ABSTRACT
Sitophilus zeamais has been identified as one of the most destructive pests of maize stored in tropical regions. While most maize hybrids are being developed, it is necessary to evaluate their resistance to this pest. This study determined the resistance of selected maize hybrids to infestation by S. zeamais. Twenty two hybrids with varying resistance to weevil infestation and two checks DUMA 41-suceptible and MTP0701-resistant were used in a randomized complete block design experiment. Assessment was done at 10, 60 and 120 days of maize storage. Data was collected on percent weevil damage, grain weight loss and number of live and dead weevils. Heritability and correlation of factors were also estimated. Analysis of variance showed significant differences (P ≤ 0.05) on weight loss. The selection of the resistant genotypes was based on percent weight loss after 60 days. KH631Q and PH4 were selected as the most resistant and moderately resistant hybrids, respectively. The resistant check MTPO701 was also found to maintain resistance to weevil attack. There was a strong positive correlation between weight loss, number of live weevils and percent damage. Moderate heritability estimates of hybrids at 60 days of storage indicated the possibility of their parents to transfer the desirable traits to subsequent generations. Therefore, parents of the resistant hybrids could be utilized in breeding programs for maize weevil resistance and be deployed to farmers for use, respectively.
Key words: Hybrid, maize, post-harvest, resistance, Sitophilus zeamais.
Maize plays a major role in peoples’ livelihood in the sub-Saharan Africa. It is an important subsistent and cash crop for a majority of the population in this region (Midega et al., 2016). Several factors including low maize yield production and population growth rate have aggravated food insecurity (Cairns et al., 2013; FAO, 2013). Another key constraint which has worsened the situation is losses due to post harvest insect pests. It is estimated that, insect pests result to losses of up to 88% of the total maize produced in a season in sub-Saharan Africa (Ojo and Omoloye, 2012). In spite of several efforts being employed to curb insect pest menace (Midega et al., 2015), challenges contributing to post harvest losses remain a huge hindrance to sufficient food production (Tefera et al., 2011). In addition to feeding, insects further contaminate maize by accumulation of excretory products. Further deterioration is witnessed when insect presence and feeding raise grain temperature and moisture contents resulting to fungal activity (Tefera et al., 2011). In Kenya, more than 75% of local maize production is provided by farmers (Kang’ethe, 2011; Mohajan, 2014). Most breeding work in maize focuses on increased yield, field pests and disease resistance. While these efforts are worth, most of the farmers produce is destroyed after harvesting due to the introduction of improved varieties accompanied by reports of increased susceptibility to storage pests (Fortier et al., 1982; Kossou et al., 1992; Ricker-Gilbert and Jones, 2015). Post-harvest losses to storage insect pests such as the maize weevil Sitophilus zeamais have been recognized as an increasingly important problem (Markham et al., 1994; Midega et al., 2016). Maize weevil aggravates shortage of maize food by causing losses of 20 to 90% in Kenya (Giga and Mazarura, 1991; Kumar and Kalita, 2017). This reduces the nutritional value, germination capacity, grain weight and marketability of such grains. As a remedy to control of these pests, synthetic insecticides have been widely used on stored grains. There is a global concern with respect to environmental hazard, chemical residues on food, insecticides resistance development and associated costs (Cherry et al., 2005; Carvalho, 2017). To this regard, host-plant resistance as a pest control measure has been recognized to be environmentally safe, economically cheaper to farmers and integrates well with other components in pest management initiatives (Chapman, 2000; Carvalho, 2017). This has directed research to the development of resistant maize varieties. There has been progress in developing maize varieties that have multiple resistances to pests and diseases and improved agronomic performance. Despite the growing effort of developing weevil resistance varieties, little has been done on identifying resistant maize hybrids. Therefore, it was necessary to evaluate hybrids for resistance to maize weevil infestation and identify resistant hybrids.
Source of maize germplasm
Maize grains used in this study were from twenty two hybrids which had been planted at the Kiboko nursery in July 2016. The genotypes used were provided by the Kenya Agricultural and Livestock Research Institute (KALRO) – Katumani. The hybrids originated from local commercial enterprises within Makueni County.
Field trial and management
The experimental materials were evaluated at KALRO-Kiboko, a research centre situated in Makueni County. The mean annual rainfall is 530 mm and is spread over two very short rainy seasons. It lies at an altitude of 975 m above sea level and between latitude 2° 25’ S and longitude 37° 72’ E. Sand-clay type of soil occupies this location. Temperatures are uniformly high with mean maximum value of 35.1°C and the minimum of 14.3°C. The twenty two hybrids were planted in the Kiboko experimental site. Field sizes were 87.5 × 18 m2 and 87.5 × 30 m2, respectively. Each plot measured 5 × 0.75 m2. Fertilizer was applied at a standard rate of 30 kg calcium ammonium nitrate (CAN) and 30 kg Di ammonium phosphate (DAP) per ha. Supplementary irrigation was administered when needed. The fields were kept free from weeds by hoe weeding. Number of rows per plot was 2 and distance between stations, 0.25 m. Treatments were laid in a randomized complete block design (RCBD) with 4 replicates.
