ABSTRACT
Dosage rates of 1.25, 2.5, 5 and 10% (w/w) of red and white locally available inert dusts were evaluated as protectants against maize weevils, Sitophilus zeamais in maize grain under laboratory conditions. Parental adult mortality, F1 progeny emergence, percent protection, percent grain damage and weight loss were measured as efficacy determining parameters. Mortality of the weevils was observed only after 4 days post exposure and mean percentage mortality of the parental weevils caused by both inert dusts were significantly (P < 0.05) higher compared to untreated controls. Significant death of adult weevils was found to be induced gradually as from day 14 post exposure by both dusts when applied at the lower rate of 1.25 and 2.5%. Mortality with higher dosage rates of 5 and 10 % was observed as from day 7 after exposure. At day 21 post exposure, all dosage rates of both inert dusts and the positive control induced 100% weevil mortality. Both inert dusts applied at higher dosages 5 and 10% caused significantly (P < 0.05) higher (52.64%) protection of maize grain against F1 progeny emergence, percent grain damage (6.33%) and weight loss (2.33). Thus, the tested inert dusts were potent at a rate of 5 and 10%, and have the potential to be used in managing maize weevils under farmer’s storage conditions following field trials.
Key words: Red inert dust, white inert dust, Sitophilus zeamais, stored maize.
In Ethiopia, maize is one of the major cereal crops grown for its food, feed, firewood and construction values (Sori, 2014). Besides, being a cereal crop, maize ranks second to tef in area coverage and first in total production in Ethiopia. Currently, it also ranks first in total production and productivity among all cereals grown in southern Ethiopia (Gemu et al., 2013). But, its productions and yields have been highly affected by an array of biotic and abiotic stresses (Tefera et al., 2011). Among biotic constraints, insect pests are most often considered as the main agents responsible for causing its losses (Adams and Schulter, 1978) and the most important of these pests in storage are coleopterons weevils (Getu and Abate, 1999; Demissie et al., 2008). To control these insect pests, synthetic pesticides have been used by the majority of smallholder farmers in Africa.
However, over the years negative attributes have been associated with synthetic insecticides use such as environmental, health and other concerns (development of resistant strains of insect pests, toxicity to beneficial organisms and like) (Harish et al., 2013). Thus, there is a need for development of locally available
(cheap) , safe and ecologically sound control alternatives such as inert dusts as benign component of integrated pest management (IPM). Besides, it was reported that inert dusts such as clay powders, sands, wood ashes, silicates, lime and dianamite have been used traditionally by farmers to protect stored grain from insect pests in developing countries (De Lima, 1987; Golob, 1997). The main merits of using inert dusts were reported to be their low mammalian toxicity, long-term protection, easier application and maintenance of grain quality (Korunic et al., 1996) and their ease of availability.
Most of the early formulations, however were not widely accepted by the grain industries in developed countries for a variety of reasons including the high rates required for mortality, variation in toxicity among target species, damage to grain handling equipment and health problems with worker exposure to dusts (Arthur, 1997). But, more recently, materials including diatomaceous earths and silica aerogels, have been developed and used increasingly in commercial storage in the developed world, replacing conventional chemicals (Golob, 1997), that is, silica-based inert materials such as silica aerogels and diatomaceous earths have been proven to be very effective in smaller quantities and such information is essential in order to establish a sustainable management strategy against insect pests of maize (Demissie et al., 2008). However, synthetic silicates, which are manufactured for industrial uses, have very high silicon dioxide content, and are very expensive and therefore, inappropriate for use as grain protectants at farmers small scale level (Golob et al., 2002). Thus, identifying useful locally available, cheap, ecologically sound and safe inert materials or dusts against storage pest’s of maize such as S. zeamais is very essential and will aid in development of sustainable management strategies. From all these facts and the attempts that have been made to divert attention away from reliance on a single control options so as to find natural, cheaper and safe materials for the control of storage pests, the present study was initiated with the following objectives:
(1) To evaluate the bio-efficacy of two colored locally available inert dusts against the most economically important insect pest of maize, maize weevils under laboratory condition and
(2) To determine the possibility of using inert dusts in storage insect pest management, particularly against maize weevils under farmers storage conditions in Ethiopia.
The study period
The study was conducted between October 2016 and June 2017 in the Insect Science Laboratory of Zoological Science Department of Addis Ababa University of Ethiopia.
