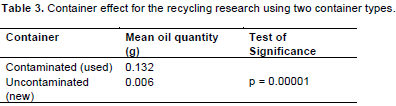ABSTRACT
Affordable, pesticide-free, and reliable maize storage containers for seed and food purposes are not available in many subsistence farming cultures. As a result, subsistence farmers lose their crop to pests and are forced to dispose of their maize for a low price right after harvest thus, robbing them of profit and food for the rest of the year. The objective of this research was to develop information to enable utilization of recycled sanitary hermetic maize storage containers. Use of these containers will allow farmers to preserve their maize for as long as they wish, using locally available resources. A market survey found edible oil containers available for sale and re-use in markets in Uganda, Tanzania, and Kenya. A laboratory study compared three cleaning methods (oil drain+45°C water(A), oil drain+ 90 to 100°C water(B), oil drain+ 90 to 100°C water + soap(C)) for cleaning soybean oil contaminated 20 L HDPE containers. Research results, indicate that using “C” will clean the containers to a cleanliness level comparable to a new container. This is an encouraging result in light of the edible oil containers available for sale and re-use in East African markets. A Ugandan field study also tested the efficacy of recycled containers for hermetic storage, and confirmed that hermetic storage using these containers is lethal to maize weevils, while preserving the quality of stored maize.
Key words: Maize storage, hermetic storage, recycled containers.
About 19% of maize produced in East Africa is lost during post-harvest storage, due to insects, fungi, rodents, and birds (PHL Network, 2009; Lindblad and Druben, 1980; Demissie et al., 2008a, b; Gregg and Billups, 2009). The highest losses are usually due to maize weevils (Sitophilus zeamais) (Holst, et al., 2000; Demissie et al., 2008a). Hermetic storage seeks to eliminate gas exchange with the outside environment and thus causes weevils to die as oxygen in the grain container is used up. It can eliminate maize weevil damage along with bird, and rodent damage (Navarro, et al., 1994; CRC, 2003; Springer, 2007; Glevitzky, et al., 2009; FAO, 2010; Yakubu, et al., 2011). If a container is to be used for hermetic maize storage, it needs to be sealable, robust, affordable, available, and free of harmful contaminants which can be transferred to stored maize. Some recycled containers may meet these criteria and be suitable for grain storage by subsistence farmers. Lindblad and Druben (1980) described the procedure to use recycled 220-L (55-gallon) oil drums for storage of grains.
Storing maize in recycled containers
Maize is grown for food. Container recycling and re-use for maize storage, therefore, requires proper selection and cleaning to prevent any toxic contents from contaminating stored maize (Schmidt and Erickson, 2008; ASM, 2009; EPA, 2009a; 2009b). Since recycled containers may have held a variety of possibly toxic materials, only food grade containers previously utilized for storage of carbonated soft drinks and triglycerides, were considered for the recycling and maize storage research (Bhatt, 2004; Shachman, 2004; Ashaye and Olusoji, 2006). But since soft drinks do not have uniform chemical contents only containers previously containing edible oil were considered in this research (Lindblad and Druben, 1980; Cleveland et al., 2001; Murdock, et al., 2003; Adhikarinayake, 2005; EPA, 2006; Mercer, 2006; Malik et al., 2006; Wiley-Blackwell, 2006; EPA, 2009c; Tsimihodimos, et al., 2009; CRU, 2010; EWG, 2011). “Clean” refers to being free from dirt or pollution, unadulterated, sanitary or pure (Merriam-Webster, Inc. 2010). However, according to Coats (2010) and others the end-products of some chemicals are more toxic than the starting chemicals, and containers previously used to store some chemicals cannot be cleaned adequately (Cleveland et al., 2001; EPA. 2006). To preserve maize quality, while preventing cross- contamination between stored maize and the associated rancid oil free radicals, as well as dirt, a procedure is needed for cleaning edible oil containers such that nearly 100% of the oil is removed (Andrikopoulos et al., 2002; Andrikopoulos, 2004; Choe and Min, 2006). This can give subsistence farmers the benefits of effective hermetic storage in a clean container (Carroll and Fulton, 2008; Murdock et al., 2003).