Grain preparation, insect culture and infestation
At harvest, sieving was done to remove any dirt, dust or broken grains. The mature maize weevil insects used for the evaluation were sourced from CIMMYT/KALRO-Kiboko post-harvest testing laboratory. The insects had been reared on commercial hybrid maize H614 under controlled conditions of 28°C and 70% relative humidity (Tefera et al., 2011). Fifty grams of grains was put in 250 ml capacity no-choice glass jars at room temperature and then thirty unsexed adult insects were introduced into the glass jars (Tefera et al., 2011). Glass jars were then covered with a lid made of wire mesh (1 ml) to allow for adequate ventilation and prevent escape of the weevils (Tefera et al., 2011).
Categories of samples
At harvest, the maize was arranged into three categories. Each category describes the time when the samples were assessed for insect damage. One category represented materials under storage for 10 days; the second had materials under storage for 60 days while the third, were materials stored for 120 days. Each category consisted of 22 entries replicated 4 times. The experimental set up for these genotypes was done at the same time.
Experimental design in the screening laboratory
Treatments were laid out in a randomized complete block design and kept on wooden shelves at room temperature in the laboratory. The experiment consisted of 22 germplasms replicated 4 times and put in 3 categories. A total of 264 samples were assessed in this experiment. MTP 0701 (resistant check) and DUMA 4 (susceptible check) to weevil infestation were incorporated in the study. Assessment of the trials was done at 10, 60 and 120 days of storage.
Data collection and assessment
Data was collected on weight of damaged and undamaged grains, live and dead weevils. On each assessment date (10, 60 and 120 days), the glass jars were opened, contents separated into grains, insects and dust using 4.7 and 1 mm sieves (Endecott Ltd UK). All maize weevils were separated and removed (by hand) from the maize at the end of these three storage periods. Separation of the damaged and undamaged kernels was done using grain tunneling and holes as the criteria (Tefera et al., 2011). These were counted and the percentage of damaged grain and grain weight loss computed. The percent damage was determined using the converted percent damage method of Baba-Tiertor (1994):
where GD is the damaged grain and WDG is the weight of damaged grain, and WDUD is the weight of damaged and undamaged grains. Weight loss was determined by the count and weight method of Gwinner et al. (1996):
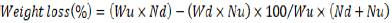
where Wu is the weight of undamaged grain, Nu is the number of undamaged grain, Wd is the weight of damaged grain, and Nd is the number of damaged grain. Genotypes were categorized as resistant (1-5%), moderately resistant (5.1-8%), moderately susceptible (8.1-10%), susceptible (10.1-13) and highly susceptible (>13.1%) after 60 days based on percentage weight loss, which was found to be a key trait of discriminating genotypes in relation to resistance (Mwololo et al., 2012; Tefera et al., 2011).
Data analysis
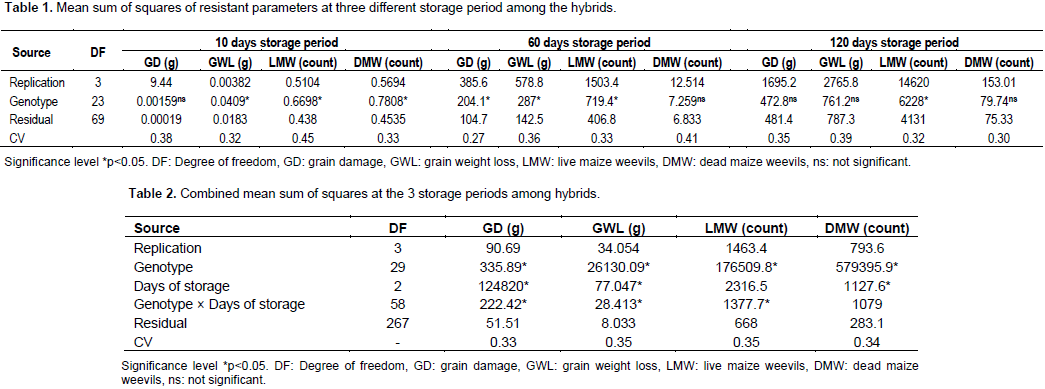
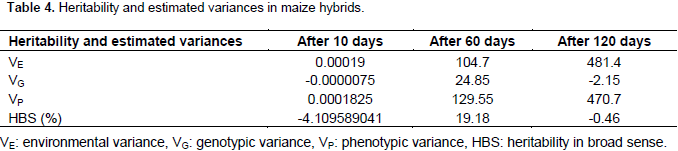