Insect’s culture
Adult
maize weevils (
Sitophilus zeamais) were collected from maize stored in various farmers’ traditional storage facilities major maize producing localities Shashogo and Sankura Districts of Southern Ethiopia and brought to the laboratory Insect Science Stream of Zoological Science Department of Addis Ababa University of Ethiopia. These test insects were cultured at 27±3°C and 55 to 70% RH (Jembere et al., 1995; Zewde and Jembere, 2010). Shone variety of maize grains were obtained from farmer’s storages of the aforementioned districts. It was the most commonly grown hybrid in the region and considered to be susceptible to insect infestation. The grains were kept at -20±2°C for 2 weeks to kill any infesting insects, cleared of broken kernels and debris and then graded manually according to size, and similar sized grains were selected for the experiment (Gemechu et al., 2013). Following the methods by Zewde and Jembere (2010) fifteen pairs of the adult of the test insects were placed in 12.1 L glass jars containing 250 g seeds. The jars were then covered with nylon mesh and held in a place with rubber bands to allow ventilation and to prevent the escape of the experimental insects. The parent of the test insects were sieved out after an oviposition time of 14 days. Then, the jars were kept under the laboratory condition until F
1 progeny emergence. The F
1 progeny, which emerged after 30 days, were sieved out and used for the experiment.
Inert dusts
Two different colored locally available inert dusts (white and red) (Figure 1) collected from rocky area of Hadiya zone of south Ethiopia were sieved to remove larger material. These inert dusts were then applied at the rate of 1.25%, 2.5%, 5% and 10% (w/w) which are equivalent to 1.25/100, 2.5/100, 5/100 and 10/100 g of maize grains following similar procedures by Tadesse (2003) and Demissie et al. (2008). These inert dusts have been used for painting of houses by local people in rural areas.

Treatment application of the inert dusts
Following similar methods used by Tadesse (2003) and Demissie et al. (2008), four different rates of each inert material were weighed and added to each 1 L glass jars containing 100 g of disinfested maize grains and shaken well to get a uniform coating. Treated and untreated controls were included. In the treated control, Malathion 5% dust at recommended rate of 0.05% (w/w) was used. In the untreated control, neither the inert dusts, nor Malathion 5% dust was used. After treatment, 20 pairs of three to seven day old unsexed experimental insects were introduced to the treated and an untreated seed in the glass jars. Then, the jars were covered with nylon mesh and held in place with rubber bands. The experiments were laid up in Completely Randomized Design (CRD) in three replications consisting of the two colored inert dusts in four rates of application. All treatments were maintained under the same laboratory conditions indicated
in insect culture section. Data’s were collected on:
Adult mortality
The data for adult mortality counts were recorded after 1, 2, 4, 7 and 14 days post-exposure, while the dead and live adults were removed from the jars after the last count at 21st day as described by Tadesse (2003) and Demissie et al. (2008).
F1 progeny assessment bioassay
The treated and control grains were also kept until emergence of F1 progeny under same experimental conditions indicated in insect capture section after mortality observation. Then the numbers of F1 progeny produced by the experimental insects were counted. Counting was stopped after 56 days from the day of introduction to avoid overlapping of generation (Zewde and Jembere, 2010).
Damage and weight loss assessment
Two days after the last F1 count at 56 days post exposure, samples of 100 grains were taken randomly from each jar and the number of damaged (grains with characteristic hole) and undamaged grains were counted and weighed. Grain damages were conveyed as a percentage of the entire number of grains in each replicate. Percentage weight losses were calculated by count and weight method following methods by FAO (1985); Boxall (1986); Haile et al. (2003) and Haile (2006) as follow:
Where U = weight of undamaged grain, D = weight of damaged grain, Nd = number of damaged grain and Nu = number of undamaged grain. As adopted by Gselase and Getu (2009), percent protection or inhibition in F1 progeny emergence (% IR) was also calculated using the following formula:

where Cn is the number of newly emerged insects in the untreated (control) jar and Tn is the number of insects in the treated jar.
