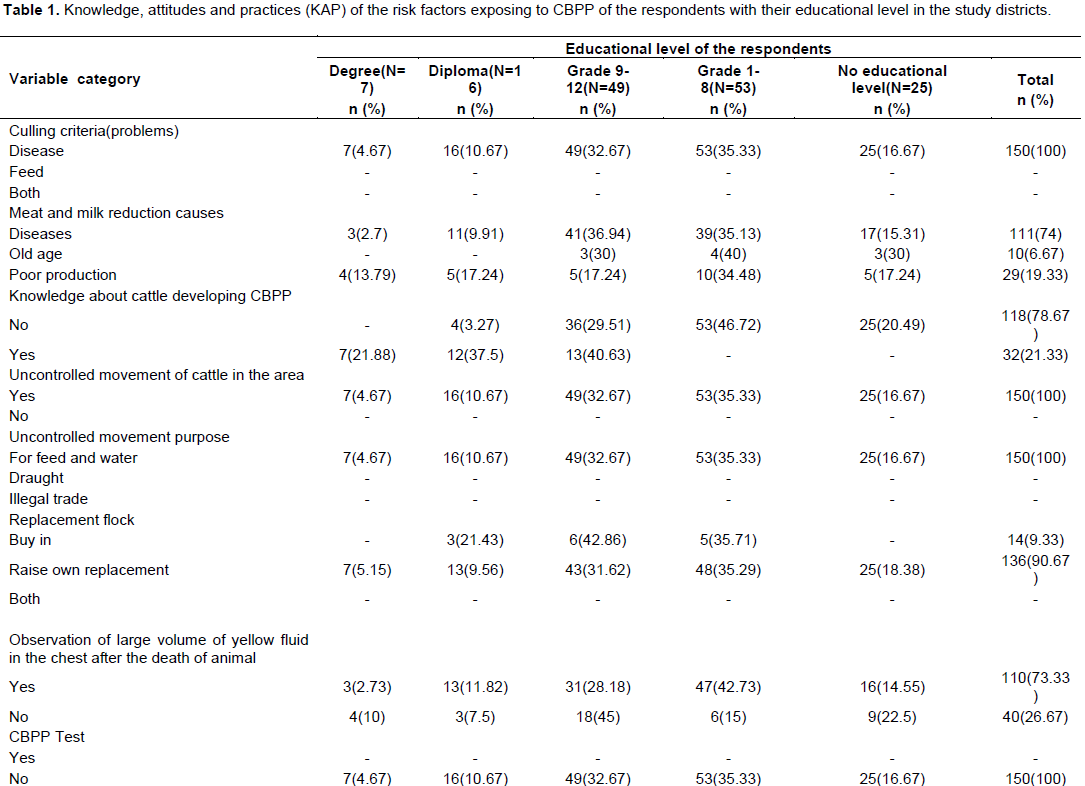
Knowledge, attitudes and practices of the respondents about contagious bovine pleuropneumonia
The educational level of the respondents involved in cattle production system in the two selected pastoral districts of Bale Zone were diverse from literate to illiterate people in that they had degree (4.67%), diploma (10.67%), grade 9 to 12 (32.67%), grade 1 to 8 (35.33%) and no formal education training (16.67%). Of the 150 HHs (herdsmen/pastoralists) that responded, 125 (83.33%) could at least read and write in English language. The study finding, astonishingly, revealed that as the disease was the only culling criteria for cattle from the rest herds than feeding system in the study settings. The study illustrated that only about 21.33% of the HHs (pastoralists/herdsmen) in the study area had knowledge about CBPP threat while about 74% of the respondents stated that the disease was the main cause of meat and milk reduction in the cattle production of the study area. All the HHs included in the study reported uncontrolled movement, and search for feed and water as the main intention for cattle movement in the study districts. In addition to this, all of the respondents (pastoralists/herdsmen) stated that as there was no CBPP test and provision of vaccine for their cattle by the government as illustrated in Table 1.
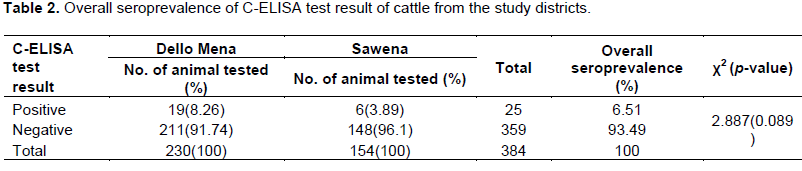
Overall seroprevalence of contagious bovine pleuropneumonia in the study districts
In the present study, an overall sero-prevalence of 6.51% was determined by the C-ELISA test. A higher sero-prevalence of 8.26% was observed in Dello Mena when compared to Sawena (3.89%) but there was no significant association between the study districts and CBPP sero-positivity as depicted in Table 2.