The data on percentage weevil damage, grain weight loss, live and dead weevils were subjected to GENSTAT 14th edition software and means separated using Fishers least significance difference at 5% probability level. Heritability was measured based on grain damage. Broad sense heritability was estimated based on Johnson et al. (1955) where by the error mean sum of squares (EMS) was considered as error variance (σ2e). Genotypic variance (σ2g) was derived by subtracting error mean sum of squares (EMS) from the genotypic mean sum of squares (GMS) and divided by the number of replications as given by the formula:
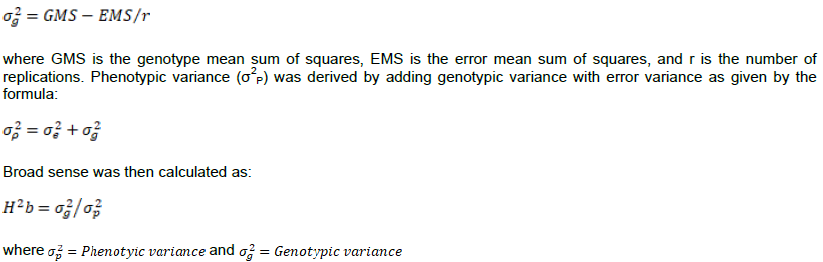
Pearson’s correlation coefficients were obtained using the GENSTAT 14th edition software. Correlations were computed to establish the interaction between grain weight loss, grain damage, live and dead weevils.
Maize weevil damage of hybrids
Genotypes did not differ significantly to weevil damage at 10 days of storage (Table 1). Maize weevil damage on genotypes after 10 days of infestation was negligible. The mean weevil damage was recorded as 0% (Table 3). It was evident that most genotypes had whole maize grains and were not damaged by the weevil. There were significant differences in hybrids response to maize weevil damage at 60 days and hence performance of hybrids to weevil damage was easily distinguished (Table 1). The mean weevil damage after 60 days of infestation was at 27%. Maize weevil damage on hybrids ranged from 4 to 48% (Table 3). After 60 days of weevil infestation, MTPO701 (resistant check) was least damaged at 4% followed by KH631Q at 9% (Table 3). The susceptible check was damaged at 29% while the highly damaged hybrid was DK8031 at 48% (Table 3). Hybrids were not significantly different in reaction to weevil damage at 120 days of storage (Table 1). Mean weevil damage in all hybrids after 120 days of storage was 51% (Table 3). Damage among hybrids varied from 28 to 67%. H513 and WH403 were the highly damaged hybrids after 120 days of weevil attack (Table 3). Damage in these hybrids was 67%. Nevertheless, MTP0701 (Resistant check) and KH631Q were the least damaged hybrids. The damage in these hybrids was 28 to 31%, respectively (Table 3). It was noted that after 120 days of weevil infestation, 6 hybrids recorded damages of less than 50%. Based on combined ANOVA analysis, significant differences in weevil damage were only recorded in storage periods. The hybrids and interaction between hybrids and storage periods were not significant (Table 2). Although no damage was recorded after 10 days, the highest damage was at 120 days of storage (Table 3). At 60 days there were significant damages on the hybrids grains but the damage peeked at 120 days. Weevil damage was consistently very low in the resistant check MTPO701 and hybrid KH631Q in all the storage periods (Table 3). They were damaged by 10 and 4%, respectively at 60 days.

Heritability and variances of weevil resistance on hybrids
Heritability in the broad sense (H2) of weevil resistance was estimated as described earlier by Johnson et al. (1955). Heritability was calculated based on the weevil damage. From the results, heritability was low and varied among storage periods. Broad sense heritability was -4% after 10 days, 19% after 60 days and -0.5% after 120days. Negative heritability was recorded at 10 and 120 days of storage (Table 4).
