ABSTRACT
Faba bean, Vicia faba L. is an important legume crop that substitute animal proteins especially in developing countries. Bean bruchids or bean weevil, Accanthoscelides obtectus Say is a major insect pest of the faba bean grains mainly in the store. An experiment was conducted in Debremarkose University to screen effective botanical oils for the management of bean bruchids on faba bean grains. The tested oils were from seeds of Noug (Guizotia abyssinica L. f.), Rapeseed (Brassica napus L.), Lantana (Lantana camara L.), Pepper (Capsicum annum L.), Tephrosia (Tephrosia vogelii Hook), Groundnut (Arachis hypogaea L.) and Castor (Ricinus communis L.). The untreated check, Acetone (solvent used to extract oils from the botanicals) and Ethiothion 5% dust (a standard check) were used for comparison. One day after treatment application, the dead bruchid in Ethiothion 5% dust, noug oil and rapeseed oil treated grains were 100, 90.85±0.63 and 78.35±0.78%, respectively indicating the fast knock down effect of the treatments. Four days after treatment, 100% of the parent bruchids were killed in all oil treated grains. The mortality of the parent bruchids four days after treatment application was 10.85+0.6% in acetone treated grains and 11.65±0.5% in untreated grains. Statistically lower number of eggs and progeny (adults) were recorded from Ethiothion 5% dust and oil treated grains than the acetone treated and untreated grains. Bean grain losses due to bruchids were 18.3±0.4% in acetone treated grains and 18.4±0.44% in the untreated check, while 0 to 5.2±0.27% losses were recorded in other treatments. Ethiothion 5% dust and the oils inhibited emergence of bruchids from 71.7±2.56 to 100±0.53%. Lower germination percentages were recorded from acetone (67.3±4.23%) treated grains and the untreated grains (66.7±3.02%). From the results of this experiment, it can be concluded that the Ethiothion 5% dust and all the tested oils can effectively control bean bruchid and recommended for the management of the same.
Key words: Bean bruchid/weevil, botanical oil, faba bean grain (Vicia faba L.), mortality, survival, progeny.
The faba bean, Vicia faba L. is one of the earliest domesticated legumes after chickpea and field pea which was vastly distributed throughout the world (Kanaji, 2007). It is an important crop used as a source of organic protein for peoples who cannot afford feeding expensive animal protein on regular basis (El-Tokhy and Kasem, 2012). However, bean storage at small scale subsistence farming level is limited due to bruchid infestations that result in heavy losses (Mushobozy et al., 2009). The common bean weevil, Acanthoscelides obtectus (Say) and the Mexican bean weevil, Zabrotes subfasciatus (Boheman) are the common insect pests in a wider area where faba bean is growing (Mushobozy et al., 2009; Taponjou et al., 2002). Their attack results in yield reduction, poor grain quality, loss of seed viability and unsuitable taste for human consumption (Prakash et al., 2008).
Farmers are using synthetic insecticides to keep faba bean grains free of insect infestation and to reduce damage during storage (Taponjou et al., 2002). They use insecticides without having appropriate knowledge on its application and drawbacks like development of resistance, environmental pollution and poisoning of fauna and flora (Prakash et al., 2008; Taponjou et al., 2002). To date, attentions were diverted to look for effective, biodegradable, simple to apply and homemade control options such as oil extracts of botanical origin for the management of bruchids (Olotuah, 2013). Oils of many plant species are known to have one or more insecticidal properties in terms of pest control such as fumigants, repellents, toxicant and oviposition deterrent among others (Mushobozy et al., 2009; Olotuah, 2013).
In Ethiopia, specifically in Amhara region small scale farmers are facing problems of storing their bean grains due to damage by A. obtectus. The faba bean grains are prone to damage by A. obtectus starting from field at the maturity of the crop and extended to storage where heavy infestation occurs. In the region, the availability of plants with insecticidal value is tremendous. Hence, the objective of this study was to screen effective botanical oils for the management of A. obtectus on the faba bean grains.
Description of the study area
The study was conducted in Eastern Gojjam zone, Debre Markose University located 300 and 265 km away from Addis Ababa and Bahir Dar, respectively. The average elevation of the study area was 2400 masl. The rainfall pattern was uni-modal with a mean annual rainfall of 1500 mm. Daily average temperature was 24°C. The major crops grown are Teff, wheat, maize, faba bean, chickpea and rough pea.
Rearing of A. obtectus
Cultures of A. obtectus were established at Debremarkose University to obtain the same age group and required numbers of adult bruchids for the experiment. The bruchids used for rearing were collected from local farmer faba bean stores and reared in three plastic pots having 5 L capacity each. Each pot was half filled with 3 kg grains to serve as food for the bruchids. Newly harvested faba bean grains with no bruchid eggs were collected from local market. The grains were carefully examined by hand lens for the presence of bruchid eggs. The selected grains were washed with potable water to avoid any obscure sources of infestation and frozen at a temperature of -4±1ºC for three weeks to protect fungal development and to ensure uniform moisture content of the grains (Ileke and Olotuah, 2012). Grains having 14% moisture contents were used as a substrate for A. obtectus rearing (Kanaji, 2007).
