Five and ten percentage rates of leaf powder of Calpurnia aurea (Ait.) Benth and Millettia ferruginea (Hochst) Baker as well as red and white local inert dusts each and their integration with resistance varieties of maize were evaluated as protectant against Sitophlus zeamais under laboratory condition. All treatments of the tested management tactics induced significantly (p < 0.05) higher adult mortality of weevils (8.33 to 71.67) at both tested rates (5 and 10%) in 2 to 4 days of post treatment application than untreated check. The maximum of these were being in integrated treatments, followed by the C. aurea and M. ferruginea, while relatively the minimum were red and white inert dusts treatments, respectively. All of the treatments of integration and other tactics applied at tested rates were also induced significantly (p < 0.05) higher (≥63.16%) inhibition of F1 progeny emergence, higher reduction in grain damage (≤6.67) and weight loss (≤1.12) of maize grain due to S. zeamais than negative control, the maximums of which were caused by integration, followed by the two botanicals, while relatively minimums were induced by inert dusts. Thus, all the management tactics tested at rates of 5% and above (10%) were potent in preventing maize grains against maize weevils and thus, they could be used in managing maize weevils at the rate of 5 and 10% under farmer’s storage conditions in Ethiopia.
Cereal crops play a major role in the livelihoods of smallholder farmers’ in sub-Saharan Africa (SSA), of which maize (Zea mays) being the most important food and cash crop for millions of people in the region (Midega et al., 2016). Besides, maize is the most important basic food grain, providing food and income to millions of resource-poor smallholders in Eastern and Southern Africa region in general and Ethiopia in particular (Tefera et al., 2011). However, in Ethiopia among other things, food security has been greatly threatened by excessive post-harvest losses grains like maize caused by storage insect pests, mainly of the maize weevil and Angoumois grain moth (Worku et al., 2012). Control of insect pests of stored grains in Africa including Ethiopia have been heavily relied on use of synthetic chemical insecticides among resource poor farmers who lack technical knowledge in the safe handling and use of them (Mvumi et al., 1995; Mvumi and Stathers, 2003). But, environmental pollution, high cost of application, direct toxicity to users, adverse effects on beneficial and non-target organisms, and increased risk to workers safety (Ofuya and Longe, 2009) have been associated as the negative attributes related to their repeated use. In addition to their health and environmental concerns, insecticides are too expensive or frequently unavailable for subsistence farmers in Africa (Demissie et al., 2008). All the aforementioned facts, along with the desire for residue-free grains by consumers, indicate the presence of urgent need for searching and developing of safe, cheap and ecologically sound management alternatives such as botanicals, inert dusts, varietal resistance and integrated pest management (IPM). Thus, the current investigation was initiated with the following objectives: to evaluate the efficacy of integration of two botanicals (Calpurnia aurea and Millettia ferruginea) and inert dusts (red and white locally available inert dusts) with resistance varieties (Melkasa-6Q, MH-138Q and SPRH) relatively to their respective pure (unitary) tactics against Sitophlus zeamais at selected rates under laboratory condition.
The study period
The study was conducted between 1 October 2016 and 30 June, 2017.
Test insect’s culture
S. zeamais adults were collected from maize grains stored in various farmers traditional storage facilities of major maize producing localities Shashogo and Sankura districts of Southern Ethiopia (Figure 1) and brought to the laboratory of Insect Science Insectary of Zoological Science Department, Faculty of Life Science, Addis Ababa University of Ethiopia. These test insects were cultured at 27 ± 3°C and 55 to 70% RH (Jembere et al., 1995; Zewde and Jembere, 2010). Shone variety of maize grains were also obtained from farmer’s storages of the aforementioned districts (Figure 1). It was the most commonly grown hybrid in the region and considered to be susceptible to insect infestation. The grains were kept at -20±2°C for 2 weeks to kill any infesting insects. Broken kernels and debris were removed and the grains were graded manually, where similar sized grains were selected for the experiment (Gemechu et al., 2013). Following the methods by Zewde and Jembere (2010), fifteen pairs of the adult of the test insects were placed in 12, 1-L glass jars containing 250 g disinfested seeds. The jars were then covered with nylon mesh and held in a place with rubber bands to allow ventilation and to prevent the escape of the experimental insects. The parent of the test insects were sieved out after an oviposition time of 13 days. Then, the jars were kept under laboratory condition until F1 progeny emergence. The F1 progeny, which emerged after 30 days, were sieved out and used for the experiment.
Description of the tested materials and their preparation
Botanicals
Plant materials (that is leaves) used for the study (Figure 5) were collected from natural habitats of Hadiya zone, Southern Ethiopia (Figure 1) and the identities of the plants were confirmed into C. aurea and M. ferruginea species at the national herbarium of Life Science Faculty of Addis Ababa University.