Research need
Little information was found on the availability and suitability of used edible oil containers in East Africa. A study was therefore needed on container sizes, prices, and availability in East Africa. A study was also needed to establish cleaning procedure for reused edible oil containers to ensure food safety.
Objectives
1. To determine the availability of used edible oil containers in East Africa suitable for hermetic maize storage.
2. To develop and test procedures for cleaning previously used edible oil containers.
3. To deploy and test the efficacy of the containers for hermetic storage, in the field.
This study consisted of a market survey, laboratory research and field study.
Market survey
To explore recycled edible oil container availability for hermetic storage in East Africa, a recycled container survey form was designed and dispatched to contacts in Uganda, Kenya, and Tanzania. The purpose of the survey was to determine availability of containers in selected East African markets which can be recycled for maize storage. Containers identified were expected to be at least five L in capacity and airtight (no holes). Container properties to be identified by the survey included previous or intended use (edible oil, soft drink concentrate, etc.), volume (L), material (plastic, steel, etc.), quantity in each market, as well as price. Each surveyor was expected to carry out the survey in at least three markets. East African Markets surveyed included (1) Jua Kali Drum Dealers (Nairobi), (2) Frere Town (Mombasa) and (3) Musila Enterprises (Kikambala Village), in Kenya; (1) Mwembe, (2) Same Center, and (3) Kwasakwasa, in Tanzania; (1) Namanve Market (Mukono-Kampala), and (2) Soko Mujinga Market (Kitale), in Uganda.
Laboratory research
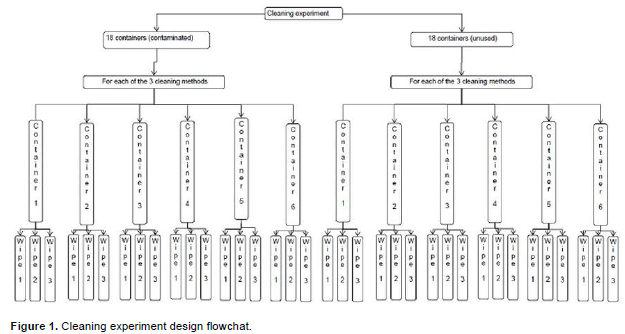
The laboratory part of the research conducted at Iowa State University was done in two stages (Figure 1). The first stage involved applying three treatments (A,B,C) to randomly assigned experimental units (20-L HDPE containers). Experimental units utilized were previously used (contaminated) and new (uncontaminated) 20-L (~5 gallon) HDPE containers. The complementary second stage utilized the goldfisch oil extraction method to measure leftover oil quantities in the 20-L containers for use in statistical analysis which allowed comparison of the three cleaning treatments.
Field study
A study simulating East African field conditions was designed and tested at Iowa State University, based on Yakubu et al. (2011), then deployed for standard field testing in Uganda (Bern et al., 2015).
Treatment definition
Cleaning of oil-contaminated containers using water, with or without soap, is a common practice in East Africa (Myers, 2006; Yakubu et al., 2011). Literature, however, suggests that cleaning, disinfecting and sanitizing containers to prevent cross-contamination of food stored within them is best done at high temperature, using soap and mechanical action, such as scrubbing and shaking (Knoxa and Walkera, 1947; Gangneux, et al., 2004; Atlas and Snyder, 2006; Sebastião, et al., 2006; CRC, 2006; Helmenstine, 2011; MTL, 2011; Patwardhan and Kelkar, 2011). Heat treatment destroys oil-splitting enzymes, arrests hydrolytic rancidity and autoxidation during vegetable oil extraction and causes oil to leach out from container surfaces (FAO, 2002). The use of soap plus hot water cleaning treatment was expected to produce similar advantages. Factors considered for this research were (i) soap and water, at two water temperatures: (control (45°C) and hot or boiling (90 to 100°C)), and (ii) container history (contaminated and new (control)). We hypothesize, therefore that cleaning with soap at high temperature (90 to 100°C), with mechanical action would be enough to clean and sanitize vegetable oil contaminated containers for maize quality preservation within them.
