This review is aimed at highlighting the issue of ethno-veterinary medicine (EVM) and the progress and limitations in its application in Zimbabwe.It is a fact that animal diseases are a major constraint to livestock production and development, particularly in communities living in marginal areas affected with endemic pathogens, vectors and diseases. These communities do not have access to modern veterinary information and services or are less economically endowed albeit coping with enormous animal health problems. Their animal health care management systems are based on people's own local and inherent indigenous knowledge of EVM. It is crucial to learn, evaluate, and without being biased and ethnocentric, promote and integrate the beneficial facets of traditional animal health care practices into current primary livestock health care delivery services. This review article highlights some developments in understanding and recourse to EVM.It also discusses a variety of issues related to the use and understanding of EVM in many different ways by different stakeholders in Zimbabwe. The review further outlines the possible way forward.
Parasites present a periodic problem particularly to grazing livestock as they are always exposed to and, therefore, constantly reinfected (Masikati, 2010). The levels of infestation for untreated animals raised in natural settings tend to fluctuate with seasonal metabolisms. It has been observed in goats and sheep that the highest levels of parasites correspond to periods of change: change in location (e.g. buildings to pasture); change in diet or use of food (e.g. lactation to maintenance diet) (Duval, 1994). The use of synthetic drugs against internal and external parasites only provides short term solutions, thus it has to be done consistently in order to maintain low parasitic concentrations in livestock. However, this is mainly possible when supported by donor aid in Zimbabwe. As long as the funds remain available, synthetic drugs will be the main methods of controlling parasites in livestock production systems (Masimba et al., 2011).The reliance on donor funds is, however, not sustainable.
In the absence of funds, farmers face the challenge of scarcity, erratic supply and/or prohibitive costs of synthetic drugs or veterinary services and they usually revert back to more appropriate and sustainable traditional systems of animal health care (Mathias and McCorkle, 2004). According to the Word Health Organisation (WHO), at least 80% of people in developing countries depend largely on indigenous practices for the control and treatment of various diseases affecting both human beings and animals (Jabbar et al., 2005). In Zimbabwe for example the Department of Veterinary Services used to supply acaricides to all communal dips in the rural areas, but since the economic depletion many dip tanks have not been operational as the Department failed to supply these acaricides (Mwale et al., 2005). Initially some farmers resorted to using commercial acaricides but the prices of these acaricides became deterrent that most farmers were unable to dip or spray their cattle. Eventually, these resource-limited farmers resorted to using locally available medicinal plants to control livestock diseases, ectoparasites and other related vectors (Mutibvu et al., 2012). Mwale et al. (2005) acknowledged that in Zimbabwe EVM is gaining recognition at the expense of conventional drugs especially because of its greater accessibility, lower costs and apparent effectiveness. The objective of this paper is to highlight the main issues in the reappraisal and to point out possible ways forward for use of EVM in Zimbabwe. It is important to first prompt a reappraisal of the potential and limitations of both ethno and modern veterinary medicine.
The major problems associated with the use of synthetic chemicals include; environmental pollution, development of resistant strains (Dipeolu and Ndungu, 1991), and delay in the development of immunity in young animals (Dipeolu, 1991). Most products are sold with English instructions for use and safe handling rather than the local language which can be understood by local users who are in most cases illiterate hence the frequency of incorrect application is high. In Kenya adulteration of commercial livestock drugs such as synthetic anthelmintics has been established to be a common practice leading to resistance of some disease causing organisms in livestock (Githiori, 2004).Resistant strains of ticks were reported to have developed against benzene hexachloride, dichlorodiphenyltrichloroethane (DDT) and toxaphane in a period ranging between 18 months to five years (Kayaa, 2000). Some internal parasites have developed resistance against deworming drugs asbenzimidazole, levamisole and even Ivermectin because of too frequent use (Duval, 1994). Studies carried out by Kayaa (2000) have also revealed that synthetic dewormers slow the decomposition of manure. The annual cost for importing acaricides has been estimated at US$9.3 million in Zimbabwe (Perry et al., 1990), US$16 million in Kenya (Tatchell et al., 1986), US$10 million in Zambia (Pegram et al., 1988), US$26 million in Tanzania (Kagaruki, 1997) and Uganda (Okello-Onen and Nsumbuga-Mutaka, 1997). The overall loss due to ticks has thus been estimated at US$720 million per year (Dipeolu and Ndungu, 1991).