Data analysis
The data’s collected for this study were managed by the Microsoft Excel package 2013 and analyzed using the Statistical Program for Social Sciences (SPSS) version 16. To observe the effects of botanicals treatments against weevils adults mortality and F1 progeny emergence, as well as grain damage and weight loss of maize grains at a particular time, appropriate statistical methods, Univariate analysis (for the former one) and one way analysis of variance (ANOVA) (for the rest of parameters measured) were used. Difference among means were stated significant when p<0.05 and highly significant when p<0.01. Data’s were not transformed. Standard errors (± SE) were given following means in tables and in the form of error bars in figures. Correlation between the treatments and the efficacy measuring parameters were determined using Pearson’s correlation of SPSS program of version 16.
Effect of inert dusts on mortality of maize weevil adults
There was no significant adult maize weevil mortality recorded for all dosages of the tested inert dusts up to 4 days post exposure and it was not recorded for the lower doses of 1.25%, and 2.5% even up to 7 days post exposure. Mean percentage mortality of the weevils caused by both the tested inert dusts admixed with maize grains at all rates were significantly (P < 0.05) higher in all dates after post treatment exposure than untreated check (Figure 2). Significantly (P < 0.05) higher (≥65%) mean percentage mortality of adult maize weevils were induced by both the tested inert dusts applied at dosages of 2.5, 5 and 10%, 14 days after treatment application. Besides, one hundred percentage weevil’s mortality was induced by both of the tested inert dusts admixed with maize grains at all doses, 21 days after treatment application which was similar to that of the positive control. Furthermore, significant death of adult weevils was found to be induced gradually (14 days after treatment) by both of the tested inert dusts applied at the lower rate (1.25 and 2.5%) in comparisons to relatively higher dosage (5 and 10%) in which it occurred 7 days after treatment. In general, the weevil’s mortality increased as both the dosage rate and days after exposure were increased (Figure 2).
The effect of inert dusts on emergence of F1 progeny, percentage protection and weight loss
The number of F1 adult progeny produced, percent grain damage and weight loss caused by S. zeamais in all treatments with the two inert dusts were significantly (P < 0.05) lower compared to negative control. At higher dosages (5 and 10%), both dusts caused significantly (P < 0.05) higher (≥ 52.64%) protection of maize grain against F1 progeny emergence, percent grain damage (≤ 6.33) and weight loss (≤ 2.33) by weevils than the lower rates (1.25 and 2.5%) (Table 1). The correlations among the treatments of the two inert dusts applied at different dosage rates and the efficacy parameters measured were found to be highly significant (Table 2). The correlations between the various treatments of inert dusts applied at different rates and the various parameters measured (the number of F1 progeny emerged, percentage grain damage and weight loss) were strongly negative (r was in the range between -0.95 and -0.790). However, the correlations between F1 progeny produced, and percent grain damage (r ≥ 0.923) and weight loss (r ≥ 0.960) of all treatments of inert dusts were strongly positive (Table 2).
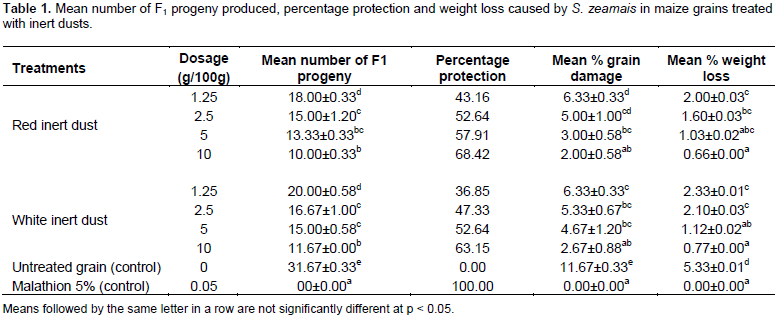

The current study has shown that the treatments of local inert dusts applied at a rate of 5 and above (10%) were more effective in terms of parental adult weevil’s mortality than lower doses. Similarly, it was reported that the mortality of the adult beetles of the four species of insects (Tribolium confusum, Trogoderma granarium, Oryzaephilus surinamensis and Rhizopertha dominica) due to treatments of four local rocky dusts namely, ninivite, kaolinite, montmorillonite and bentonite increased with the increase of concentration used (Al-Iraqi and Al-Naqib, 2006). Besides, Tadesse (2005) and Ibrahim (2017) indicated that filter cake or Melkabam (by-products of Aluminum sulfate factory) applied at dosages of 0.5% (w/w) and above (5%) caused higher level of mortality of weevils. Demissie et al. (2015) added that inert materials involving clays have been effective on stored-product insects at high rates (>10 g /kg of grain) and suggests as they might be viable protectants grain in underdeveloped countries. The present study has also indicated that the treatments of local inert dusts applied at 5 and 10% rates induced significantly higher protection of maize grains against maize weevils attack, in terms of F1 emerged, percent grain damage and weight loss than the rest lower doses. Besides, it has also revealed that all treatments of the local inert dusts induced higher protection of grains against weevils than untreated check.