Experimental 20 L HDPE containers
The 36 experimental units (containers) utilized for the research each had a net weight of about 16 kg (35 lb) when full and normally contain about 15.88 kg of oil claimed to be 100% pure soybean oil (Columbus Foods, Chicago, Illinois). Eighteen were new and unused from Columbus Vegetable Oils, 30E. Oakster Street, Des Plaines, IL and eighteen were recycled used containers obtained from Ames and Des Moines, IA Chinese restaurants. They were made of high density polyethylene (HDPE), with a resin classification or recycling code of 2 (ACC, 2007; Bakers and Chefs, distributed by Sam's West, Inc. 608 S.W. 8th Street, Bentonville, AR). HDPE has a melting point of 130 to 135°C, a tensile strength of 4550 psi, and can withstand temperatures of 120°C for short periods or 110°C continuously (EOS/EDS, 2000; Antec, 2001; Dow 2009; Dynalab, 2011).
Quantification of vegetable oil remnants
Average weight of the new containers was 296.50 g. On average, contaminated containers treated with “A”, “B”, and “C” lost weight equaling 10.45, 14.23, and 20.06 g oil, respectively. The follow up wiping treatment using weighed cheesecloth strips to wipe container interior indicated that on average, contaminated containers treated with “A”, “B”, and “C” contained 0.249, 0.142, and 0.004 g, remnant oil, respectively. In cleaning contaminated containers, to which “C” was employed as treatment, enough soap was added to remove all the oil, irrespective of whether leftover oil is free flowing or bound tightly to the container internal surfaces. Therefore, the soap quantity necessary to remove nearly 100% oil contaminant was based on that. Since water at 45°C and 90 to100°C was utilized in dissolving congealed oil before draining, the oil dissolved in the water flowed out, readily reducing the leftover oil in the drained containers.
Experimental soap and water
The preliminary soap quantification involved determining the cleansing power of Ivory soap available with these containers, and quantifying the number of grams of the soap that effectively removes each gram of the vegetable oil contaminant. From an initial soap quantification experiment, it was determined that 1 g of soap removed almost 1 g of oil, from oil contaminated experimental units. However, three times that amount of soap (3 g soap/g oil) was utilized with hot water for cleaning the containers, in order to account for variability in the history of containers collected from the field. Deionized (demineralized) water was used for this research.
Oil removal treatments
Stage one of the research involved adding the assigned and calculated treatment quantities, followed by application of 3.78 L of deionized water to each EU. The mixture was then shaken at the onset and at 5 min intervals afterwards for a total of 1.5 h, and emptied. Cans were then rinsed three times and turned upside down for 48 h to allow water to flow out and dry from inside. The low temperature treatment (“A”) involved just adding water at 45°C to assigned EUs, shaking at 5 min intervals, and emptying it at the end of the treatment application period. The high temperature treatment (“B”) application involved addition of 3.78 L of water at 90 to 100°C to assigned EUs, while the soap treatment (“C”) involved addition of the calculated quantities of soap to the assigned EUs, followed by 3.78 L of water at 90 to 100°C, and shaking. Since these treatments involve only use of soap at various temperatures, they should be repeatable in Africa.
Oil residue measurements
The second stage, follow-up, treatment involved tying about 1.7 g of absorbent cheese cloth from Prym Creative, Estopilla, Prym Consumer USA, Inc., Spartanburg, SC, to the end of a wood stick and using the cloth end to wipe each of three pre-assigned 229 mm x 229 mm areas (right, left, bottom) of the interior of each experimental container, in order to determine the level of oil remnant left. New cheesecloth was installed on the stick prior to each wiping. The oil remnant levels were determined by quantifying the oil content of the cheesecloths using the goldfisch (35001-00, LABCONCO, Kansas City, MO. 1637) hexane oil extraction method. The second stage was performed after the EUs had dried, following the initial treatment application.