Dried and ground materials
Following the methods by Gebre-Selase and Getu (2009), the fresh plant materials (leaves) of a known weight were kept in a well-ventilated room under shade for 2 to 3 weeks depending on weather conditions and dried. Dried materials were ground to fine powder using mortar and pestles. Then they were sieved to remove larger material (Figure 5) (Tekie, 1999; Jembere, 2002).
Admixture bioassay with botanicals
Following the methods by earlier scientists (Gebre-Selase and Getu, 2009; Zewde and Jembere, 2010), 100 g of disinfected maize grains of shone varieties (that were disinfested using the same procedure as indicated in insect culture section) were introduced into 1 L glass jars that were treated differently with the powdered peels of the test plant (that is 5% and 10% leaf powder of the test plant) for treatment of powder (Figure 5). Malathion (5%) dust was used as the positive control at a dosage of 0.05 g/100 g maize grain and untreated grains were served as the negative control. The jar contents were shaken thoroughly for 5 min to ensure uniform distribution of the treatments with grain surface. After treatment, 20, three to seven day old experimental insects of unsexed were introduced to the treated and untreated seeds in the glass jars. The jars were covered with nylon mesh and held in place with rubber bands. The treated and control grains were then kept under same experimental condition indicated in insect capture section. All treatments of powders were arranged in Completely Randomized Design (CRD) in three replications.
Inert dusts
Red and white inert dusts (Figure 4) that have been used by local people for painting of houses in rural areas were obtained from rocky area of Hadiya zone of South Ethiopia (Figure 1). These were sieved to remove larger material. These inert dusts were then applied at the rate of 5 and 10% (w/w) which are equivalent to 5 g/100 g and 10 g/100 g of maize grains following similar procedures by Tadesse (2003) and Demissie et al. (2008).
Treatment application of the inert dusts
Following similar methods used by Tadesse (2003) and Demissie et al. (2008), the aforementioned two different rates of each inert material (Figure 4) were weighed and added to each 1 L glass jars containing 100 g of disinfested maize grains and shaken well to get a uniform coating. Treated and untreated controls were included. In the treated control, Malathion 5% dust at recommended rate of 0.05% (w/w) was used. In the untreated control, neither the inert dusts, nor Malathion 5% dust was used. After treatment, 20 pairs of three to seven day old experimental insects of unsexed were introduced to the treated and an untreated seed in the glass jars. Then, the jars were covered with nylon mesh and held in place with rubber bands. The experiments were laid up in a Completely Randomized Design (CRD) in three replications consisting of the two colored inert dusts in two rates of application.
Integration of botanicals inert dusts and resistant varieties treatment application
Following the methods by Gebre-Selase and Getu (2009) and Zewde and Jembere (2010), 100 g of disinfected the top three resistant varieties of maize grains (Figure 6), were placed in 1 L glass jars, and were treated differently with 1.25 and 2.5% doses (rates) of the leaf powders of each of the test plants (Figure 5). Each plastic jars containing a total of 100 g of grains treated with respective concentrations plant leaves powder was also be admixed with the aforementioned selected rates each of the two colored inert dusts (red and white) (Figure 4) following similar procedures adopted by Ibrahim (2017) and Ibrahim and Sisay (2012), that is, each botanicals and inert dusts were integrated at the rates 5% and 10% with 100 g of the three resistant varieties. Untreated grains of the aforementioned three varieties were kept under similar conditions and served as a negative control. Besides, Malathion (5%) dust at recommended dose of 0.05% (w/w) was served as positive standard cheek. The jars were then covered with nylon mesh and held in a place with rubber bands to allow ventilation and to prevent the escape of the experimental insects. The corresponding glass jars were shaken well for five minutes in order to have a uniform mixture of treatments (Jembere et al., 1995; Zewde and Jembere, 2010). Twenty randomly picked newly emerged 3-7 days unsexed adult experimental insects were introduced to each treated and untreated jars and were kept for fourteen days of oviposition in the laboratory at 27 ± 5oC and 55-70% RH (Derera et al., 2001; Tadesse and Basedow, 2005; Jembere et al., 1995; Zewde and Jembere, 2010). Each experiment was arranged in a completely randomized design with three replications.
Adult mortality assessment of the experiments
The adult mortality counts were performed after 1, 2, 3 and 4 days after treatment exposure. All dead and live adults were taken away from the jars after thirteen days of oviposition.
F1 progeny assessment of the experiments
The treated and control grains were kept until emergence of F1 progeny under same experimental condition indicated in insect culture section. Then the numbers of F1 progeny produced by the experimental insects were counted. Counting were stopped after 56 days from the day of introduction to avoid overlapping of generation following similar procedures of previous scientists (G/selase and Getu, 2009; Zewde and Jembere, 2010).