About 100 unsexed adults of A. obtectus were added to each jar assuming that 50% of the adults are females and the rest are males as the sex ratio for the series of sex ratio experiments was 1:1 (Ileke and Olotuah, 2012). The jars were covered with muslin cloths held in place by rubber bands. Rearing was conducted at 28±1.5ºC and 65±5% RH (Ileke and Olotuah, 2012; Kanaji, 2007). The temperature used for rearing was adjusted by electric power, while the follow up record was taken by Thermo hygro and a thermometer. Frequent inspection of the culture for emergence of the progeny was carried out daily starting from twenty two days after parent bruchids introduction (Bhardwaj and Verma, 2012). The newly emerged one day old adult F1 progenies were used for the experiment (Bhardwaj and Verma, 2012).
Treatment preparation
The botanical seeds were collected from the localities nearby Debre Markos University. Oils were extracted from various plant species using acetone. The plant species used for the experiment were seed of Noug (Guizotia abyssinica L.f.), rapeseed (Brassica napus L.), Lantana (Lantana camara L. (Sensu lato)), pepper (Capsicum annum L.), Tephrosia (Tephrosia vogelii Hook.), Groundnut (Arachis hypogaea L.) and Castor oil (Ricinus communis L.) (Table 1). Untreated check, Ethiothion 5% dust (standard check) and Acetone (a solvent to extract the oils) were used in the experiment for comparison.
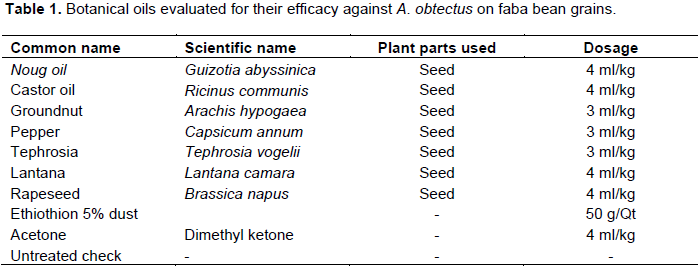
The seeds were dried under shade and grinded with pestle and mortar to obtain fine dusts. The dusts were further grinded until it passed through 0.5 mm perforated sieves (Ileke and Olotuah, 2012). The dusts were dissolved in acetone in a two liters plastic pot to separate oils from fine dusts. Each mixture was stayed for one month and shaken daily for 15 min (Bhardwaj and Verma, 2012). The liquid that contains oil and acetone was float on the upper surface of the containers. Each container was pierced with scissor at the site of layer formation between dust residues and the floated liquids. The liquids that float on the surface of the containers were come out through the hole prepared between the layers. Each liquid was collected and filtered again using muslin cloths. The oils and solvents were exposed to air for two days to evaporate the solvent (Acetone). Subsequently, the oils collected from each botanical were stored separately in clean vials at a temperature of 4°C in a refrigerator until use (Ileke and Olotuah, 2012).
A concentration of 3 to 4 ml/kg oils were added in one litter plastic pots containing 500 g of the faba bean grains. The pots containing faba bean grains, the botanical oils, Ethiothion 5% dust and acetone were vigorously shaken and rolled for one minute to coat the treated grains uniformly (Ileke and Olotuah, 2012).
Effects of botanical oils on parent adult bruchid fertility and mortality
The treated bean grains in each pot were infested with 24 hour old 20 unsexed adult of A. obtectus (Ileke and Olotuah, 2012). The pots were covered with muslin cloth to allow sufficient aeration, to protect escape of A. obtectus and entrance of other pests. The experimental design was a Completely Randomized Design (CRD) in three replications. Parent adult A. obtectus mortality and the number of eggs laid per 20 grains were recorded from each pot 1, 2, 4, 8 and 10 days after treatment application. In each subsequent count, dead bruchids were counted and removed from each pot. Ten days after treatment application all dead and alive bruchids were counted and removed from the pots.
Emergence of adult progeny of A. obtectus
Bruchid progenies inspection and continuous follow up started from 22 days after treatment application. Subsequent counts of dead and alive adult bruchids were done. The bruchids were separated from bean grains with the help of three mm sieve made from wire mesh (Swella and Mushobozy, 2007). Data on grains with and without eggs per 20 randomly selected grains were taken for three months with ten days interval.
Percentage protection
Protective efficacy of the botanical oils was calculated based on the emerged progenies using Taponjou et al. (2002) and El-Ghar et al. (1987) procedures. Percentage reduction to adult emergence or inhibition rate (% IR) was calculated as:
Where: Cn = the number of newly emerged insects in the untreated pot, and Tn = the number of newly emerged insects in the treated pot.