Survey results
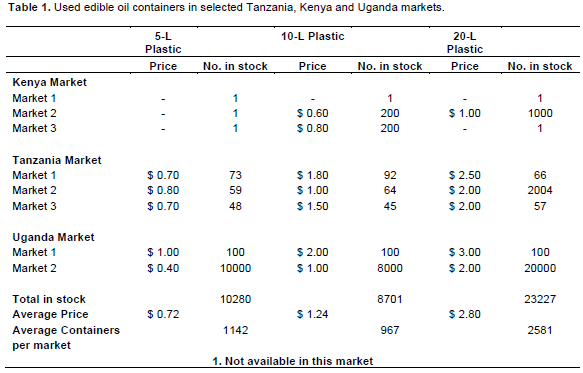
The recycled container survey identified a total of 55522 containers that met the survey criteria. However, only 42208 (76%) of the containers had edible oil residue or were earmarked for edible oil storage (Table 1). These ranged in storage capacity from 5 to 20 L, with prices ranging from $0.72 (US) to $2.08 (US), based on size. On average, the number of recyclable plastic containers per market was 1142 (5 L), 967 (10 L), and 2581 (20 L), respectively.
Laboratory research results
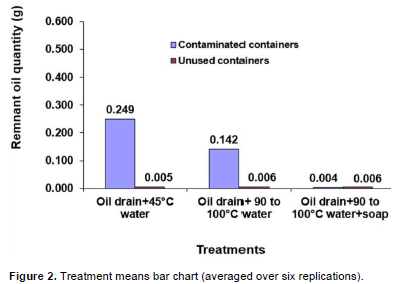
Each treatment was assigned six containers, and three wipes (interior bottom, right, and left) were taken from each of the containers. Therefore, each of the bars in Figure 2 represent the mean of the wipes from 18 (six 20 L containers*3 wipes) container locations assigned to each of the treatments. The figure shows results obtained from cleaning recycled and new containers with three treatments (A, B, C). It indicates a strong treatment effect, and shows that while treatments “A” and “B” left behind significant (p<.0001) amounts of oil, treatment “C” as well as the control treatments (A, B, C, applied to unused containers) had an insignificant (p = 0.8992, 0.8633, 0.8448, and 0.8526, respectively) oil remnant, following cleaning treatment application.
Since analysis was done at the 0.05 significance level (Dallal, 2003), where p < 0.05 is sufficient evidence for accepting a hypothesis of treatment effect, the laboratory results conclude that there is a treatment difference between treatments A, B (contaminated containers) versus treatment C (contaminated containers) and all the control treatments. Any level of cross contamination between stored food and storage container surface contaminants is unacceptable (Shachman, 2004; Onsongo, et al., 2005; EPA, 2009a, b). The un-contaminated containers utilized in this research are reference standards for clean containers. Based on the results, using 3 g (0.031 moles) of Ivory soap (99.4% pure) to clean each g of soybean oil in the contaminated containers, will remove enough oil to prevent rancidity and cross-contamination of soybean oil with maize that would be stored in them.
A total of 108 (18 wipes*6 treatments) samples were analyzed by the goldfisch oil extraction method, using 54 (108/2) wipes obtained from each of the contaminated and unused containers. The remnant oil quantity (Figure 2) shows the average of 18 (3 wipes/container for six containers) wipes obtained from six containers assigned to each treatment. Treatment “C” had the lowest oil remnant (0.004 g) following cleaning of the containers, in relation to “A” (0.249 g) and “B” (0.142 g).
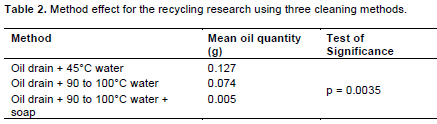
Treatment C’s oil remnant (0.004 g), from contaminated containers compare favorably with those for control treatments (from unused containers), which had 0.005 g, 0.006 g, and 0.006 g of oil remnants, respectively for (i) “A”, (ii) “B”, and (iii) “C”. Method by container interactions shows a clear difference between the effect of cleaning methods on the two container types (Table 2). For contaminated containers, cleaning with “C” produced the cleanest containers, followed by “B”, and “A”, respectively. However, there is no distinguishable difference in the oil remnant for uncontaminated containers cleaned using all three methods. Significant container effects are also reflected in oil quantity means (Table 3), for contaminated and uncontaminated containers.