Similarly, Demissie et al. (2008) reported that in addition to causing adult mortality, the different inert dusts either completely hindered or significantly reduced progeny emergence, after testing the efficacy different inert dusts against the maize weevil. Significantly, higher mortality effect of the tested inert dusts at rates of 5% and above (10%) following 14 and 21 days after treatment against parental weevils in the present study could probably be related to their slow acting and non-toxic mode of action. Similarly, it has been reported that inert dusts are chemically unreactive and thus, used for managing storage insect pests by killing physically than chemical means (Abd EL-Aziz, 2011). Moreover, it was shown that the action caused by inert dust is progressive, and extended post treatment exposures for longer period against insect pests in treated grains significantly decrease the rates required to kill the population of insects (McLaughlin, 1994). Chakraverty et al. (2003) explained that inert dusts act slowly and take 20 or more days to cause insect mortality.
The present study has indicted that the treatments of local inert dusts applied at 5 and 10% induced significantly higher protection of maize grains against maize weevils attack. This fact has revealed that the local inert dusts measured at rates of 5% and above (10%) were potent in preventing maize grains against maize weevils attack. Thus, these inert dusts could be used in managing maize weevils as safe, ecologically sound and cheap management alternative to synthetic chemicals under subsistence farmer’s storage conditions in the study area and Ethiopia in particular and elsewhere with similar pest problems in general. However, their potency against weevils under subsistence farmer’s storage condition need further study before wide implementation of outcomes this study. Findings of this study have revealed that the two local inert dusts applied at rate 5 and above (10%) induced significantly high mortality following 14 days and 100% mortality following 21 days of treatment application and thus, they were concluded as slow acting substances.
The authors have not declared any conflict of interests.
REFERENCES
|
Abd EL-Aziz SE (2011). Control strategies of stored products pests. J. Entom. 8:101-122.
Crossref
|
|
|
|
Adams JM, Schulter GM (1978). Losses Caused by Insects, Mites and Micro-organisms. In: Harris KL, Lindblad CG (Eds.). Postharvest grain loss assessment methods. New York, American Association of Cereal Chemists pp. 83-95.
|
|
|
|
Al-Iraqi RA, Al-Naqib SQ (2006). Inert dusts to control adults of some stored product insects in stored wheat. Raf. J. Sci. 17:26-33.
|
|
|
|
Arthur FH (1997). Grain protectants: current status and prospects for the future. J. Stored Prod. Res. 32:293-302.
Crossref
|
|
|
|
Boxall RA (1986). A critical review of the methodology for assessing farm level grain losses after harvest. Report of the TDR G191. P 139.
|
|
|
|
Chakraverty A, Mujumdar AS, Raghavan GS, Ramaswamy VHS (2003). Hand book of postharvest technology. Cereals, fruits, vegetables, tea and spices. Marcel Dekker, Inc. New York. P 907.
|
|
|
|
De Lima CPF (1987). Insect pests and post-harvest problems in the tropics. Ins. Sci. Appl. 8:673-676.
|
|
|
|
Demissie G, Swaminathan R, Ameta OP, Jain HK, Saharan V (2015). Biochemical basis of resistance in different varieties of maize for their relative susceptibility to Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae). J. Stored Prod. Post-Harvest Res. 6:1-12.
|
|
|
|
Demissie G, Tefera T, Tadesse A (2008). Efficacy of Silicosec, filter cake and wood ash against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) on three maize genotypes. J. Stored Prod. Res. 44:227-231.