Seed weight loss determination
On the 90th day of treatment application, 1000 grains were randomly taken from each treatment. The selected grains were separated and categorized into damaged grains with exit holes and undamaged grains. Grains with and without exit holes were counted and weighed separately and the obtained data were used to calculate the percentage weight loss. Percentage weight losses were determined by the count and weigh method applied by Gwinner et al. (1996):
Where: Wμ = weight of undamaged grains; Nμ = number of undamaged grains; Wd = weight of damaged grains; Nd = number of damaged grains.
Germination test
The effect of botanical oils on faba bean grain germination was done three months after treatment application. Faba bean grains from the untreated check, acetone and the standard check were used for comparison. Fifty grains were randomly selected from each treatment. Grains from each treatment were treated separately with sodium hypochlorite (Clorox 10%) for one minute to eliminate fungal contamination. Grains treated with sodium hypochlorite were washed by water for one minute to avoid physical damage. The seeds were placed on moist filter paper in petri dish for seven days. The number of sprouted seeds was counted seven days after incubation. Subsequently, germination percentage was determined using the following formula (Gwinner et al., 1996):
Statistical analysis
Analysis of variance (ANOVA) was done according to Gomez and Gomez (1984) procedures. All obtained data were transformed using square-root, logarithmic and arc sign transformations before the analysis. Tukey’s studentized range test (HSD) at p=0.05 was used to separate significant means. SAS (SAS 9.2) and MS Excel 07 soft wares were used for these analyses. Results were reported using back transformed values.
Number of eggs laid by adult parent female bruchid on treated and untreated faba bean grains
The number of eggs laid by parent bruchidson treated (the oils, the Ethiothion 5% dust and the Acetone) and untreated grains are shown in Table 2. Parent bruchids were started mating with in the first days of an experiment due to their eggs were recorded on day one after treatment application. Significantly (p<0.05) higher number of bruchid eggs were laid on untreated check (7.33±0.53) and acetone (7.5±0.68) treated faba bean grains compared to the botanical oils (0.33±0.33 to 3.17±0.48) and Ethiothion 5% dust treated grains which did not yield any bruchid egg one day after treatment. The current result was similar to that of Ibrahim (2012) who reported sesame, olive and sunflower oils effectively decreased the egg laying capacity of C. maculates on chickpea grain starting from treatment application up to the end of the experiment. Similar results were reported by Abdulahi (2011) who tested four levels of Balantie aegyptiaca leaf extract and found that the botanical treatments were an efficient ovipositional deterrent compared to the untreated check. Tabu et al. (2012) conducted similar experiment that bruchids inhibited oviposition on chickpea grains treated by seed powders of Azadiracta indica at 2% (w/w) and Chenopodium ambrosiodes at 4% (w/w) starting from day one of treatment application up to three months.
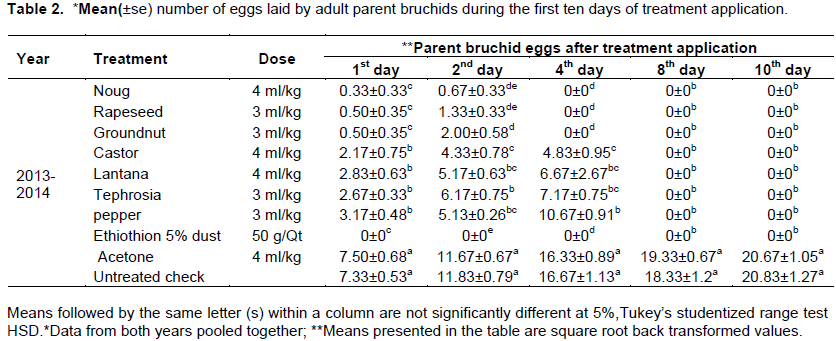
Two days after treatment the number of eggs laid by bruchids on noug oil and rapeseed oil treated the faba bean grains were 0.67±0.3 and 1.33±0.33, respectively. Significantly (p<0.05) higher number of bruchid eggs were laid on pepper (5.13±0.26), lantana (5.17±0.63) and tephrosia (6.17±0.75) than the rest of the oil treatments. The highest number of bruchid eggs were recorded on untreated grains (11.83±0.79) and acetone (11.67±0.67) treated faba bean grains. Similar results were reported by Uddinll and Sanusi (2013) such that no bruchid eggs were recorded on cowpea grains treated with groundnut and palm kernel oils starting from two days after treatment application. Yahaya et al. (2013) reported similar result on chickpea grains treated with groundnut or palm oil at the rate of 2.5 to 3 ml/kg which significantly reduced C. maculates oviposition.