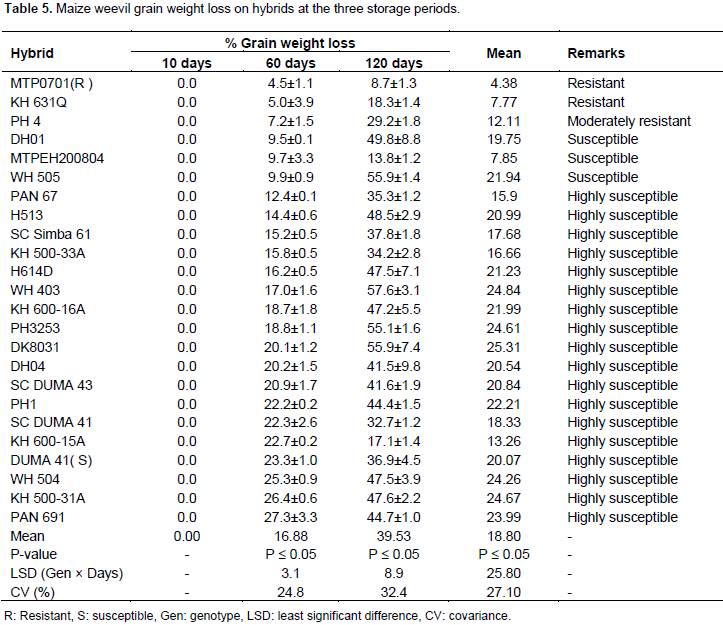
Maize weevil grain weight loss of Hybrids
There was no significant difference in weight loss among hybrids after 10 days of storage. Grain weight loss in all hybrids was considerably low and less than 1% after 10 days (Table 5). The mean grain weight loss of all hybrids was 0%. At this storage period, the hybrids grain weight loss varied from 0 to 0.01. The susceptible check DUMA 41 was not damaged after 10 days of storage (Table 5). The hybrids showed differences in percentage grain weight loss after 60 days of storage. The mean grain weight loss after 60 days of storage was at 17%. The grain weight loss of hybrids varied from 4 to 27%. According to the criteria of categorizing genotypes (Mwololo et al., 2012; Tadele et al., 2011) 1 genotype was resistant, 1 was moderately resistant, 3 were susceptible and 17 were highly susceptible (Table 5). As expected, the resistant check, MTP0701 had the least damage and hence recorded the least grain weight (Table 5). The susceptible check DUMA 41 recorded a weight loss of 23% and hence was grouped among the highly susceptible genotypes. Among the hybrids, PAN 691 and KH 500-31A had the most grain weight loss. The weight loss in these hybrids was 27 and 26%, respectively. At this stage about 45% of hybrids had lost more than 20% of the grain weight. The hybrids did not differ significantly in grain weight loss after 120 days of storage. At this stage, the hybrids had lost more grain weight than after 60 days, and the mean loss was at 39.5%. This storage period recorded the highest grain weight loss which varied from 8.7 to 57.6% (Table 5). The resistant check; MTP0701 had the least weight loss at this storage period. On the contrary, PH3253, DK8031, WH 505 and WH 403 lost the most grain weight at this period. The grain weigh loss in these hybrids was above 50% (Table 5). From combined ANOVA results, the hybrids did not differ significantly in grain weight loss and in the interaction of hybrids and storage days. Grain weight loss increased gradually after the 60 days of storage and was most observed at 120 days in all hybrids. The least and most grain weight loss was recorded after 10 and 120 days, respectively (Table 5).
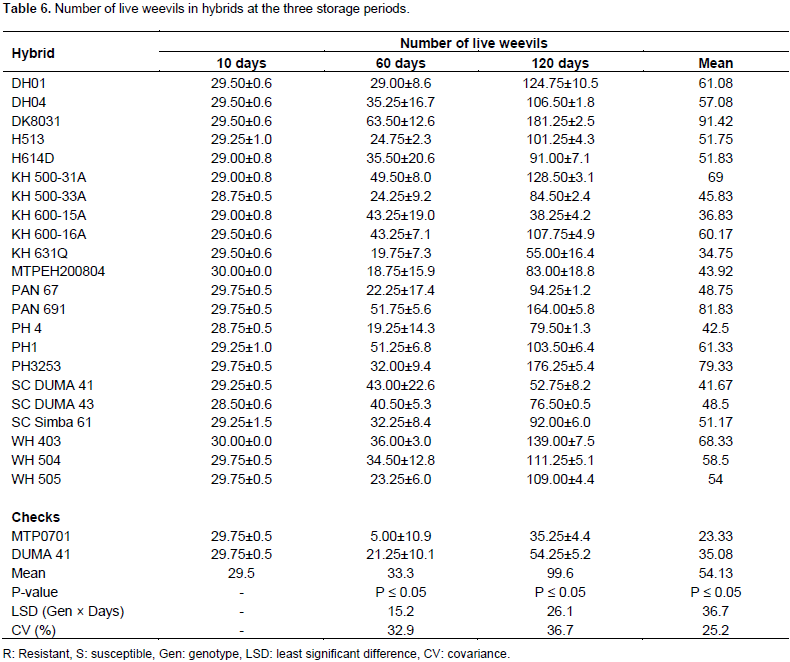
Number of live weevils in hybrids
After 10 days of storage, hybrids did not show differences in number of live weevils present in the grains. At the start of the experiment, 30 live insects had been introduced in each glass jar containing grains of hybrids. After 10 days of storage the live insects were ranging from 29 to 30 (Table 6). This showed that only one weevil had died in most hybrids. However, 12 hybrids retained the number of live insects introduced at the start of the experiment. There were significant differences in number of live weevils among hybrids when hybrids were stored for 60 days. At this storage period, a mean of 33 live weevils was recorded. Nonetheless, the number of live weevils varied from 5 to 64 at this storage period.The least number of weevils was recorded in resistant check MTPO701. This check had a mean number of five weevils after 60 days of storage. In this check, number of live weevils had reduced by 25. Similarly, in nine hybrids, live weevils had reduced by between 