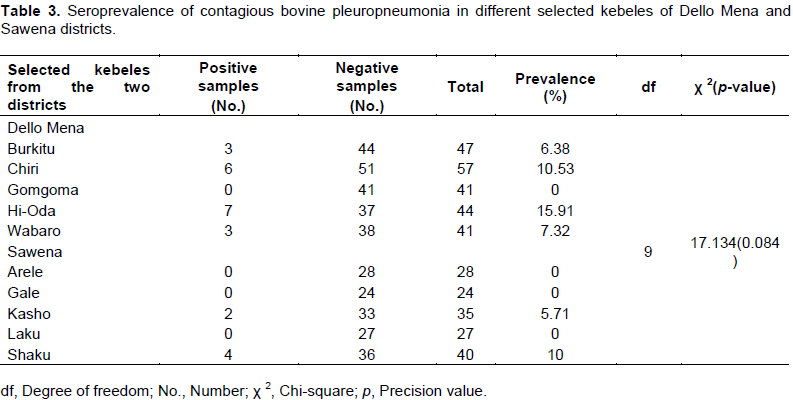
Sero-positivity of contagious bovine pleuropneumonia in selected kebeles of the study districts
Of 5 kebeles selected from Dello Mena district, Hi-Oda had the highest CBPP sero-positivity (15.91%) while Shaku (10%) from Sawena district had the highest sero-positivity among the 5 kebeles selected.
However, no CBPP sero-positive cattle were found in 4 kebeles (one (1) from Dello Mena and three (3) from Sawena) of the study districts. There was no significant association (p>0.05) between the selected kebeles of the study pastoral districts and CBPP sero-positivity (Table 3).
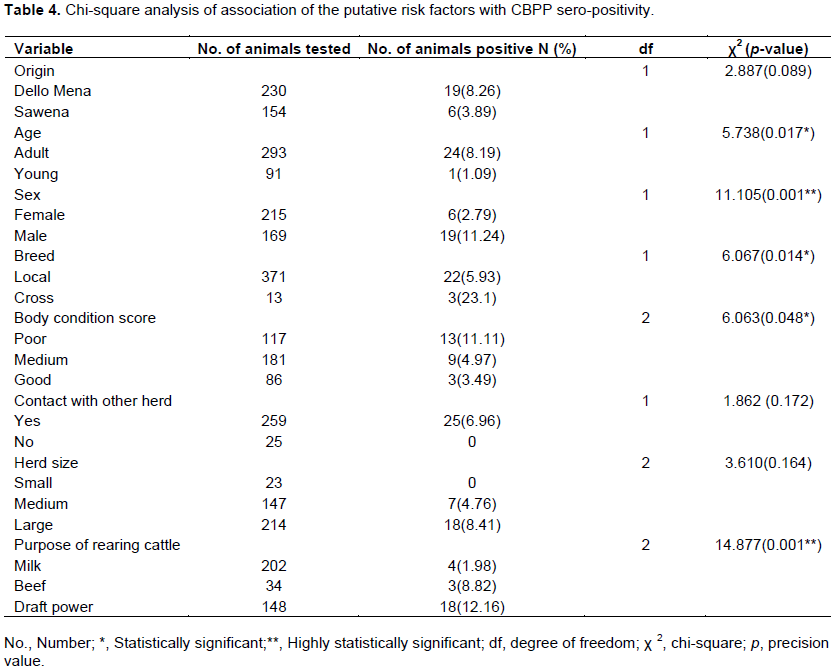
Chi-square analysis of association of the putative risk factors with contagious bovine pleuropneumonia sero-positivity
A chi-square analysis revealed age, sex, breed, body condition score and purpose of rearing cattle were significantly associated (p< 0.05) with CBPP sero-positivity among the putative risk factors considered during the study as indicated in Table 4.