Cultural livestock health care practices
Ethno-veterinary medicine (EVM) provides alternatives for controlling both internal and external parasites in livestock production systems that are environmentally friendly, relatively cheap and not prone to development of resistant parasitic strains. Ethno-veterinary medicine involves the use of indigenous beliefs, knowledge, skills, methods and practices pertaining to the health care of animals (Mathius-Mundy and McCorkle, 1989).Perhaps of more importance is the fact that herbal remedies are known to be broad spectrum and therefore may be a future answer to resistance development of pathogens to conventional drugs (Mwale et al., 2005). Ethnoveterinary medicine knowledge varies from region to region as well as between and within communities. Documentation of this traditional knowledge is limited in many developing countries including Zimbabwe. Storage of this knowledge is solely depended on the collective memory of just a few entrusted persons within communities for it is just not common 'knowledge' for everybody. It has been transmitted across generations by an oral tradition which according to Matekaire and Bwakura (2004) is in danger of extinction. A survey conducted by Masimba et al. (2011) also reiterated that the knowledge of ethnoveterinary practices was mostly passed down generations orally. Their study revealed the sources of EVM knowledge as grandparents and parents (61.5%), friends (20.5%), neighbours (12.5%), and others (6.5%). The situation is worsened by rapid socio-economic, technological and environmental changes (Sarasan et al., 2011). Documentation of ethno-veterinary practices is critically urgent so that the knowledge can be preserved, plants conserved and sustainably managed for the control of livestock diseases.
As wide spread as it is, the practice of EVM has lagged behind that of its counterpart (modern veterinary medicine) mainly because the practice was secretly done and its information hidden in the gray literature (Mathias, 2004). Ethno-veterinary practice has its own limitations as well. Over-harvesting threatens the diversity of habitats which includes semi-arid woodland and savanna habitats. Diversity is also threatened by high deforestation rates and overexploitation (Wanzala et al., 2005). Efforts are needed to propagate and cultivate those species most at risk. A better scientific understanding of where these plants grow and how they work will optimise their collection and sustainable use. Also all plants are not necessarily harmless, so a better understanding of their chemistry can help to evaluate the associated risks. For example, nicotine, a well known compound in tobacco plants is actually insecticidal and the plant is consequently used for this purpose by some farmers. However, nicotine is also well known for its toxicity to man and so the use of tobacco plants for this purpose needs to be carried out with care (Mathias, 2004). As indicated earlier EVM knowledge is not universally recognized as a valid method of disease control as it differs from region to region as well as within communities. It has been developed through trial and error and deliberate experimentation, therefore the practice is less systematic and less formalized (Matekaire and Bwakura, 2004).In Zimbabwe as in other African countries many veterinarians and decision makers have not examined its potential and/or have been trained to ignore or ridicule it. There is still need for the validation, documentation and acknowledgement of EVM in Zimbabwe among other tropical countries.
Merits of ethno-veterinary medicine
However, despite these limitations, EVM has been scientifically proven to control a wide spectrum of common livestock diseases such as diarrhea, wounds, coccidiosis and reproductive disorders (Mlambo et al., 2011; Mwale et al., 2005; Matekaire and Bwakura, 2004). In recent years a growing number of researchers from varied fields have studied, valued, confirmed, validated and documented the potential effectiveness of the traditional animal health management systems in native and local communities (Wanzala et al., 2005).Research has also provided an understanding of the plant chemistry and modes of action for plant species already used by many farmers. Research results have revealed several pesticidal plants that can be used reliably and safely to treat livestock. Legal registration of these botanical products is usually not required for their promotion. This approach offers sustainable strategies directed towards developing sound and appropriate animal health care systems suitable and relevant to rural communities in improving livestock performance and production and, hence, livelihood(Stevenson et al., 2010).