There were no extra bruchid eggs after four days of treatment application than the two days of experiment on Ethiothion 5% dust, noug oil, rapeseed oil and groundnut oil treated grains. Based on Okonkwo and Okoye (2008) finding, essential oils of D. tripetela and P. guineense achieved 100% mortality of C. maculates and S. zeamais in 24 h after treatment application as a result no extra eggs were recorded till death of the parent insects. Statistically higher numbers of bruchid eggs were recorded on oils of castor (4.83±0.95), lantana (6.67±2.6), tephrosia (7.17±0.75) and pepper (10.67±0.91) treated faba bean grains four days after treatment. The numbers of bruchid eggs were significantly higher than the rest of the treatments in untreated (16.67±1.13) and acetone (16.33±0.89) treated grains four days after treatment (Table 2). Yahaya et al. (2013) indicated that, significantly lower number of C. maculates eggs were recorded on cowpea grains treated with botanical oils (0-4.5+1) compared to 62.87+0.2 eggs laid in the control plot.
Eight days after treatment, no extra eggs of bruchids were recorded in Ethiothion 5% dust, and oil treated faba bean grains. However, the numbers of bruchid eggs laid in acetone treated and untreated grains were recorded to 19.33±0.67 and 18.33±1.2 eggs, respectively. The number of A. obtectus eggs laid in acetone (20.67±1.05) treated and the untreated (20.83±1.27) faba bean grains were slightly increased ten days after treatment (Table 2). Ibrahim (2012) remarked that sesame (98.49%), olive (96.54%) and sunflower (95.37%) oils at the rate of 7.5 ml/kg (v/w) were effectively deterring bruchids oviposition on chickpea grain. Comparable result was reported by Shukla et al. (2007) that Murraya koenigii L. and Eupatorium canabinum L. dusts deter bruchid oviposition by 90.62 and 86.46%, respectively. Sangeeta and Apte (2015) reported that green gram grains treated with botanical oils were 100% free from parent
Callosobruchus maculates infestation.
Effects of botanical oils on the survival of adult parent bruchids
The oils killed parent bruchids started from day one after treatment application. Parent bruchids died in Ethiothion 5% dust, noug oil and rapeseed oil were 100, 90.85±0.63 and 78.4±0.78%, respectively within the first day of experiment. 28±0.52 to 70.85±0.85% the parent bruchids were died in groundnut, lantana, tephrosia and pepper oil treated grains. On the contrary, 100% of the parent bruchids were survived in acetone and untreated check treated grains day one after treatment application (Table 3). Olotuah (2013) conducted similar experiment to control C. maculates (F.) that the oils were killed 100% of the parent bruchids with in six minutes while100% of the parents survived in the untreated check. Chickpea grains treated with dusts of A. indica at 2% (w/w) were killed parent bruchids 80, 91.67 and 93 67% after one, two and four days respectively (Taba et al., 2012). Tadele et al. (2014) also confirmed that botanical dusts were completely eradicated soldiers and worker termites a day after treatment. Okonkwo and Okoye (2008) evaluated the efficacy of D. tripetela dusts at 1.5 to 2.5 g/kg to manage S. zeamais on maize grains that 100% of the parent weevils were died at (P < 0.05) within 24 h of treatment.
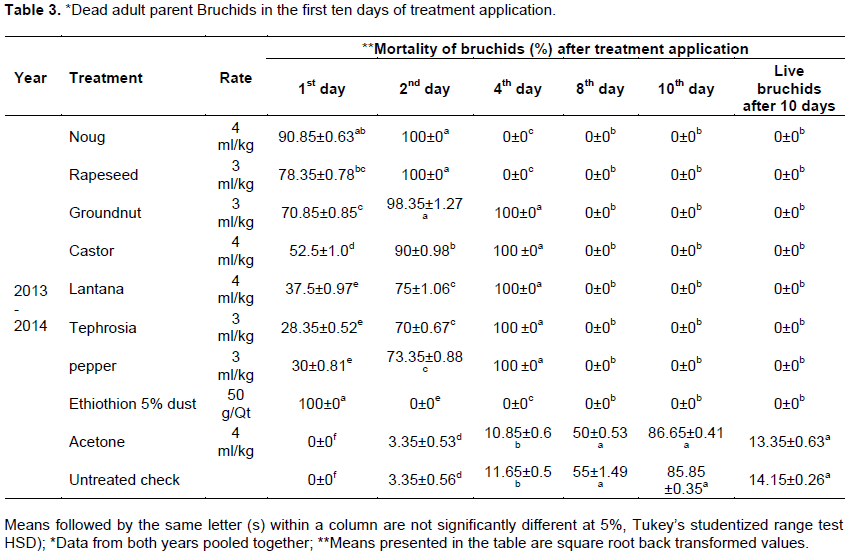
Treatments like Ethiothion 5% dust, noug, rapeseed and groundnut oils were statistically shown similar results that 98.35±1.27% to 100% of the parent bruchids were died after two days of treatment application. The rest of the botanical oils were shown superior efficacy (70±0.67 to 90±0.98%) to kill parent bruchids than acetone treated and untreated check faba bean grains that 96.65% of the parents were continued to exist. Four days after treatment 100% of the parent bruchids were died in Ethiothion 5% dust and oil treated faba bean grains. In acetone and the untreated check 88.65 and 88.35% of the parent bruchids respectively were alive to lay their eggs (Table 3). Similar result was reported by Bhardwaj and Verma (2012) and Tabu et al. (2012) management of beetles by botanical dusts, edible oils and inert materials were appeared to be more effective compared with control after four days of treatment. According to Yohannes et al. (2013) 85 to 100% parent bruchids were eradicated by botanical dusts within seven days after treatment application. C. ambrosiodes leaf dusts at 4% (w/w) killed the parent bruchids 88.34 and 91.67% after two and four days of treatment application (Taba et al., 2012).