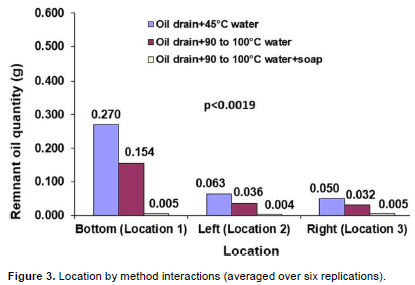
Location by method interactions
Location by method interactions show more leftover oil for location 1 (interior, container bottom), compared to location 2 and 3 (interior, left and right container sides, respectively), which are almost alike in remnant levels (Figure 3). This is because oil usually settles to the bottom of its holding container, and the bottom is expected to hold more oil for this reason. The figure also reflects differences in treatment effects, with treatment “C” being the most effective. This is obvious from the leftover oil differences between contaminated containers assigned to treatments “A”, and “B” compared to those assigned to the (i) control treatments, and (ii) treatment “C” assigned to contaminated containers. “C” produced as much clean as control treatments, and since new containers and contaminated containers assigned to “C” had virtually no oil, their remnant oil levels remain close to zero. The leftover oil levels for “A” and “B” are about the same, although, slightly lower for “B”. The lack of a clear trend in control treatments is expected since control EUs had no oil contaminants at the onset.
Field test results
The Ugandan field study confirms the superiority of hermetic storage over open-air maize storage (Bern et al., 2015).
Based on the results of this study, the following conclusions can be drawn:
1. Used, edible oil containers are readily available in East African (Kenya, Tanzania, and Uganda) markets for use in hermetic grain storage. 5 to 20 L, recyclable plastic containers exist with prices ranging from $0.72 to $2.08, and markets had 1,142 (5 L), 967 (10 L), and 2,581 (20 L) such containers respectively, on average per market.
2. Previously used edible oil containers can be recycled following cleaning with soap plus water at 90 to100°C, and mechanical action, for safe hermetic maize storage.
3. Rancidity and associated negative health effects can be eliminated using the cleaning procedures outlined in the research, while preserving maize quality for the end-user.
4. Hermetic storage can be implemented successfully using recycled edible oil containers, to meet the needs of subsistence farmers.
The authors have not declared any conflict of interests.
REFERENCES
|
Adhikarinayake TB (2005). Thesis. Wageningen University. Chapter 7.Technical and economic feasibility of airtight ferrocement bin for on-farm storage of paddy in Sri Lanka. The Netherlands.
|
|
|
|
Andrikopoulos NK, Kalogeropoulos N, Falirea A, Barbagianni MN (2002). Performance of virgin olive oil and vegetable shortening during domestic deep-frying and pan-frying of potatoes. Int. J. Food Sci. Technol. 37(2):177-190.
Crossref
|
|
|
|
|
Andrikopoulos NK (2004). Oxidative stressed frying fats and oils. Potential role for health. 4th International symposium on deep-frying: "healthier and tastier deep fried foods", January 11-13, Hagen, Germany, pp. 11-12.
|
|
|
|
|
ASM (American society for microbiology) (2009). Pathogens and toxins in foods: challenges and interventions. Juneja VK, Sofos JN (Eds.). Herndon, VA: ASM Press, pp. 420.
|
|
|
|
|
Atlas RM, Snyder JW (2006).Handbook of media for clinical microbiology. CRC Press, P 105.
Crossref
|
|
|
|
|
ACC (American Chemistry Council) (2007).Plastic packaging resins. Plastics division.Plastic packaging resins. Arlington, VA (USA).
|
|
|
|
|
ANTEC (2001). Plastic: The lone star. Conference proceedings.Volume II. Materials. Society of Plastics Engineers Technical Conference. Boca Raton, FL: CRC press, P 2705.
|
|
|
|
|
Ashaye OA, Olusoji OC (2006). Evaluation of some quality attributes of soybean oils in Ibadan. Afr. J. Agric. Res. 1(1):017-019.
|
|
|
|
|
Bern CJ, Yakubu A, Rosentrater KA, Brumm TJ (2015). Hermetic Storage Systems for Maize (Mısır için Hermetik Depolama Sistemi). 9(68):74-81.
|
|
|
|
|
Bhatt BI (2004). Stoichiometry. India: Tata Mcgraw Hill Education, pp. 127-178.
|
|
|
|
|
Carroll NJ, Fulton JR (2008). Extension methods to teach hermetic storage in West-Central Africa. In 2008 Providence, Rhode Island, June 29–July 2, 2008. Am. Soc. Agric. Biol. Eng. P 1.
|
|
|
|
|
Choe E, Min DB (2006). Mechanisms and factors for edible oil oxidation. Comprehensive Rev. Food Sci. Food Saf. 5:169.