Crossref
|
|
|
|
Food and Agriculture Organization (FAO) (1985). Prevention of post-harvest food losses: A training manual, FAO, Training Series No. 11, Rome, Italy.
|
|
|
|
Gselase A, Getu E (2009). Evaluation of botanical plants powders against Zabrotes subfasciatus (Boheman) (Coleoptera: Bruchidae) in stored haricot beans under laboratory condition. Afr. J. Agric. Res. 4:1073-1079.
|
|
|
|
Gemechu F, Santiago DR, Sori W (2013). Laboratory evaluation of cotton (Gossypium hirsutum) and Ethiopian mustard (Brassica cariata) seed oils as grain protectants against maize weevil, Sitophilus zeamais M. (Coleoptera: Curculionidae). Afr. J. Agric. Res. 8(32):4374-4379.
|
|
|
|
Gemu M, Getu E, Yosuf A, Tadess T (2013). Management of Sitophilus zeamais Motshulsky (Coleoptera: Ciurculionidae) and Sitotroga cerealella (Olivier) (Lepidoptera: Gelechidae) using locally available inert materials in southern Ethiopia. Greener J. Agric. Sci. 3:508-515.
Crossref
|
|
|
|
Getu E, Abate T (1999). Management of maize stem borer using sowing date at Arsi-Negele. Pest Manage. J. Eth. 3:47-51.
|
|
|
|
Golob P (1997). Current status and future perspectives for inert dusts for control of stored product insects. J. Econ. Entom. 33:69-79.
Crossref
|
|
|
|
Golob P, Farrell G, Orchard JE (2002). Crop post-harvest: science and technology. Volume 1. Principles and practice. Blackwell science Ltd, a Blackwell publishing, USA. P 577.
|
|
|
|
Haile A (2006). On-farm storage of chickpea, sorghum and wheat in Eritrea. Dry lands Coordination Group Report No. 42. DCG, Norway. P 35.
|
|
|
|
Haile A, Selassie DG, Zereyacob B, Abraham B (2003). On-Farm Storage Studies in Eritrea. Dry lands Coordination Group Report No. 28. Noragric, the center for international environment and development studies at the Agricultural University of Norway, Norway. P 55.
|
|
|
|
Harish G, Nataraja MV, Ajay BC, Holajjer P, Savaliya SD, Gedia MV(2013). Comparative efficacy of storage bags, storability and damage potential of bruchid beetle. J. Food Sci. Technol. 51:4047-4053.
Crossref
|
|
|
|
Ibrahim AY (2017). Evaluation of different rates of filter cake (by-products of Aluminum sulphate factory) for the management of the maize weevil, Sitophilus zeamais Mostch (Coleoptera: Curculionidae) on stored maize at Bako, western Ethiopia. Acad. Res. J. Agric. Sci. Res. 5:36-43.
|
|
|
|
Jembere B, Obeng-Ofori D, Hassanali A (1995). Products derived from the leaves of Ocimum kilimandsharicum (Labiate) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull. Entomol. Res. 85:361-367.
Crossref
|
|
|
|
Korunic Z, Fields PG, Kovacs MIP, Noll JS, Lukow OM, Demianyk CJ, Shibley KJ (1996). The effect of diatomaceous earth on grain quality. Post-harvest Biol. Technol. 9:373-387.
Crossref
|
|
|
|
McLaughlin A (1994). Laboratory trials on desiccant dust insecticides. Proceedings of the 6th international working conference on stored product protection. pp. 638-659.
|
|
|
|
Sori W (2014). Effect of selected botanicals and local seed storage practices on maize insect pests and health of maize seeds in Jimma Zone. Singapore J. Sci. Res. 4:19-28.
Crossref
|
|
|
|
Tadesse A (2003). Studies on some non-chemical insect pest management options on farm-stored maize in Ethiopia. Ph.D. Thesis, Giessen University, Germany. P 225.
|
|
|
|
Tadesse A (2005). Filter cake: a promising inert dust for the control of the maize weevil (Sitophilus zeamais) and the bean bruchid (Challosobruchus chinensis L.). Program and abstract of thirteenth annual conference of the crop protection society of Ethiopia, CPSE, August 11-17. Addis Ababa.
|
|
|
|
Tefera T, Mugo S, Tende R, Likhayo P (2011). Methods of screening maize for resistance to stem borers and post-harvest insect pests. International Maize and Wheat Improvement Center (CIMMYT), Nairobi, Kenya.
|
|
|
|
Zewde DK, Jembere B (2010). Evaluation of orange peel citrus sinensis (L) as a source of repellent, toxicant and protectant against Zabrotes subfasciatus (Coleoptera: bruchidae). Momona Ethiop. J. Sci. 2(1):61-75.
Crossref
|