The dead bruchids in acetone and the untreated check after eight days of experiment were 50±0.53 and 55%±1.49, respectively. The numbers of dead parent bruchids were increased in acetone and the untreated check with time due to natural death was expected. As a result, the dead parent bruchids were 85.85±0.35% in untreated check and 86.65±0.41% in acetone ten days after treatment application. 14.15±0.26 and 13.35±0.63% of the parent bruchids in the untreated check and acetone treated grains respectively were continued and alive after ten days of the experiment (Table 3). Waktole (2014) was reported similar finding that a dust prepared from Chenopodium spp. was achieved 66.67% mortality of S. zeamais parents at par with Acetellic 2% dust (70.39%). Sangeeta and Apte (2015) report was also confirmed that C. maculatus were not survived in any of the green gram grains treated with botanical oils. Shukla et al. (2007) report indicated that parent bruchids were effectively controlled by M. koenigii and E. canabinum dusts of leaves.
Effects of botanical oils on adult progeny emergence and the capacity of adult progeny in egg laying
Eggs laid by bruchids on bean grains were vary due to various efficacy of treatments. Numbers of eggs laid in noug, rapeseed and groundnut oils were statistically lower (0±0) at par with Ethiothion 5% dust treated grains. Yahaya et al. (2013) reported that palm, groundnut and coconut oils treated chickpea grains at the rate of 4 ml/kg completely inhibited C. chinensis infestation for three months of storage periods. Sangeeta and Apte (2015) indicated that green gram grains treated with botanical oils were free from bruchid eggs one year after treatment application while100% of the grains were infested with the insect eggs within the same period at the same conditions of experiment.
Significantly lower numbers of bruchid eggs were laid on castor, lantana, tephrosia and pepper oil (37.67±3.16 to 98±8.19 eggs) treated grains compared to 498.3±41.5 eggs in untreated check and 493.3±41.1 eggs in acetone treated grains. The numbers of eggs laid in acetone and untreated check were fivefold that of pepper oil shows better numbers of eggs laid than other oil treatments (Table 4). A comparable result was reported by Abdulahi (2011) that the eggs laid by bruchids on cowpea grains treated with actellic 2% and botanical dusts were statistically lower (88.6±4.6 to 203.6+ 5.9) than acetone (798.6±27.4) and untreated check (794.3+15). Shukla et al. (2007) report was in line with the current finding that bruchids were laid lower numbers of eggs on 2% (w/w) leaves dusts of M. koenigii (17.67±5.5) and E. canabinum (22.34±4.5) treated chickpea grains compared to control (127.67±8.05).
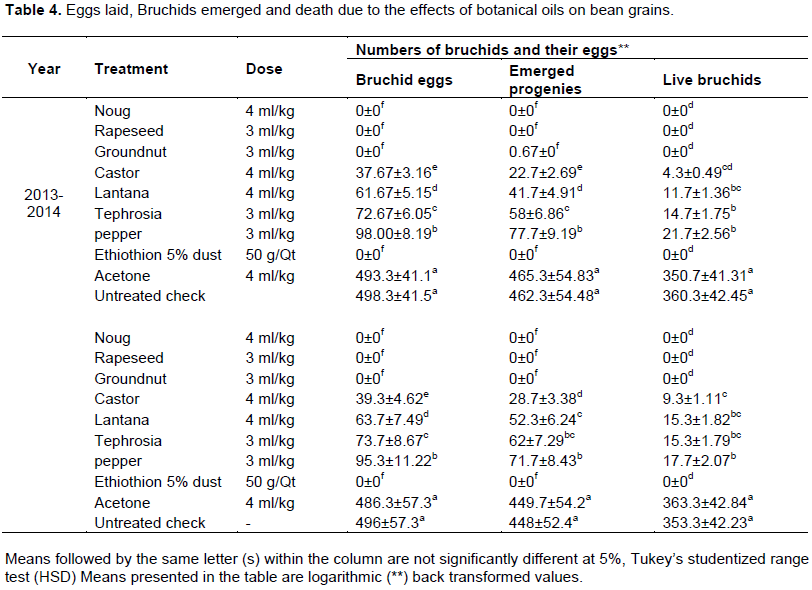
There was no bruchid progeny emerged in oil treatments like noug, Rapeseed, Groundnut and Ethiothion 5% dust treated grains (Table 4). Okonkwo and Okoye (2008) were reported the same result that there was no F1 S. zeamais progeny emerged on maize grains treated with leaves dusts of D. tripetela and Piper guineense till three months of experiment. According to Okonkwo and Okoye (2008) seed oils of D. tripetela and P. guineense treated maize and Cowpea grains were completely suppressed the emergence of F1 progenies and given protection up to 4 months of storage.