Damage and weight loss assessment of the experiments
Two days after the last F1 count of 56 days, samples of 30 grains were taken randomly from each jar and the number of damaged (grains with characteristic hole) and undamaged grains were counted and weighed. Damaged grains were expressed as a percentage of the total number of seeds in each replicate. Percentage weight losses were calculated by count and weight method following similar procedures by of previous scientists (Gebre-Selase and Getu, 2009; Zewde and Jembere, 2010) as:
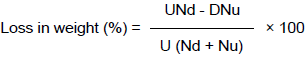
where U = weight of undamaged grain, D = weight of damaged grain, Nd = number of damaged grain and Nu = number of undamaged grain
Percentage protection of the experiments
Following similar procedures by previous scientists (G/selase and Getu, 2009), percent protection or inhibition rate in F1 progeny emergence (% IR) was calculated using the following formula:
IR (%) = (Cn-Tn) ×100 / Cn
where Cn is the number of newly emerged insects in the untreated (control) jar and Tn is the number of insects in the treated jar.
Data analysis
Data on adult mortality, F1progeny emergence, and grain damage and weight loss were managed with the Microsoft Excel version 2013 and then were subjected to analysis of variance (ANOVA) of SPSS Version 16. The effect of treatments of unitary tactics and integration on the aforementioned parameters was analyzed using appropriate statistical method, Univariate or two-way analysis of variances. Significant differences between means of different treatments and time of exposure were separated using Tukey's studentized (HSD) test at 5% confidence interval. Difference among means were stated significant when p<0.05 and highly significant when p<0.01. Correlation between the treatments and the efficacy measuring parameters like weight loss and others were determined using Pearson’s correlation of SPSS program of version 16.
Comparison of integration with others in terms parental weevils mortality
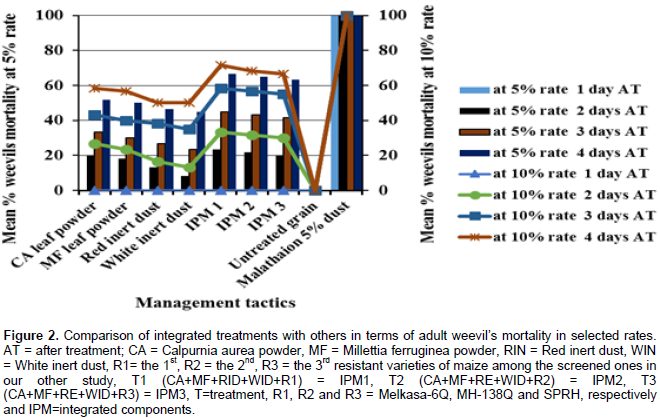
Percentage adult weevil’s mortalities were found to be increased both with increased dosage (concentration) and days after treatment exposure for all treatments of all tested tactics, the maximum were being in integrated treatments, followed by the two botanicals leaf powders, while the minimum were in inert dusts in all of 1 to 4 days (Figure 2). All treatments of the tested management tactics induced significantly (p < 0.05) higher adult mortality of weevils (8.33 to 71.67%) at both of the selected rates (5 and 10%) in 2 to 4 days of post treatment application than untreated check. The maximum of these was being in integrated treatments, followed by the C. aurea and M. ferruginea, while relatively the minimum was red and white inert dusts treatments, respectively. Besides, significantly (p < 0.05) higher weevil’s mortality (45.00 to 71.67) was also observed in all treatments of all the tested management tactics applied at both selected doses, 4 days after treatment exposure, followed by 3 days after treatment application (23.33 to 58.33) than 2 days after treatment (8.33 to 33.33). However, no adult weevil’s mortality was recorded in all treatments of the tested tactics applied at both the selected rates 1 day after treatment (Figure 2).