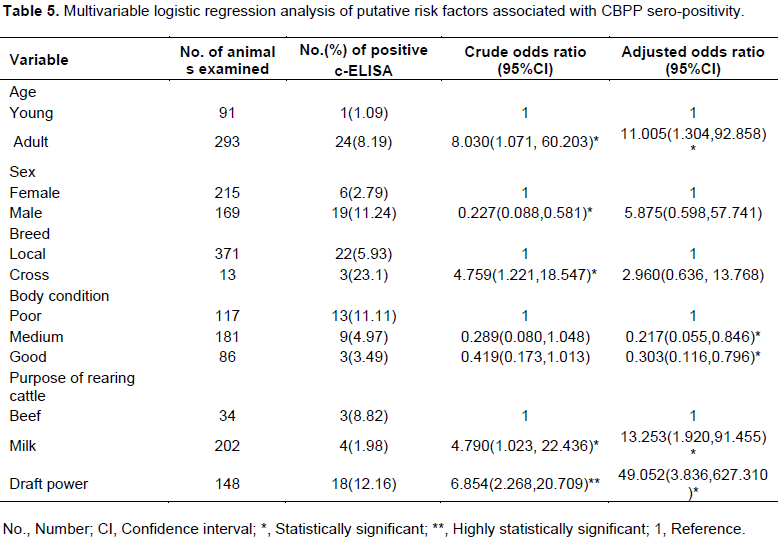
Multivariable logistic regression analysis of putative risk factors associated with CBPP sero-positivity
The logistic regression analysis of the putative risk factors indicated that cattle used for the purpose of draft power were more likely to be infected (AOR=49.052; 95% CI: (3.836-627.310) by CBPP disease than cattle used for milk and beef as depicted in Table 5.
Knowledge, attitudes and practices of the respondents about contagious bovine pleuropneumonia
The studyd is covered the local name of CBPP to be “dhukkuba sombaa” in Dello Mena and Sawena districts of Bale Zone. The high level of literacy among the HHs (pastoralist/herdsmen) in which 83.33% were literate disagrees with the report of Tahir (2001), who stated that pastoralists and their children have little or no access to formal education. The HHs (pastoralists/herdsmen), astonishingly, responded that as the disease was the only culling criteria for cattle from the rest herds than feeding system in the study settings. Communities in this study had a low (21.33%) level of awareness of CBPP when compared with the work of Tambuwal et al. (2011) and Billy et al. (2015) who reported a high level in the two transboundry states of north-western Nigeria (65.0%) and in Kaduna State of Nigeria (88.9%), respectively. This low level of awareness could be attributed to the rare outbreaks of CBPP.
About 111(74%) of the respondents (pastoralists/herdsmen) stated that the disease was the main cause of meat and milk reduction in the cattle production system of the study areas. All of the HHs (pastoralists/herdsmen) included in the study linked uncontrolled movement, and search for feed and water as the main intention for cattle movement in the study districts. In addition to this, all of the respondents (pastoralists/herdsmen) stated that as there was no CBPP test and provision of vaccine for their cattle by the government. In contrast to this finding, Aliyu et al. (2000) in northern Nigeria, Tambuwal et al. (2011) in the two transboundry states of north-western Nigeria and Billy et al. (2015) in Kaduna State of Nigeria reported 14.2, 32.5 and 36.7% vaccination coverage, respectively. The absence of CBPP test and vaccination coverage in the study area is possibly due to decreased awareness of the disease and absence of veterinary services.
Sero-epidemiology and putative risk factors exposing to contagious bovine pleuropneumonia
The present serological study established the presence of antibodies of CBPP among sampled cattle from two pastoral districts ,Dello Mena and Sawena, of Bale Zone. An overall sero- prevalence of 6.51% was obtained using c-ELISA from the two districts.
This finding is comparable to 9.4% in Borena (Ahmed 2004), 9.7% in south-western Kenya (Schnier et al., 2006), 9.1% in northwest Ethiopia (Gashaw, 1998) and 4% in and around Adama, central Ethiopia (Kassaye and Molla, 2013). However, the overall sero-prevalence was lower than the work of Daniel et al. (2016) in Western Oromia (28.5%), Gedlu (2004) in Somali Regional State (39%) and Dejene (1996) in North Omo (56%) of Ethiopia. The variation in prevalence reported from different parts of Ethiopia and other countries could be because of differences in agro ecological systems, cattle management and production systems, population density, sample size and the types of tests used to determine the sero-prevalence (Daniel et al., 2016).