The maximum numbers of bruchid progenies emerged from castor, lantana, tephrosia and pepper oils were 28.8±2.69, 52.3±4.91, 62±6.86 and 77.7±9.19, respectively. Statistically higher numbers of bruchid progenies were recorded in acetone (465.3±54.83) and the untreated check (462.3±54.48) (Table 4). Abdulahi (2011) was reported related result on numbers of bruchids emerged from actellic 2% dust and botanical dust treated cowpea grains were ranged from 9.3±1.2 to 40.3±7.9 compared to acetone (698±24.46) and control (672.3±19.6). The similar result was reported by Regmil and Dhoj (2011) that numbers of bruchids emerged in oil and dust treated chickpea grain were significantly lower (0 to 2292±204.6) compared to control (4713±204.6). Tabu et al. (2012) reported similar results on the numbers of progenies emerged from A. indica at 2% (w/w) and C. ambrosiodes at 4% (w/w) were 42 and 24.33 respectively compared to 320 bruchids were recorded from untreated check.
A comparable result was reported by Waktole (2014) that S. zeamais progenies emerged from maize grains treated with Chenopodium leaves dusts at 10% (w/w) and control were 20 and 80% of the recorded eggs respectively. Based on Ibrahim (2012) experiment sesame seed oils treated chickpea grain at 5 and 7.5 ml/kg were decreased adult emergence by 96.03 and 96.22% respectively compared to the untreated check. A similar result was reported by Yahaya et al. (2013), e.g., applications of groundnut or palm oil at the rate of 2.5 to 3 ml/kg on cowpea grains were significantly suppressed C. maculates progeny emergence.
Effect of botanical oils, Ethiothion 5% dust and acetone treatments on the germination and grain weight loss by bean bruchid
The oil treatments and Ethithion 5% dust were statistically shown lower percentage weight loss than untreated check and acetone treated bean grains. There was no weight loss recorded in Ethiothion 5% dust, noug, rapeseed and groundnut oil treated grains. Higher weigh losses were recorded from tephrosia oil (3.2+0.51%) and pepper oil (5.2±0.27%) treated bean grains than the rest of the oil treatments, however, the loss recorded from untreated check was 3.5 to 18.4 folds exceeded than the oil treatments and the standard check (Table 5). Regmil and Dhoj (2011) reported comparable results on chickpea grains treated with oils and botanical dusts were no loss compared to 38.44%+9.43 chickpea grain weight loss was recorded in the control treatments. Loth et al. (2007) were recorded significantly lower bean seed weight loss (0 o 8.54%) in Ngongwe, pyrethrum flower and garlic powder treated grains than 14.64% in the untreated check after three months of experiment.
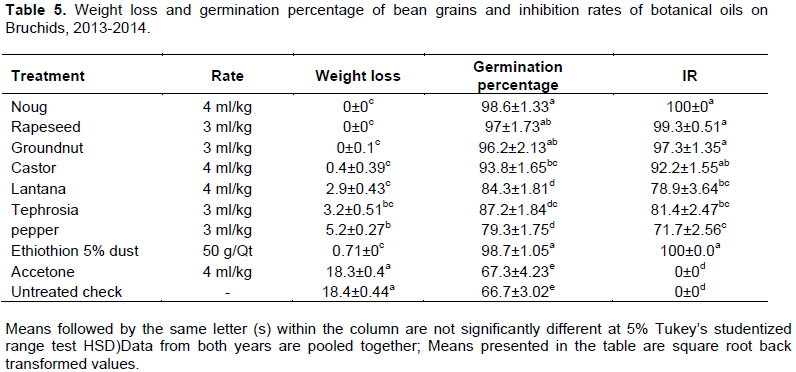
Higher bean grain germination was recorded from Ethiothion 5% dust (98.7±1.05%), noug (98.7±1.33%), rapeseed (97±1.73%) and groundnut oil (96±2.13%) treated grains. All the treatments were statistically shown better bean grains germination than acetone and untreated check after three months of the experiment (Table 5). Treatments like oils and Ethiothion 5% dust inhibited emergence of bruchids from 71.7±2.56 to 100% compared to 0±0% in acetone and untreated check. Mushobozy et al. (2009) were conducted similar research on common bean to manage Zabrotes sabfasciatus that the oils treatments were shown superior efficacy to inhibit emergence of bruchids and better germination (87.5 to 95%) ability of grains. As Taba et al. (2012) reported that on A. indica at 2% (w/w) and C. ambrosiodes at 4% (w/w) seed dust treated chickpea grains were effectively germinated to 100 and 98.33% respectively. Ibrahim (2012) was added that 80 to 93% of the chickpea grains treated with botanical oils were effectively germinated after the 90 days of experiment.