Integrating ethno-veterinary medicine in modern livestock health care systems
Like any other knowledge systems, EVM is very dynamic in its management and practice (Wazala et al., 2005). As a result of this dynamism, many ethnopractitioners find themselves in a situation where they complement EVM with modern veterinary medicine, especially in cases where EVM is limited and or may be deemed dysfunctional (Martin et al., 2001; Mathias, 2004).
Enhancement of this approach is most likely to spur research and development of EVM and undoubtedly enable it to make immense strides in the development of the livestock industry. This however, requires good knowledge and understanding of EVM limitations and successes, needs and circumstances of local practitioners, mutual understanding and co-operation between conventional veterinarians and ethnopractitioners and respect to Intellectual Property Rights (IPR).
According to a survey conducted in Gutu District, Zimbabwe by Masimba et al. (2011) none of the households relied on conventional medicines alone in treating poultry diseases. Ninety five percent of the households used traditional medicines only whilst the rest employed a combination of traditional and conventional remedies.
Common herbs used for ethno-veterinary disease control
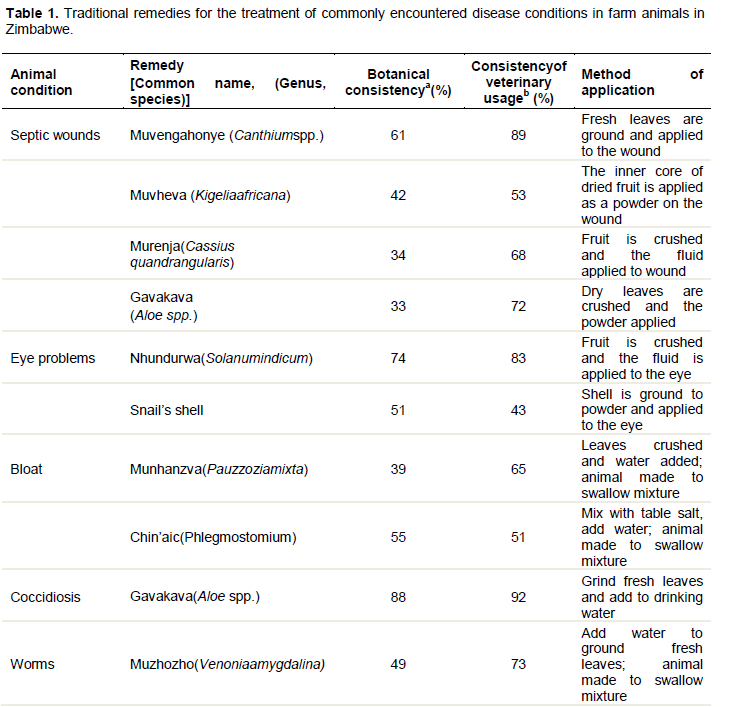
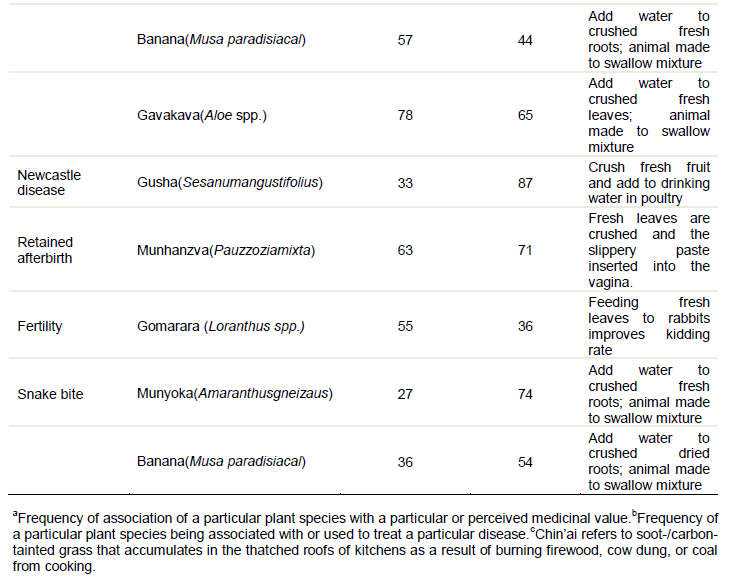
Mari et al. (2005) carried out a diagnostic survey of the use of herbal plants in poultry health management in a small-scale farming area in Masvingo, Zimbabwe. They revealed the therapeutic value of some of the indigenous practices and emphasized on the need to document indigenous knowledge systems before they are lost. Their surveys showed that the majority of farmers were familiar with pesticidal plants, but very few actually used them. This highlights the need to foster the actual use of these plants, to maximise the benefits they can bring to resource-limited farmers. They listed herbs that are commonly used by smallholder farmers to treat livestock in Zimbabwe. The listed herbs included: Boswellia serata (frankincense), Adansonia digitata (baobab lemonade), Adenium multiflorum(impala lily), Aloe spicata, Aloe vera (burn plant), Cussonia arborea (Chibwabwa/Chipombola), Cycnium adonense (the ink plant), Cyperus articulatus (jointed flatsedge), Allium sativum (garlic), A. spicata, Lycopersicon esculentum, Myrothamnu sflabellifoilius, Lannea stullmannii, Ficus burkei, Sarcostemma