ABSTRACT
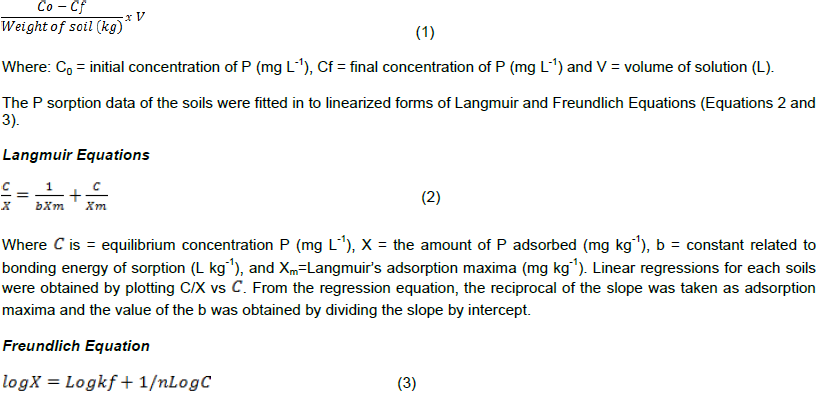
Soil chemical forms of Phosphorus and P sorption capacities of Fincha Sugar Estate soils were studied using samples collected from luvisols, vertisols and fluvisols of the farm. P forms were sequentially extracted following standard P fractionation procedure. In sorption study, 3 g of soil sample was equilibrated with 30 ml of 0.01 M CaCl2 containing various amounts of KH2PO4. Data were fitted into Langmuir and Freundlich equations. Results revealed that organic P (Po) accounted for the larger proportion of total P than total active inorganic P fraction in 66.7% of soils. The ratio of organic carbon to Po was < 200 in all soils indicating net mineralization of Po. Ca-P was dominant in luvisols and vertisols whereas Fe-P was dominant in fluvisols. P sorption data of all soils fitted well with Langmuir equation but the P sorption data of these soils were found not to fit with Freundlich equation. Langmuir adsorption maxima and external P requirement (EPR) of soils ranged from 227.3-344.8 and 63-153.8 mgkg-1 respectively. Luvisols and vertisols which account for more than 95% of the farm had EPR values < 150 mgkg-1 and hence they were classified as low P sorbing soils. This implies that high P sorption is not P limiting factor in the estate. The result further indicates that current P fertilizer application rate of 30 kg P ha-1 being practiced across all soil types of the farm needs to be revised after validating the EPR values estimated in this study for each soil both in greenhouse and in the field at Fincha Sugar Estate.
Key words: P fractions, P sorption, Langmuir equation, Freundlich equation.
Phosphorus (P) is the second most important limiting nutrients for crop production in Ethiopia next to nitrogen. Nutrient mining including P due to continuous cropping, soil erosion, inadequate use of organic and inorganic nutrients are some of the cause of declining soil P content (Wakene and Heluf, 2003).
Deficiency of P in soils may also occur due to conversion of added soluble P fertilizer into unavailable form for plant uptake and this phenomenon is called P sorption/fixation (Ravikovitch, 1986). According to Gichangi (2007) P sorption is defined as a loss of orthophosphate from soils solution due to either adsorption or precipitation reaction. Soil P limitation due to sorption/fixation is a major problem in highly weathered tropical acid soils dominated by aluminum and iron oxides (Sanchez et al., 1997). The same authors compared the P requirements of 200 soil samples collected from West, east and Southern Africa based on their P sorption isotherm and they found thatto be in order of Anidsol >Oxisol >Ultisols >Alfisols >Entisols. High P-sorbing soils are characterized by clayey topsoil having red color as indicative of Fe and Al oxides and such soils make the bulk of soils in smallholder farms of Africa. This implies that P sorption is the major factor that limits P bioavailability in most African soils most of which are acidic.
The extent of P sorption greatly varies from soil to soil owing to their differences in physicochemical properties (Brady and Weil, 2002) and management (Moazed et al., 2010). Thus, understanding the P-sorption characteristics of soils are important for designing appropriate management strategies and predicting fertilizer requirements that are need to be applied (Zhang et al., 2005).
However, little or no information is available on sorption capacities of Ethiopian with the notable exception of the work of Sahlemedhin and Ahmed (1983), Duffera and Robarage (1999) and Birru et al. (2003). Thus, it is important to determine the P sorption capacities of as many arable soils of Ethiopia as possible and identify properties affecting the sorption characteristics of soilsas such information serve as a basis for improving the P nutrition of crops. Moreover, the P sorption capacities of soils are mostly determined by fitting sorption data of different soils into commonly known Langmuir and Freundlich equations (Sparks, 2003). However, the degree to which the P sorption data of soil fit to these equations vary from soil to soil. Thus, it is necessary to test and identify best fitting equation that describes a sorption characteristic of for a particular soil.
The chemical forms of P in which it exists in soils is another factor that greatly influence bioavailability of P and have environmental consequences (Rubio et al., 1998).The forms of soil P are broadly categorized into organic (Po) and inorganic (Pi) P forms. Organic P can account for 5-95% of total P (TP) content of soils. Soil Po is derived mainly from manures, plant material, and products of microbial decomposition. On the other hand, the Pi originates from the addition of inorganic fertilizers and weathering of primary minerals such as apatite and secondary minerals such as Ca and/or Mg phosphates and Fe and Al phosphates (Hedley et al., 1982). The Po and Pi forms are further categorized into several different forms. However, the types and quantities of different forms of P that occur in a particular soil is the function of parent material, management and climate conditions. For instance, in acidic soils Fe and Al bound P is the predominant forms of soil P whereas Ca bound P is the major forms of Pi in calcareous soil. Knowledge on the different forms of soils is important to know the P supply potential of the soils, the degree of weathering of a particular soil, mineralization rate of Po and the extent of P sorption/fixation capacities of soils (Samadi, 2006).