Higher bean grain germinations were achieved form treatments of better inhibition rate on bruchids emergence and their correlation was strongly positive (Figure 1). Okonkwo and Okoye (2008) conducted similar experiment on essential oils of D. tripetela and P. guineense to control C. maculates and S. zeamais and their finding was indicated that 100% of the parent insects mortality were observed within the first 24 h as well as the insects were inhibited to lay their eggs and emerge to adult stages. Tabau et al. (2012) also indicated that A. indica at 2% (w/w) and C. ambrosiodes at 4% (w/w) seed dust treated chickpea grains were inhibited emergence of bruchids 86.85 and 92.36% respectively. In general oils and Ethiothion 5% dust were shown lower faba bean grain weight loss, better germination percentage and better seedling performance even after germination than acetone treated and untreated faba bean grains.
Ethiothion 5% dust and oils were eradicated 30±0.81 to 100% parent bruchids day one after treatment application while 100% of adult bruchids were survived in acetone and untreated check treated grains. The parent bruchids were totally eradicated in Ethiothion 5% dust and oil treated faba bean grains four days after treatment application. The numbers of dead bruchids in acetone and the untreated check treated grains were only 10.85±0.6 and 11.65±0.5%, respectively after four days of experiment. Ten days after treatment application in acetone (13.35±0.63%) treated grains and untreated check (14.15±0.26%) of the parent bruchids were survived. The numbers of eggs laid by parent bruchids during the first ten days of experiment were significantly lower in Ethiothion 5% dust (0±0) and oil (0.67 to 10.67±0.91) treated bean grains. However, very significantly higher numbers of bruchid eggs were laid in acetone (20.67±1.05) and the untreated check (20.83±1.27) and the eggs were continued to lay till ten days of experiment.
Bean grains treated with Ethiothion 5% dust and oils were shown lower numbers of eggs laid by bruchids and lower numbers of progeny emerged adults than acetone and untreated check (Table 4). Statistically lower percentage of bean grain weight loss, no adult bruchids emergence and better germination percentage of faba bean grains were recorded from Ethiothion 5% dust, noug, rapeseed and groundnut oil treated grains. Higher weight losses of faba bean due to bruchids and lower germination percentages of the grains as well as freely emergence of bruchids were recorded from acetone treated and the untreated check grains.
Based on the results recorded, the botanical oils tested were effective to control bruchids from day one to 90 days of experiment. The botanical oils were effectively controlled parent bruchids and their progenies. The numbers of eggs laid by parent bruchids and their progenies were effectively inhibited by the oils evaluated up to the whole durations of the experiment. As a result bean grains treated with the oils were effectively sprouted (79.3±1.75 to 98.7±1.05%).
It is needed to assess efficacy, biodegradability, chemistry of the evaluated botanical oils and their effect on bruchids biology on faba bean and other pulse grains at different agro-ecological zones started from field to storage conditions to be used as one component of Integrated Pest Management (IPM) to manage bruchids and other storage insect pests. The farmers can be used to manage bruchids of based on the evaluated botanical oils due to their availability and ease of extraction without adversely affecting the health of farmers, other consumers and the environment.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdulahi N (2011). Studies on the efficacy of leaf extract of Balanites aegyptiaca on the ovipositon and survival of immature stages (larvae and pupae) of Callosobruchus maculates (F.) on treated cowpea seeds. Bavero J. Pure Appl. Sci. 4:40-43.
|
|
|
|
Bhardwaj A, Verma SC (2012). Evaluation of vegetable oils against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae) infesting pea seeds (Pisium sativum L.). J. Pest Manage. Hortic. Ecosyst. 18:46-53.
|
|
|
|
El-Ghar G, El-Shelken AEG, Gesa EEL (1987). Effectiveness of some plant extracts as surface protectant of cowpea seed against the pulse beetle, Calosobruchus chinensis. (L.). Phytoparasitica 17:109-113.
Crossref
|
|
|
|
El-Tokhy F, Kasem A (2012). Studies on fertilizer requirements on faba bean. A Thesis submitted to Cairo University Department of Agronomy, Faculty of Agriculture, in Partial Fulfillment of the Requirement for the Degree of Master of Science. pp. 1-13.
|
|
|
|
Gomez KA, Gomez AA (1984). Statistical Procedures in Agricultural Research. Johnwiley and Sons. 2nd Edn. New York. P. 680.
|
|
|
|
Gwinner JR, Harnisch J, Muck O (1996). Manual of the Prevention of Post-harvest Grain Losses. Deutsche Gesellschaft fur Tecnische Zusammenarbeit (GTZ) GmbH. P 338.