1 and 12 weevils (Table 6). However, in 14 hybrids number of weevils had increased. DK8031 had the highest number of live weevils. This hybrid had doubled the number of live weevils to 64 at this storage period (Table 6). Hybrids did not differ significantly in number of live weevils after 120 days of storage (Table 1). At this stage, live weevils ranged from 35 to 181. The mean number of live weevils had tripled after 60 days and was at 99 (Table 6). At this stage, 15 hybrids had at least tripled number of weevils than the introduced. The least number of live weevils were in hybrids, KH 600-15A and SC DUMA 41. The number of live weevils in the two hybrids was at 38 and 52, respectively. The resistant check MTP0701 had the least number of weevils (35 weevils on average). The susceptible check DUMA 41 had 54 weevils at this stage. However, 2 hybrids had lesser number of live weevils than the susceptible check (Table 6). In the combine ANOVA, significant differences in the number of weevils were recorded between storage periods and hybrids. However, the interaction of the two factors was not significant (Table 2). The live weevils were retained at 10 days but increased after 60 days of storage. However, at 120 days of storage, the live weevils had tripled the introduced number. In all storage periods, the highest number of live weevils were at 120 days of storage and lowest at 10 days (Table 6).
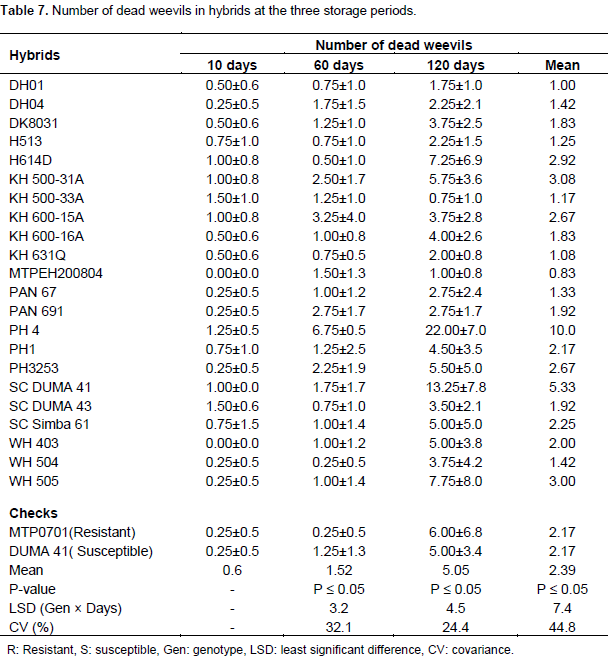
Number of dead weevils in hybrids
At 10 days of storage, hybrids were significant for number of dead weevils. However, only one weevil had died in 11 hybrids. The rest of the hybrids had live weevils (Table 7). Hybrids did not show significant differences in number of dead weevils after 60 days (Table 1). At this storage period, dead weevils had increased and were ranging from 0 to 7 (Table 7). Resistant check MTPO701 and WH504 lacked dead weevils at this stage. The remaining hybrids had at least one or two dead weevils after 60 days of storage (Table 7). The number of dead weevils was also found to be insignificant among hybrids after 120 days of storage (Table 1). At this stage, dead weevils were at an average of 5 but varied from 1 to 22. At 120 days of storage, the highest numbers of dead weevils were in hybrid PH4. The check MTP0701 and DUMA 41 had dead weevils averaging at 5 and 6, respectively (Table 7). In the combined analysis, significant differences in number of dead weevils were only reported in storage periods. The highest number of dead weevils was recorded after 120 days of storage. The number of dead weevils was considerably lower than live weevils at the three storage periods (Tables 6 and 7).
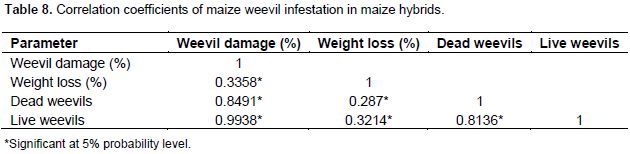
Correlations
There was a significant correlation among all the factors. In all the factors, numbers of live weevils were strongly correlated to percent of weevil damage. The correlation coefficient in these factors was 0.99. Unlike the inbred lines where weight loss and percent of weevil damage had the most association, correlation coefficient was low in these factors (r=0.34) in hybrids (Table 8). Live weevils correlated well with dead weevils giving a coefficient of 0.81 while dead weevils and percent of weevil damage resulting in coefficient of 0.85 (Table 8).