viminale, Capsicum annum, Parinaria curatellifolia, Albizia gummisera, Albizia adianthifolia, and soot. Of these A. vera was reported to be arguably the most important as it is found in many geographical regions and is effective against a wide range of diseases and ailments (Mwale et al., 2005). This is attributed to its several pharmacological properties which include antibacterial, antifungal, antivenin as well as strong immunological properties (Mwale et al., 2005). Mwale et al. (2005) also reported that A.vera contain acetylated mannans or acemannan sugars which are responsible for boosting immunity in animals, hence Alloe species has wide range of uses. Externally A. vera has been used to treat wounds (mixed with used engine oil in rural areas), skin irritations including burns, bruises and abrasions as well as general inflammatory skin disorders (Mwale et al., 2005). The authors went on to indicate that A. vera has anti-allergy and anti-inflammatory properties because of the presence of glycoproteins and anthraquinones which block the regeneration of thromboxanes and inhibit bradykinin. Table 1 shows common animal conditions and their perceived remedies as recorded by Matekaire and Bwakura (2004):
Small-scale farmers’ attitude towards veterinary services
Matekaire and Bwakura (2004) reported results from a survey they carried out in Mashonaland East, West and Central, Zimbabwe. The survey revealed that approximately 95% of communal area farmers never avail themselves of the veterinary services offered by the Government except for cattle dipping, which is legally mandatory. The farmers cited prohibitive costs of drugs and services provided, while others perceived the veterinary health service as an organization that destroys livestock in the event of disease outbreaks, and thus would not seek assistance from them. According to the study carried out by Matekaire and Bwakura (2004) traditional animal healers in Zimbabwe have less to offer in the treatment and control of epidemic and endemic infectious diseases like foot and mouth disease, rinderpest, anthrax, and acute life-threatening bacterial diseases. However, they can cope with a reasonable spectrum of common symptoms and conditions such as septicaemia, diarrhoea, wounds, colds, worms, and reproductive disorders.In addition, they have been successful with numerous bacterial diseases including coccidiosis, mycobacteriosis, plague and a wide spectrum of coliform diseases.