The present study revealed that cattle from the two pastoral districts (origin) of Bale Zone was negatively associated with CBPP sero-positivity (p>0.05) and the results showed a relatively higher individual cattle sero-prevalence of CBPP in Dello Mena (8.26%) compared to Sawena (3.89%) district. A relatively higher sero-positivity of CBPP recorded in Dello Mena when compared to that of Sawena district could be attributed to more cattle that were sampled from Dello Mena, the presence of larger herds and communal grazing areas, making contact with infected animals more likely. This justification could be similar with the study conducted in Somali Regional State by Gedlu (2004). Of 10 kebeles selected from the two districts, the highest sero-epidemiology of CBPP was observed in Hi-Oda Kebele (15.91%) while no CBPP sero-positivity was found in four kebeles of the two pastoral districts. In contrast to this finding Daniel et al. (2016) reported the highest sero-positivity of CBPP in Gobbu Sayyo (40.3%) than in Bakko Tibbe (19%) and Horro (5.7%) districts of Western Oromia while Gashaw (1998) reported the highest seroprevalence of CBPP in Banja (66. 3%) than in Dangila (41.7%) and Denbecha (33.3%) districts of Western Gojam and Awi Zone.
Age was considerably associated with the sero-positivity of CBPP as detected by C-ELISA, which corroborates with the finding of Kassaye and Molla (2013) who reported that age was found significantly (p<0.05) associated with the occurrence of CBPP in which a high sero-prevalence was recorded in aged (9.5%) animals than young (3%) at export quarantine centers in and around Adama. In addition to this, the study conducted by Boelaert et al. (2005) revealed that increasing age is a surrogate measure of repeated exposure.
But adult CBPP cattle carriers’(7.2%) showed a relatively higher numerical value over the young (4.4%) this connotes to previous studies conducted by Mtui-Malamsha (2009) and Ikpa et al. (2010). This could be associated to the fact that chronic stages of the disease are usually seen in adult cattle as the age progresses (Olabode et al., 2013).
A relatively higher sero-positivity rate among the male (11.24%) compared to the female animals (2.79%) was significantly associated with the sero-positivity of CBPP (p<0.05). Though our result revealed that as sex of the animal was positively associated with the sero-positivity of CBPP, the sero-epidemiology of CBPP reported in male and female cattle of the two pastoral districts was lower than that of Daniel et al. (2016) who reported the prevalence of 30.9% in male and 27.04% in female animals in Western Oromia with no significant difference (p > 0.05) in the occurrence of CBPP. This could be attributed to lowered immunity following continuous reproductive stress as buttressed by the age frequency of the disease in adult cows (Olabode et al., 2013). Our finding also contradicts the work of Schnier et al. (2006) and Olabode et al. (2013), who reported a significantly higher prevalence in female animals, in the Maasai ecosystem of south-western Kenya and Kwara state of Nigeria, respectively.
A higher seroprevalence was recorded in cross (23.1%) than in local breeds (5.93%) in the present study. The difference was also positively associated with CBPP sero-positivity (p<0.05). This finding coincided with that of Daniel et al. (2016) who reported a higher sero-positivity of CBPP in cross (37.2%) than in local breeds (27.4%) but their finding revealed a negative association of breed with sero-positivity of the disease(p>0.05).
The body condition score of the animals was positively associated (p<0.05) with the occurrence of the disease in which a relatively higher sero-positivity was recorded in animals with poor body condition score (11.11%) than in animals with medium (4.97%) and good body condition score (3.49%). This finding is consistent with the study of Atnafie et al. (2015) who reported a significant association of body condition score with the CBPP sero-positivity in abattoirs at Bishoftu and export oriented feedlots around Adama in which a high seroprevalence was recorded in animals with poor body condition (18%) than in medium (12.5%) and good body condition score (6.4%), which was significantly associated with the occurrence of the disease (p<0.05). Nevertheless, our finding do not agree with the work of Daniel et al. (2016) who reported no significant association of body condition score with CBPP sero-positivity.
The current study established that CBPP sero-positivity was insignificantly associated with the contact of the animals with other herds (at watering points and animals brought into herds). There was no any suggestion on previous studies that is parallel with this finding in Ethiopia. Though the contact of the animals with other herd was negatively associated with the CBPP sero-positivity, the higher seroprevalence was observed in animals in contact with other herd at watering points and communal grazing (6.96%) than in animals with no contact with the other herds(confined in the feedlots/tied in the garden). This could be explained by the contact structure, herd size and seasonal herding practices influence CBPP disease distribution patterns (Mariner et al., 2006).