Maize weevil damage of Hybrids
The present study showed great variation in hybrid resistance to weevil attack after 60 days of storage and hence hybrids could easily be separated on the basis of resistance at this storage period. Minimum and insignificant damage was witnessed after 10 days whereas maximum damage observed after 120 days of storage. Such variation in maize weevil has been earlier identified to exist in maize genotypes from eastern, southern and western Africa (Gafishi et al., 2012). This resistance could either be due to physical factors such as grain hardness or antibiosis as a result of biochemical compounds which are toxic to the insects (García-Lara et al., 2004; Siwale et al., 2009; Munyao, 2015). Resistance mechanisms have been classified into three categories: non-preference for oviposition, food or shelter (also called antixenosis), antibiosis referred to the adverse effect of the plant on the biology of the pest, and tolerance or the plant’s ability to repair, recover or withstand infestation (Derera et al., 2001; Dhliwayo et al., 2005). Biochemical properties such as a- amylase- and protease-inhibitors (Dari et al., 2010) and phenolics (Sori and Keba, 2013), may explain the basis of resistance to weevil infestation in this study.
Heritability of maize weevil resistance
Estimate of heritability assists breeders to allocate resources necessary to effectively select for desired traits and to achieve maximum genetic gain with little time and resources. Heritability is recommended to be considered in association with genetic advance to predict the effect of selecting superior crops varieties. In this study heritability for weevil resistance in hybrids was low. Dhliwayo et al. (2005) reported that inheritance of weevil resistance is complex and heritability is likely to be small to moderate. Low heritability indicates slow progress in selection for this character. This may explain why resistance to maize weevil resistance is influenced by additive and non-additive gene effects. Also, low heritability levels could be because most of the evaluated hybrids were being developed for other agronomic traits and hence weevil resistance was not considered as a primary factor during selection.
Maize weevil grain weight loss of hybrids
At 10 days, there was no damage while at 120 days most genotypes had attained maximum damage. However, after 60 days of storage it was easy to identify and categorize genotypes into groups of resistance and susceptibility. Therefore, the hybrids KH631Q and PH4 were identified as resistant and moderately resistant, respectively. It has been reported that resistant varieties offer a sustainable, cost effective and environmental friendly way to reduce damage by S. zeamais under storage conditions (Gu et al., 2008). Gafishi et al. (2012) also identifies host plant resistance to storage pests as a component of integrated pest management that is particularly important in developing countries, where maize is stored under often inappropriate conditions due to lack of knowledge or resources. Therefore, the identified resistant varieties can be used as a source of resistance in breeding programs and subsequently be adapted by smallholder farmers to diversify the basis of resistance to this pest. The selected resistant hybrids exhibit genetic factors which confer resistance to maize weevil attack. Within the first storage period (10 days), none of the varieties suffered any significant damage or weight loss. But beyond that, there were increases in weevil numbers, leading to increased weight losses. According to Wangui (2016), despite the shape, size and hardness of the grain, its chemical and nutritional composition are important primarily in resisting insect attack and damage; the length of exposure of the grain to the pest may also affect the level of infestation of maize varieties by S. zeamais. This therefore, results in increased grain weight losses. Grain weight losses were generally lower at 10 days of storage with higher losses being at 60% at 120 days. Hossain et al. (2007) reported that grain loss of 12 to 20% is common, but up to 80% has been reported for untreated kernels.
Number of live weevils in hybrids
The number of live insects remained unchanged after 10 days since new insects had not emerged within this short period. According to Gafishi et al. (2012), complete development for the life cycle of the maize weevil averages 36 days. This could also mean that short storages of studied hybrids up to two weeks are also possible. However, at 60 days number of maize weevils decreased and increased in some hybrids. The hybrids with highest number of weevils were mostly damaged as it was the case for DK8031. This variety has been found to be susceptible to maize weevil damage and hence a favourable host for maize weevil (Kalunde, 2011). In this study, numbers of live S. zeamais varied with the maize hybrid varieties used. Therefore, the shortest developmental times occurred on the varieties which had the largest number of weevils emerging. On the other hand, the longest developmental times occurred in varieties with the least number of live weevils. The development of an insect is influenced by nature of food the insect is reared on. Generally, more eggs are laid on and development is faster on a more favourable than a non-favourable hosts. Increased maize weevil emergence is a result of high susceptibility of a genotype on which weevils can feed easily and therefore produce many eggs and progeny. From the study, the number of live weevils is also primarily significant in assessing resistance varieties to maize weevil.