Most of the non-conventional treatment of ailments involved the use of plant parts except for the use of soot (Mwale et al., 2005). Matekaire and Bwakura (2004) also reported that every household made use of A.vera. Over 95% of the households acknowledged the use of Sarcostemma viminale intreating ailments in poultry.Plant part used was similar across all the four villages, implying this knowledge is common and generally shared among households within a common setting (Matekaire and Bwakura, 2004).
Ethnoveterinary control of external parasites
Ticks are external parasites on cattle that cause tick worry and disturbs animals from reaching their potential productivity (Norval et al., 1994). They also cause tick borne diseases (TBD) which reduce cattle productivity and, if left untreated, may result in their death. Conventional control for TBD vectors in Zimbabwe involves the use of conventional acaricides as cattle dips or sprays. Regular acaricides treatment of cattle is expensive and so, for economic reasons, the Government of Zimbabwe is no longer enforcing a policy of strict tick control (Norval et al., 1994). It is likely that reduced tick control will result in the spread of Amblyomma ticks to previously uninfested areas (Norval et al., 1994). Farmers ought to make use of medicinal plants that have been proven to be effective against ticks.
Preliminary surveys carried out by Madzimure et al. (2011) reported Lippia javanica (Verbencaceae), Strychnos spinosa and Solanum panduriforme (incanum) as plants with acaricidal properties in Zimbabwe. Subsequent studies by Madzimure et al. (2011) revealed that L. javanicais effective in controlling cattle ticks that cause morbidity and spread fatal blood diseases in livestock. Tick counts on cattle treated with plant extracts at an application rate of 10% were as low as on cattle treated with the standard Amitrazbased commercial product (Tickbuster). While some tick parasitism remained on these cows (approximately 10% of that found on untreated cows), peripheral blood samples showed no haemoparasites in the treated cattle, implying that animals did not suffer from clinical tick-borne diseases after treatment with L.javanica extracts. Further studies were carried to confirm efficacy and determine toxicity of these plant species. In this experiment the efficacy and toxicity of the plants were evaluated against a positive control of an Amitraz based commercial acaricide (Tickbuster). Water leaf extracts (5, 10 and 20% w/v) and fruit extracts in the same concentrations at v/v were sprayed on Mashona animals for six weeks and daily tick counts collected. The efficacy trials carried out at Henderson Research Station showed that all the plants had acaricidal properties (P <0.05). There was no significant difference between Tickbuster and 10% L. javanica treatment. In the other plant species the 5% treatment was more effective than the higher concentrations. Acaricidal activity was attributed to the various chemical constituents of the plants.
In the toxicity experiments water extracts of the plant materials were orally administered to sexually mature Balb/c mice. Overall mortality data was high in the S. spinosa treatments (83.4%) and relatively lower in L. javanica (37.5 %) (Nyahangare et al., 2012). Acute toxicity was attributed to some secoiridoids in S. spinosa and methylxanthines in L. javanica. The results show that while the plant extracts are pesticidal they have potential negative health effects to users if they are used incorrectly (Stevenson et al., 2010). More chemistry work still needs to be done though for optimal exploitation of the acaricidal plants.
Tephrosia vogelii
Literature suggests that Tephrosia vogelii (TV) can be used as an acaricide. Researchers at Makoholi Research Station, in collaboration with ICRAF, tested and verified the effectiveness of T. vogelii as a biopesticide on cattle in 2009. It was concluded from preliminary results that TV is a very promising biopesticide that, with ascertainment and further testing, could be disseminated to a wider audience.
Indeed, it is true that ignoring ethno-veterinary medicine in today's development would mean losing a very important and special component in life history of mankind that definitely would have made a difference for better. However, although EVM practices are evidently gaining popularity in various Zimbabwean communities, a lot of claims have not been validated by research. Research is also necessary as some plants used in EVM may have deleterious health implications on humans and animals; hence, advice on their safety should accompany promotion of their use. Documentation of EVM is critical but it offers resourcesto poor farmers an effective, low cost, sustainable and environmentally friendly pest management strategy.