Herd size of the animals sampled from the study pastoral districts was found negatively associated (p>0.05)) with the occurrence of the disease. However, a relatively higher sero-positivity of CBPP was observed in larger (8.41%) than in medium herd sizes of the cattle sampled (4.76%) while no CBPP sero-positivity was observed in cattle from small herd sizes. This area needs an indebt study to unveil the factors responsible for this difference.
Reductions in the production of beef, milk and draft power were considered as morbidity losses. The present study established the association of the purpose of rearing cattle (beef/milk/draft power) with CBPP sero-positivity. A relatively higher seroprevalence of CBPP was observed in animals (bull/oxen) used for draft power (12.16) than the cattle used for beef (8.82%) and milk (1.98%). This revealed that the animals (bull/oxen) used for draft power was most likely infected by the disease. There was no any hint in previous studies that is parallel with this finding elsewhere and it needs an indebt study to divulge the factors responsible for the variation of CBPP sero-positivity in cattle used for beef, milk and draft power. Tambi et al. (2006) discussed in their review the loss in draft power resulted in economic turn down and was estimated as the product of the number of infected oxen and the number of workdays per year while the loss in beef production by infected animals was used as a surrogate for the absence of weight gain since diseased animals are assumed not to gain weight. They may even lose weight depending upon the severity of the infection. In addition to this, the economic significance of CBPP in animals reared for the purpose of milk (reduction in milk production) was also estimated from the number of infected reproductive females.
These are the animals that show clinical signs, estimated as the product of the number of reproductive females at risk and the transition rate from exposed to the state of infection. The rate of transition from exposed to state of infection was obtained from Mariner et al. (2006).
This study established a relatively high seroprevalence of CBPP in cattle in two pastoral districts of Bale Zone in south eastern part of Ethiopia, suggesting the disease could be causing considerable economic losses through morbidity and mortality. A relatively higher seroprevalence was observed in Dello Mena when compared to Sawena district with insignificant difference. In addition, age, sex, breed, body condition score and purpose of cattle rearing were significantly associated with CBPP sero-positivity. However, origin, contact with other herds and herd size of the cattle were insignificantly associated with CBPP sero-positivity. The occurrence of the disease may cause restriction on the trade of animals and animal products internationally, affecting the export earnings of the country, thereby threatening the livelihood of the farmers and national agricultural economy. In conclusion, the prevailing CBPP sero-positivity in the two districts indicates the importance of CBPP in the pastoral cattle production system of the study settings.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed I (2004). Epidemiological study of contagious bovine pleuropneumonia in Borana pastoral areas using complement fixation test and competitive enzyme-linked immunosorbent assay. Thesis for degree of Master of Veterinary Epidemiology, Faculty of Veterinary Medicine, University of Addis Ababa, Debrezeit, Ethiopia.
|
|
|
|
Alemayehu G, Leta S, Hailu B (2014). Low seroprevalence of contagious bovine pleuropneumonia (CBPP) in bulls originated from Borena pastoral area of Southern Ethiopia. Anim. Vet. Sci. 2(6):213-217.
Crossref
|
|
|
|
|
Aliyu MM, Obi TU, Egwu GO (2000). Prevalence of contagious bovine pleuropneumonia (CBPP) in Northern Nigeria. Prev. Vet. Med. J. 47:263-266.
Crossref
|
|
|
|
|
Arsham H (2007). Questionnaire design and survey sampling. Acessed July, 2015.
|
|
|
|
|
Atnafie B, Goba H, Sorri H, Kasaye S (2015). Seroprevalence of contagious bovine pleuropneumonia in abattoirs at Bishoftu and export oriented feedlots around Adama. Global Vet. 15(3):321-324.
|
|
|
|
|
Bamhare C (2000). Contagious bovine pleuropneumonia surveillance in vaccinated areas: Namibia experience with contagious bovine pleuropneumonia vaccines prepared from the T1-44 and T1-SR Strains. In: Report of second meeting of the FAO/OIE/OAU/IAEA consultative group on Contagious Bovine Pleuropneumonia (CBPP). Rome, Italy. pp. 79-87.
|
|
|
|
|
Beyene D (1997). Seroepidemiological investigation of contagious bovine pleuropneumonia in Illubabor and Wellega. DVM Thesis. Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia.
|
|
|
|
|
Billy IL, Balami AG, Sackey AKB, Tekdek LB, Sa'idu SNA, Okaiyeto SO (2015). Awareness, knowledge and practices of pastoralists towards contagious bovine pleuropneumonia in Kaduna State, Nigeria. J. Vet. Med. Anim. Health 7(9):296-301.