Number of dead weevils in hybrids
The number of dead weevils was relatively low in hybrids confirming the reports of Abebe et al. (2009) who also found a low mortality rate of maize weevils. However, the rate of mortality of weevils has been revealed not to be a good indicator for weevil resistance among maize varieties.
Correlations
Four resistance measures were used in this study in order to carefully evaluate the hybrids resistance to maize weevil and to draw confidently accurate conclusions. The correlations found between the four resistant measures are presented in the results. The results showed that percent damage, percent grain weight loss and number of live weevils are associated with weevil resistance. Therefore, hybrids that were less damaged, had less weight loss and fewer numbers of live insects were considered resistant to maize weevil attack. Also, this implies that increased numbers of weevils results in increased grain damage and hence more grain weight loss. This strong association between the factors has been reported before by Zunjare et al. (2014) and Derera et al. (2010).
This variation in response to the maize weevil attack among hybrids gives evidence of genetic diversity for breeding that exists in the parents utilized in making the hybrids. This offers a great opportunity to exploit the variability with the aim of reducing post-harvest insect-pest losses through genetic improvement. The selected resistant hybrids should also be used in areas considered to be maize weevil hotspot.
The authors have not declared any conflict of interests.
The author sincerely thanks Kenya Agricultural and Livestock Research Organization (KALRO)-Katumani and International Livestock Research Institute/Biosciences of East and Central Africa (ILRI/BecA) for funding his MSc program. Technical staffs of both organizations are greatly acknowledged for their unwavering thoughtful support during the entire project period.
REFERENCES
|
Abebe F, Tefera T, Mugo S, Beyene Y, Vidal S (2009). Resistance of maize varieties to the maize weevil Sitophilus zeamais (Motsch.)(Coleoptera: Curculionidae). Afr. J. Biotechnol. 8(21).
|
|
|
|
Baba-Tierto N (1994). Ability of powder and slurries from ten plant species to protect stored grains from attack by Prostephanusus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus oryzae (L). (Coleoptera: Curculionidae). J. Stored Prod. Res. 30:297-301.
Crossref
|
|
|
|
Cairns JE, Hellin J, Sonder K, Araus JL, MacRobert JF, Thierfelder C, Prasanna BM (2013). Adapting maize production to climate change in sub-Saharan Africa. Food Security 5(3):345-360.
Crossref
|
|
|
|
Carvalho FP (2017). Pesticides, environment, and food safety. Food Energy Security 6(2):48-60.
Crossref
|
|
|
|
Chapman RF (2000). Entomology in the Twentieth Century. Ann. Rev. Entomol. 45:261-285.
Crossref
|
|
|
|
Cherry AJ, Bantino A, Djegui D, Lomers C (2005). Suppression of the stem borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest Manage. 50:67-73.
Crossref
|
|
|
|
Dari S, Pixley KV, Setimela P (2010). Resistance of early generation maize inbred lines and their hybrids to maize weevil [(Motschulsky)]. Crop sci. 50(4):1310-1317.
Crossref
|
|
|
|
Derera J, Pixley KV, Giga DP (2010). Appraisal of protocol for the rapid screening of maize genotypes for maize weevil resistance. Afr. J. Entomol. 18:1.
Crossref
|
|
|
|
Derera J, Pixley V, Giga DP (2001). Resistance of maize to the maize weevil. I. Antibios. Afr. Crop Sci. J. 9:431-440.
|
|
|
|
Dhliwayo T, Pixley KV, Kazembe V (2005). Combining ability for resistance to maize weevil among 14 southern African maize inbred lines. Crop sci. 45(2):662-667.
Crossref
|
|
|
|
Food and Agriculture Organization (FAO) (2013). Crop Prospects and Food Situation. Accessed 15.03.16.
View
|
|
|
|
Fortier G, Amason J, Lambert JDH, McNeil J, Nozzolill C, Philogene B (1982). Local and Improved corns in small farm agriculture in Belize, C. A; the taxonomy, productivity and resistance to Sitophilus zeamais. Phytoprotection 21:68-78.
|
|
|
|
Gafishi KM, Karungi J, Asea G, Gibson P (2012). Determination of the heterotic groups of maize inbred lines and the inheritance of their resistance to the maize weevil. Afr. Crop Sci. J. 20(1).
|
|
|
|
García-Lara S, Bergvinson DJ, Burt AJ, Ramputh AI, Díaz-Pontones DM, Arnason JT (2004). The role of pericarp cell wall components in maize weevil resistance. Crop Sci. 44(5):1546-1552.
Crossref
|
|
|
|
Giga DP, Mazarura UW (1991). Levels of resistance to the maize weevil, Sitophilus zeamais (Motsch.) in exotic, local open-pollinated and hybrid maize germplasm. Int. J. Trop. Insect Sci. 12(1-2-3):159-169.
|
|
|
|
Gu H, Edwards OR, Hardy AT, Fitt GP (2008). Host plant resistance in grain crops and prospects for invertebrate pest management in Australia: an overview. Austr. J. Exp. Agric. 48:1543-1548.