Crossref
|
|
|
|
|
Cleveland CB, Oliver GR, Chen B, Mattsson J (2001). Risk Assessment under FQPA: Case Study with Chlorpyrifos. NeuroToxicol. 22(5):699-706.
Crossref
|
|
|
|
|
Coats JR (2010). Toxicology 550. Pesticides in the environment. Iowa State University. Class notes. Spring 2010.
|
|
|
|
|
CRC (CRC Press) (2003). Handbook of postharvest technology: cereals, fruits, vegetables, tea, and spices. In: Chakraverty A, Mujumdar AS, Ramaswamy HS (Eds). Boca Raton, FL: CRC Press, pp.188.
|
|
|
|
|
CRC (2006). Handbook of lubrication and tribology: Volume I. Application and maintenance, Second Edition. George E. Totten (Eds). Boca Raton: CRC press, pp. 1-11.
|
|
|
|
|
CRU (2010). Clean water for Washington. Why the concern about agricultural contamination in groundwater?
|
|
|
|
|
Dallal GE (2003). Why P=0.05? Retrieved on December 9, 2011 from
View.
|
|
|
|
|
Demissie G, Teshome A, Abakemal D, Tadesse A (2008a). Cooking oils and ''Triplex'' in the control of sitophilus zeamais motschulsky (coleoptera: curculionidae) in farm-stored maize. J. Stored Prod. Res. 44:173-178.
Crossref
|
|
|
|
|
Demissie G, Tefera T, Tadesse A (2008b). Importance of husk covering on field infestation of maize by sitophilus zeamais motsch (coleoptera:curculionidea) at Bako, Western Ethiopia. Afr. J. Biotechnol. 7(20):3777-3782.
|
|
|
|
|
EPA (2006). Finalization of interim reregistration eligibility decisions (IREDs) and interim tolerance reassessment and risk management decisions (TREDs) for the organophosphate pesticides, and completion of the tolerance reassessment and reregistration eligibility process for the organophosphate pesticides pp. 44-69.
|
|
|
|
|
EPA (2009a). List of contaminants & their maximum contaminant level (MCLs). Retrieved on September 17, 2011, from
View.
|
|
|
|
|
EPA (2009b). Food. Food safety. Food contaminants & adulteration. Data on Benzene in soft drinks and other beverages. Data through May 16, 2007.
|
|
|
|
|
EPA (2009c). Sprill prevention, control and countermeasure (SPCC) Rule Amendments. Who is subject to the SPCC rule?
|
|
|
|
|
EWG (Environmental working group) (2011). The power of information. Chemical index. Chemicals.
|
|
|
|
|
Dow (The Dow Chemical Company) (2009). Product safety assessment. High density polyethylene (HDPE) resins.
|
|
|
|
|
Dynalab (2011). Plastic properties of high density polyethylene (HDPE).
|
|
|
|
|
EOS/EDS (Electrical Overstress - Electrostatic Discharge) symposium proceedings. 22 (2000). ESD association in collaboration with IEEE, Anaheim: California. September 28-30, 1999, NY: Rome, 22: 2B.1.3.
|
|
|
|
|
FAO (2002). Small scale palm oil processing in Africa. FAO agricultural services bulletin 148:12.
|
|
|
|
|
FAO (2010). Rice: Grain storage hermetically sealed systems.Technology for agriculture. Proven technologies for smallholders.
|
|
|
|
|
Gangneux J, Noussair L, Bouakline A, Roux N, Lacroix C, Derouin F (2004). Experimental assessment of disinfection procedures for eradication of Aspergillus fumigatus in food. Blood, 104(7).