Crossref
|
|
|
|
|
Boelaert F, Speybroeck N, de Kruif A, Aerts M, Burzykowski T, Molenberghs G, Berkvens, DL (2005). Risk factors for herpesvirus-1 sero-positivity. Prev. Vet. Med. 69:285-295.
Crossref
|
|
|
|
|
Daniel G, Abdurahaman M, Tuli G, Deresa B (2016). Contagious bovine pleuropneumonia: Seroprevalence and risk factors in Western Oromia, Ethiopia. Onderstepoort J. Vet. Res. 83(1):a958.
Crossref
|
|
|
|
|
Dejene W (1996). Contagious bovine pleuropneumonia (CBPP): Prevalence and evaluation of post-vaccination immune response (North Omo, Konso and Dirashe Regions/Ethiopia). Thesis for degree of Doctor of Veterinary Medicine, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia.
|
|
|
|
|
DMDAO (2015). Dello Mena District Agricultural Office.
|
|
|
|
|
FAO (2002). Recognizing contagious bovine pleuropneumonia. Food and Agriculture Organization of the United Nations Animal Health Manual, FAO, Rome. 13:3-17.
|
|
|
|
|
Gashaw T (1998). Epidemiological survey of CBPP in Awi and Western Gojjam zone of Amhara Region and comparison of CFT and C-ELISA for the diagnosis of CBPP. Thesis for degree of Master of Veterinary Epidemiology, Faculty of Veterinary Medicine, Addis Ababa University and Freie Universität, Berlin.
|
|
|
|
|
Gedlu MG (2004). Serological, clinical and participatory epidemiological survey of contagious bovine pleuropneumonia in Somali Region, Ethiopia. MSc Thesis. Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia. P 3.
|
|
|
|
|
Ikpa LT, Ankeli PI, Jambalang AR, Atuman YJ, Akalusi Y, Nwankpa N (2010). Detection of Contagious Bovine Pleuropnuemonia (CBPP) in mixed cattle breed farm in Bauchi state, Nigeria. In: the proceedings of the 47th Nigerian Veterinary Medical Association (NVMA) Conference held in Markurdi, Benue State on Monday 4th-Friday 8th October, 2010. pp. 171-172.
|
|
|
|
|
Issa A (2004). Epidemiological study of contagious bovine pleuropneumonia in Borena pastoral area using complement fixation test and competitive enzyme-linked immune sorbent assay (ELISA). MSc Thesis. Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia.
|
|
|
|
|
Kassaye D, Molla W (2013). Seroprevalence of contagious bovine pleuropneumonia at export quarantine centers in and around Adama, Ethiopia. Trop. Anim. Health Prod. 45:275-279.
Crossref
|
|
|
|
|
LeGoff C, Thiaucourt F (1998). A competitive enzyme linked immune sorbent assay for the specific diagnosis of contagious bovine pleuropnuemonia. Vet. Microbiol. 60:179-191.
Crossref
|
|
|
|
|
Litamoi JK (2000). Overview of contagious bovine pleuropneumonia vaccine production and quality in Africa. In: Report of second meeting of the FAO/OIE/OAU/IAEA consultative group on contagious bovine pleuropneumonia (CBPP). Rome, Italy.
|
|
|
|
|
Mariner JC, McDermott J, Heesterbeek JAP, Thomson G, Martin SW (2006). A model of contagious bovine pleuropneumonia transmission dynamics in East Africa. Prev. Vet. Med. 73:55-74.
Crossref
|
|
|
|
|
Mtui-Malamsha NJ (2009). Contagious Bovine Pleuropneumonia (CBPP) in the Maasai ecosystem of south-western Kenya: Evaluation of seroprevalence, risk factors and vaccine safety and efficacy. A thesis submitted for the degree of Doctor of Philosophy College of Medicine and Veterinary Medicine. The University of Edinburgh.
|
|
|
|
|
NAHRC (2000). Report on contagious bovine pleuropneumonia study in Ethiopia. Sero surveillance results, National Animal Health Research Centre Ethiopian Agricultural Research Organization (EARO), Sebeta, Ethiopia.