Crossref
|
|
|
|
Gwinner J, Harnisch R, Muck O (1996). Manual on the prevention of post-harvest seed losses, post-harvest project, GTZ, D-2000, Hamburg, FRG P 294.
|
|
|
|
Hossain F, Boddupalli PM, Sharma RK, Kumar P, Singh BB (2007). Evaluation of quality protein maize genotypes for resistance to stored grain weevil Sitophilus oryzae (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 27:114-121.
Crossref
|
|
|
|
Johnson HW, Robinson HF, Comstock RE (1955). Estimation of genetic and environmental variability in soybeans. Agron. J. 47:314-318.
Crossref
|
|
|
|
Kalunde FT (2011). Diversity of storage insect pests in maze and susceptibility of maize varieties to maize weevil. A thesis submitted to University of Nairobi.
|
|
|
|
Kang'ethe E (2011). Situation analysis: improving food safety in the maize value chain in Kenya. Report prepared for FAO. College of Agriculture and Veterinary Science, University of Nairobi, Nairobi.
|
|
|
|
Kossou DK, Bosque-Pérez NA, Mareck JH (1992). Effects of shelling maize cobs on the oviposition and development of Sitophilus zeamais Motschulsky. J. Stored prod. Res. 28:187-192.
Crossref
|
|
|
|
Kumar D, Kalita P (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 6(1):8.
Crossref
|
|
|
|
Markham RH, Bosque-Perez NA, Borgemeister C, Meikle WG (1994). Developing pest management strategies for the maize weevil Sitophilus zeamais, and the larger grain borer Prostephanus truncatus, in the humid and sub-humid tropics. FAO Plant Protection. Bull. 42:97-116.
|
|
|
|
Midega CA, Murage AW, Pittchar JO, Khan ZR (2016). Managing storage pests of maize: Farmers' knowledge, perceptions and practices in western Kenya. Crop Protection 90:142-149.
Crossref
|
|
|
|
Midega CAO, Bruce TJ, Pickett JA, Khan ZR (2015). Ecological management of cereal stem borers in African smallholder agriculture through behavioural manipulation. J. Ecol. Entomol. 40(Suppl. 1):70-81.
Crossref
|
|
|
|
Mohajan HK (2014). Food and nutrition scenario of Kenya. Am. J. Food Nutr. 2(2):28-38.
|
|
|
|
Munyao WM (2015). Xenia effect on resistance to maize weevil and larger grain in maize (Doctoral dissertation, Kenyatta University).
|
|
|
|
Mwololo JK, Okori P, Mugo S, Tadele T, Otim M, Munyiri SW (2012). Resistance to the maize weevil (Sitophilus zeamais Motsch) among maize inbred lines. In RUFORUM Third Biennial Conference, Entebbe, Uganda, 24-28 September 2012. RUFORUM.
|
|
|
|
Ojo JA, Omoloye AA (2012). Rearing the maize weevil, Sitophilus zeamais, on an artificial maize-cassava diet. J. Insect Sci. 12:1-9.
Crossref
|
|
|
|
Ricker-Gilbert J, Jones M (2015). Does storage technology affect adoption of improved maize varieties in Africa? Insights from Malawi's input subsidy program. Food policy 50:92-105.
Crossref
|
|
|
|
Siwale J, Mbata K, Mcrobert J, Lungu D (2009). Comparative resistance of improved maize genotypes and land races to maize weevil. African Crop Science Society, Uganda. Afr. Crop Sci. J. 17:1-16.
|
|
|
|
Sori W, Keba T (2013). Differential resistance of maize varieties to maize weevil (Sitophilus zeamais Motschulsky) (Coleoptera: Curculionidae) under laboratory conditions. J. Entomol. 10:1-12.
Crossref
|
|
|
|
Tefera T, Mugo S, Likhayo P (2011). Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil Sitophilus zeamais and the Larger Grain Borer Prostephanus truncatus. Afr. J. Agric. Res. 6(10):2249-2254.
|
|
|
|
Wangui KA (2016). Utilization of lighted candle and sealing methods in metal silos for management of the larger grain borer, prostephanus truncatus (horn) (coleoptera; bostrichidae) in stored maize. (Doctoral dissertation, Department of Plant Science and Crop Protection, University of Nairobi).
|
|
|
|
Zunjare R, Hossain F, Thirunavukkarasu N, Muthusamy V, Jha SK, Kumar P, Gupta HS (2014). Evaluation of specialty corn inbreds for responses to stored grain weevil (Sitophilus oryzae L.) infestation. Ind. J. Genet. Plant Breed. 74:564-567.
Crossref
|