Crossref
|
|
|
|
|
Glevitzky M, Dumitrel GA, Perju D, Popa M (2009). Studies regarding the use of preservatives on soft drinks stability. Chem. Bull. "POLITEHNICA" Univ. (Timisoara). 54(68)1.
|
|
|
|
|
Gregg BR, Billups GL (2009). Seed conditioning. management. Enfield, NH. Sci. publish. 1:184.
|
|
|
|
|
Helmenstine MA (2011). How Do Detergents Clean?
|
|
|
|
|
Holst N, Meikle WG, Markham RH (2000). Grain injury models for Prostephanus truncatus (Coleoptera: Bostrichidae) and Sitophilus zeamais (Coleoptera: Curculionidae) in rural maize stores in West Africa. J. Econ. Entomol. 93(4)1338-1346.
Crossref
|
|
|
|
|
Knoxa R, Walkera J (1947). Bacteriological investigation of the washing and sterilization of food containers. J. Hygiene 45:151-158.
Crossref
|
|
|
|
|
Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, DeCock K, Rubin C, Kenya Aflatoxicosis Investigation Group (2005). Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya. Environmental Health Perspectives: 113. Retrieved February 20, 2007 from
Crossref
|
|
|
|
|
Lindblad C, Druben L (1980). Storage methods. Small farm grain storage. Volume III. Appropriate technologies for development. Manual M-2/ VITA publications manual series no. 35E. Arlington: VITA.
|
|
|
|
|
Malik VS, Schulze MB, Hu FB (2006). Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 84:274-288.
|
|
|
|
|
Merriam-webster (2010).
|
|
|
|
|
MTL (Mid-Continent Testing Laboratories, Inc.,) (2011). Limiting factors on a diesel engine's oil life. Rapid City, SD (USA): 1.
|
|
|
|
|
Mercer C (2006). Cadbury's benzene secret recall.
|
|
|
|
|
Myers D (2006). Surfactant science and technology. Hoboken: John wiley & Sons, Inc., publishing, P 122.
|
|
|
|
|
Murdock LL, Dogo SD, Ntoukam G, Kitch L, Shade RE (2003). Preservation of cowpea grain in sub-Saharan Africa—Bean/Cowpea CRSP contributions. Field Crops Res. 82:169-178.
Crossref
|
|
|
|
|
Navarro S, Donahaye E, Fishman S (1994). The future of hermetic storage of dry grains in tropical and subtropical climates. In: Highley, E., Wright EJ, Banks HJ, Champ BR (Eds). Proceeds of the 6th international working conference on stored product protection held at Canberra, Australia. Cambridge: CAB International, pp. 130-138.
|
|
|
|
|
Patwardhan N, Kelkar U (2011). Disinfection, sterilization and operation theater guidelines for dermatosurgical practitioners in India. Indian J Dermatol Venereol Leprol. 77(1)83-93.
Crossref
|
|
|
|
|
PHL Network (2009). Post harvest losses information system.
|
|
|
|
|
Shachman M (2004). The soft drinks companion: a technical handbook for the beverage industry. Boca Raton, FL: CRC Press, pp. xv-116.
Crossref
|
|
|
|
|
Schmidt RH, Erickson DJ (2008). FSHN04-09. Sanitary Design and Construction of Food Equipment. University of Florida. IFAS extension. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida.
|
|
|
|
|
Springer (2007). Lentil: an ancient crop for modern times. In: Yadav SS, Mcneil D, Stevenson, PC (Eds). New York, NY: Springer, pp. 407.
|
|
|
|
|
Sebastião J, Pint S, Lanças FM (2006). Hydrolysis of corn oil using subcritical water. J. Braz. Chem. Soc.17(1)85-89.
Crossref
|
|
|
|
|
Tsimihodimos V, Kakaidi M, Elisaf M (2009). Cola-induced hypokalaemia: pathophysiological mechanisms and clinical implications. Int. J. Clin. Pract. 63:833-835.
Crossref
|
|
|
|
|
Wiley-Blackwell (2006). Carbonated soft drinks: formulation and manufacture. In: Ashurst PR, Steen, P (Eds). Hoboken: Wiley-Blackwell, pp. 1-79.
|
|
|
|
|
Yakubu A, Bern CJ, Coats JR, Bailey TB (2011). Hermetic on-farm storage for maize weevil control in East Africa. Afr. J. Agric. Res. 6(14):3311-3319.
|
|