|
|
|
|
|
OIE (2001). Diagnostic tests for contagious bovine pleuropneumonia (CBPP). Report of the scientific Committee on Animal Health and Animal Welfare. Office International des Épizooties (OIE), Paris. 2-8.4.
|
|
|
|
|
OIE (2014). Contagious bovine pleuropneumonia. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris, France: World Organization for Animal Health; Chapter, 2.4.9.
|
|
|
|
|
Olabode HOK, Mailafia S, Adah BMJ, Nafarnda WD, Ikpa LT, Jambalang AR, Bello RH (2013). Serological evidence of contagious bovine pleuropneumonia antibodies in trade cattle (Bos indicus) sold in Kwara state-Nigeria. Online Int. J. Microbiol. Res. 1(1):14-19.
|
|
|
|
|
Otte MJ, Nugent R, Mcleod A (2004). Transboundary animal diseases: Socio-economic impacts and institutional response. FAO Livestock policy discussion paper 9.
|
|
|
|
|
Regassa F (2001). Herd prevalence of contagious bovine pleuropneumonia, bovine tuberculosis and dictyocaulosis in Bodji
|
|
|
|
|
Rovid SA (2008). Contagious bovine pleuropneumonia. The center for food security and public health, Iowa State University. College of Veterinary Medicine. http://www.cfsph. A state. Edu. Accessed July, 2015.
|
|
|
|
|
Schnier C, Mtui-Malamsha NJ, Cleaveland S, Kiara H, Grace D, McKeever DJ (2006). Contagious bovinepleuropneumonia seroprevalence and associated risk factors in the Maasai ecosystem of south-western Kenya. Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, August 6-11, 2006, Cairns, Australia.
|
|
|
|
|
SDAO (2015). Sawena District Agricultural Office
|
|
|
|
|
Tahir G (2001). Current mandates, activities, achievements, constraints and recommendations. A position paper presented at National Workshop on the strengthening the livestock component of the Unified Agricultural Extension System (UAES). Organized by the National Livestock Projects Division, Department of Livestock and Pest Control Services, Federal Ministry of Agriculture and Natural Resources, held at Arewa House Kaduna, 14th May.
|
|
|
|
|
Takele G (1998). Epidemiological survey of contagious bovine pleuropneumonia in Awi and Western Gojam zone of Amhara Region and comparison of CFT and C-ELISA for the diagnosis of contagious bovine pleuropneumonia. MSc Thesis. Addis Ababa University and Free University of Berlin.
|
|
|
|
|
Tambi EN, Maina OW (2004). Regional impact of contagious bovine pleuropneumonia in Africa. In: Regional Workshop on Validation of Strategies to Control CBPP in Participative PACE countries. Conakry, Guinea.
|
|
|
|
|
Tambi NE, Maina WO, Ndi C (2006). An estimation of the economic impact of contagious bovine pleuropneumonia in Africa. Rev. Sci. Technol. Off. Int. Epiz. 25(3):999-1012.
Crossref
|
|
|
|
|
Tambuwal FM, Egwu GO, Shittu A, Sharubutu GH, Umaru MA, Umar HU, Mshelia PC, Garba S (2011). Vaccination coverage and prevalence of contagious bovine pleuropnuemonia (1999-2008) in two transboundary states of north-western Nigeria. Nig. Vet. Med. J. 32:169-173.
|
|
|
|
|
Thrusfield M (2007). Sample size determination. In: Veterinary Epidemiology. 3rd ed., UK: BlackwellScience Ltd. pp.185-189.
|
|
|
|
|
Wade A, Yaya A, El-Yuguda AD, Unger H, Nafarnda Daniel W, Ikechukwu ES, Egwu GO (2015). The Prevalence of Contagious Bovine Pleuropneumonia in Cameroon: A Case Study Garoua Central Abattoir, Cameroun. J. Vet. Med. Res. 2(4):1029.
|
|
|
|
|
Wondimu D (1996). Contagious bovine pleuropneumonia: Prevalence and evaluation of post vaccination immune response (North Omo, Konso and Dirashe Regions/Ethiopia). DVM Thesis. Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia.
|
|
|
|
|
Yaya A, Manso-Silvan L, Blanchard A, Thiaucourt F (2008). Genotyping of Mycoplasma mycoides subsp. mycoides small colony by multilocus sequence analysis allows molecular epidemiology of contagious bovine pleuropneumonia. Vet. Res. 39:14.
Crossref
|
|