Comparison of integrated treatments with others in terms of F1 progeny emerged, percent protection, percent grain damage and weight loss
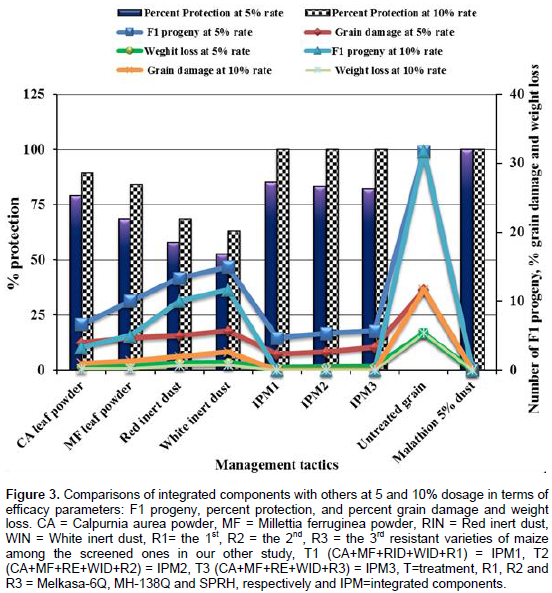
The number F1 progeny emerged, percent grain damage and weight loss were found to be significantly (p < 0.05) lower in all treatments of the tested management tactics than untreated check (Figure 2). All of the treatments of integration and other tactics applied at all rates also induced significantly (P < 0.05) higher (≥ 63.16%) inhibition of F1 progeny emergence, higher reduction in grain damage (≤ 6.67) and weight loss (≤ 1.12) of maize grain due to S. zeamais as compared to negative control. The maximums of these were caused by integration treatments, followed by the two botanicals tested, while relatively the minimums were induced by inert dusts. Besides, hundred percentage F1 progeny emergence inhibition, no percent grain damage and weight loss of maize grains were observed in all of the treatments of the integration tactics applied at dosage of 10% likewise that of Malathion 5% dust (Figure 3).

The present study has revealed significantly higher mortality of maize weevils 4 days after treatment exposure in all treatments of integration and other tactics tested at 5 and 10% doses, the maximums that were being in integration, followed by botanicals, while relatively the minimum was in inert dusts. This finding suggests that all the tested control tactics can be used in maize management maize weevils. Similarly, Bayih (2014) also reported that the unitary and binary botanical formulations at lower and higher rates were effective against Zabrotes subfasciatus. Besides, it was indicated that the powders of Plectranthus glandulosus and Azadirachta indica in isolation as well as at their different proportions of their binary combinations generally caused significant mortality to adult Callosobruchus maculatus and S. zeamais compared to the controls (untreated grain) (Katamssadan, 2016). It was also reported that combinations of different rates of Malathion 5% dust and neem seed powder caused higher weevils mortality than the untreated control (Ibrahim and Sisay, 2012). In his preliminary study, Demissie (2006) also reported that the combined use of minimum rates of Chenopodium plant powder, botanical triplex, silicosec and filter cakes with weevil tolerant varieties has reduced grain damage at Bako of Ethiopia. Ibrahim (2015) also indicated that the rates of mortality of weevils in all of the combinations of Malathion dust and Mexican tea powder and their respective pure treatments were significantly higher than that of untreated check. It was also shown that the combination of neem seed powder and Malathion at the proportions of 40+20% and 50+10% on maize were additive with respect to the mortality caused to Sitotroga cerealella Olivier (Ibrahim, 2014).
The present study has also shown that the integration the different management tactics tested induced significantly higher protection of maize grains against weevils attack than unitary tactics tested for about two months. This might be due the enhanced mortality effect in integrated treatments than unitary tactics tested, which in turn might be probably due to the presence of great possibility of synergism of morality factors in integration than those applied unitarily. Similarly, it was also reported that for the reason of their synergetic effects, integration of one management method with other sustainable method could provide long lasting solution to losses in storage (Dobie, 1977). Besides, it was also reported that combining (integrating) of different botanicals has enhanced their potency in controlling stored grain insect pests than those applied unitarily (Agona and Muyinza, 2003; Bayih, 2014). In his preliminary study, Demissie (2006) also reported that the combined use of minimum rates of Chenopodium plant powder, botanical triplex, silicosec and filter cakes with weevil tolerant varieties has reduced grain damage at Bako of Ethiopia. Katamssadan (2016) also indicated that the binary combinations of the powders reduced progeny emergency more than each botanical applied alone, with Neem Azal being more potent than P. glandulosus. According to him, all the binary combinations of the powders completely suppressed progeny production in S. zeamais. Ibrahim (2017) also indicated that integrating neem seed and Mexican tea powder provided significant protection to maize from the maize weevil. It was also reported that combinations of different rates of Malathion 5% dust and neem seed powder caused higher weevils mortality than the untreated control (Ibrahim and Sisay, 2012).
From the present study, it is possible to conclude that all the management tactics tested at the rates of 5% and above (10%) were potent in preventing maize grains against maize weevils attack, though integration was the most effective among them, followed by botanicals and inert dusts. Thus, all of the tested tactics could be used in managing maize weevils as safe, ecologically sound and cheap management alternative to synthetic chemicals at a rate of 5% and above (10%) under subsistence farmer’s storage conditions in the study area and Ethiopia in particular and elsewhere with similar pest problems in general. Besides, it also confirmed that integration rather than unitary one could be used under subsistence farmer’s storage conditions to achieve better protection of stored maize against maize weevils.
The authors have not declared any conflict of interests.