|
|
|
|
Ibrahim YM (2012). Efficacy of some plant oils against stored-product pest cowpea weevil, Callosobruchus maculates (Coleoptera: Bruchidae) on chickpea seeds. Persian Gulf Crop Prot. 1(1):4-11.
|
|
|
|
Ileke KD, Olotuah OF (2012). Bioacttivity of Anacardium occidentale (L.) and Allium sativum (L.) powder and oil extracts against cowpea Bruchid Callosobruchus maculates (Fab.) (Coleoptera; Chrysomelidae). Int. J. Biol. 4:96-103.
|
|
|
|
Kanaji GAD (2007). A study of bruchid resistance and its inheritance in Malawian dry bean germplasm. A Thesis submitted in fulfillment of the requirements of the degree of Doctor of philosophy (PhD) in plant breeding. Submitted to Kwazulu University, Natal Republic of South Africa.
|
|
|
|
Loth SM, Elice NL, Shazia OWM, Robert NM (2007). Effectiveness of local botanicals as protctants of stored beans (Phaseolus vulgaris L.) against bean bruchid (Zabrotes subfasciatus Boh) (genar: Zabrotes. Family: Bruchidae). J. Entomol. 4:210-217.
Crossref
|
|
|
|
Mushobozy DMK, Nganilevanu G, Ruheza S, Swella GB (2009). Plant oils as common bean (Phaseolus vulgaris L.) seed protectants against infestations by Mexican bean weevil Zabrotes subfasciatus (Boh.). J. Plant Prot. Res. 49:35-40.
Crossref
|
|
|
|
Okonkwo OU, Okoye WI (2008). The efficacy of four seed powders and the essential oils as protectants of cowpea and maize grains against infestation by Callosobruchus maculates (Fabricus) (Coleoptera: Bruchidae) and Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) in Nigeria. Int. J. Pest Manage. 42(3):143-146.
Crossref
|
|
|
|
Olotuah OF (2013). Comparative use of botanical oil extracts in pest management. Agric. Biol. J. North Am. 4(4):419-421.
Crossref
|
|
|
|
Prakash A, Rao J, Nandagopal V (2008). Futures of Botanical pesticides in rice, wheat, pulses and vegetable pest management. J. Biopesticides 1:154-169.
|
|
|
|
Regmil H, Dhoj Y (2011). Eco-friendly management of pulse beetle. J. Agric. Environ. 12:81-90.
|
|
|
|
Sangeeta G, Apte SD (2015). SEffects of botanicals (oils) on green gram (Vigna radiate L. Wilczek) against Callosobruchus maculates (Fab). Eur. J. Exp. Biol. 5(8):31-33.
|
|
|
|
Shukla R, Srivastava B, Kumar R, Dubey NK (2007). Potentials of some botanical powders in reducing infestation of chichpea by Callosobruchus chinensis L. (Coleoptera Bruchidae). J. Agric. Technol. 3(1):11-19.
|
|
|
|
Swella GB, Mushobozy DMK (2007). Evaluation of the efficacy of protectants against cowpea bruchid Callosobruchus maculates (F.) on Cowpea seeds (Vigna unguiculata (L.) Walp.). J. Plant Prot. Sci. 43:68-72.
|
|
|
|
Tabu D, Selvaraj T, Singh SK, Mulugeta N (2012). Management of Adzuki bean beetle (Callosobruchus chinensis L.) using some botanicals, inert materials and edible oils in stored chickpea. J. Agric. Technol. 8(3):881-902.
|
|
|
|
Tadele S, Habtamu A, Mulugeta N (2014). Effects of some botanicals against termite, macroterms Spp (Isoptera: Termitidae) under laboratory conditions. Int. J. Sustain. Agric. Res. 1:52-57.
|
|
|
|
Taponjou LA, Adler C, Bouda H, Fontan DA (2002). Efficacy of powder and essential oil from Chenopodium ambrostodes leaves as post-harvest grain protectants against six- stored product beetles. J. Stored Prod. Res. 38:395-402.
Crossref
|
|
|
|
Uddinll RO, Sanusi SA (2013). Efficacy of olive oil, Soybean oil and palm kernel oil in the control of Callosobruchus maculates (F.) in stored cowpea (Vigna unguculata L. Walp). J. Agro-res. 13:67-72.
|
|
|
|
Waktole S (2014). Effects of selected botanicals and local seed storage practices on maize insect pests and health of maize seed in Jimma Zone. Singapore J. Sci. Res. 4:19-28.
Crossref
|
|
|
|
Yahaya MM, Bandiya HM, Yahaya MA (2013). Efficacy of selected seed oils against Fecundity of Callosobruchus maculates (F.) (Coleoptera: Bruchidae). Int. J. Sci. Technol. 8:513-321.
|
|
|
|
Yohannes E, Setu B, Tigist S, Samuel S (2013). Evaluation of different botanicals for the management of cowpea Bruchid (Callosobruchus maculates) in North-Eastern Ethiopia. Arch. Phytopathol. Plant Prot. 46:1331-1337.
Crossref
|