In this regard, there is limited information on the chemical forms of P in the soils of Fincha Sugar Estate soils, Western Ethiopia. Thus, experiments were conducted to quantify the different P fractions of Fincha Sugar Estate soils, to determine their P sorption capacities and external P requirements; and to evaluate the ability of Langmuir and Freundlich equations in describing the P sorption data of the Fincha Sugar Estate soils.
Site descriptions
The experimental soils were collected from Finch sugar estates which are under Ethiopian Sugar Corporation. Geographically Fincha Sugar Estate is located in Nile basin of western Ethiopia lying between of 9°30’ to 10°00’ of North of latitudes, and 37° 15’ to 37°30’ of East of longitude. It was established in 1995 and situated at a distance of 340 km west-east of Addis Ababa, the capital of Ethiopia. It has an altitude in the range of 1350-1600 masl and average annual precipitation of 1300 mm. The sugar cane farm of Fincha has total area of 8004 ha as of 2007 (Getahun et al., 2013). Based on FAO-UNESCO classification system, the soils of FINCHA are belong to luvisols, vertisols and fluvisol (Getahun et al., 2013) and the former two soils accountfor more than 95 % of the total area of land planted with sugarcane.
Soil sampling and preparation
Random soil samples from each soil type of Fincha Sugar Estate farm were collected following standard procedure described in Brook (1983) in which surface soil (0-20 cm) were taken using augur from thirty replicated pointsby waking in a zigzag manners.Then all the thirty samples collected from each soil type were transferred in to clean plastic bucket and mixed thoroughly to make a composite sample. From the composite sample 1 kg of subsample from each soil were taken and brought to Fincha Sugar Estate soil laboratory. In the laboratory the samples were air dried, grounded to pass 2 mm sized sieve and preserved for analysisof physicochemical properties, P fractionation and P sorption isotherm experiments.
Analytical procedures
The processed soil samples were analysed for selected physicochemical properties following procedures described in Jones (2001) in which texture determined by Bouyoucous hydrometer method, pH was measured in 1:2.5 soil water solution by pH meter, organic carbon (OC) by wet digestion method (Anderson and Ingram, 1996). Soil available P was determined by Olsen method (Olsen et al., 1954). Exchangeable cations were extracted by 1N NH4OAC and in the extract; Na and K were determined by flame emission spectrophotometer whereas Ca and Mg were determined by atomic absorption spectrophotometer.
Phosphorus fractionation experiment
The total P was extracted with HClO4 digestion technique (Jackson, 1964) whereas the inorganic P fractions were sequentially extracted, soluble P with 1 M NH4Cl, aluminum bound P (Al-P) with 0.5 M NH4F, iron bound P (Fe-P) with 0.1 M NaOH and calcium bound P (Ca-P) with 0.25 M H2SO4. The organic p was estimated by the difference between P extracted with 1N H2SO4 for calcinated and non-calcinated soil. Phosphorus in all extracts was determined spectrophotometrically by ascorbic acid-molybdate method (Olsen et al., 1954).
Phosphorus sorption experiment
Three grams of soil (Duffera and Robarge, 1999) from each sample was transferred in to 50 ml capacity plastic bottles and equilibrated with 30 ml of 0.01 M CaCl2 solution containing 0, 1, 5, 10, 15 and 20 mg P L-1 in the form of KH2PO4. The bottles were shaken at 25°C on reciprocal shaker for 24 h (Graetz and Nair, 2000). Then, the suspensions were filtered through Whatman filter paper No. 42 and the concentration of P in the clear extract was determined by ascorbic acid-molybdate method (Olsen and Sommers, 1982). The amount of adsorbed P by each soil was calculated using Equation 1.
Where: X (mg kg-1) = Amount of P adsorbed per unit mass of soil, and C (mgL-1) is the equilibrium concentration, Kf (mg kg-1) = Capacity factor (Shayan and Davey, 1978) and 1/n = a constant related to bonding energy (Siradz, 2008). The linear graph and regression equation for each soil was obtained by plotting LogC against LogX. The slope and intercept were taken as 1/n and Kf respectively.
The external P requirements (EPR) of the soils were determined by substituting the desired P concentration (0.2 mg P L-1) in the soil solution into the fitted Langmuir and Freundlich equations (Dodor and Oya, 2000). The soil solution concentration of P at 0.2 mg L-1(SPC) provides P adequately to for many crops if it is maintained throughout the growing period and medium. This concentration P is known as standard soil solution concentration of P (Chaudhary et al., 2003). A soil with EPR < 150 mgkg-1 of soil at SPC are classified as low P and those soil with EPR values >150 mgkg-1 of soils are classified as high P sorbing soils (Fox, 1981).
Statistical analysis
Data on soil properties, soil P fractions and sorption indices were subjected to Pearson correlation analysis using SAS software (SAS, 2000) to determine the relationship between soil properties, P fractions and P sorption indices.
Some of the physicochemical properties of experimental soils
Some of the physicochemical properties of soil samples of Fincha sugar states are summarized in Table 1. The soil pH was in the range of 6.6 to 7.3 with mean value of 6.9. Thus, the soils of Fincha Sugar Estate are classified as neutral in its reaction. OC content varied between 1.3- 1.6% with mean value of 1.5%. According to Jones (2001) soil with OC contents between 1-2% are in low category and hence all soils of Fincha Sugar Estate are classified as low in their OC contents. Available P (Olsen) contents of all samples were in the high range (Tekalign and Haque, 1991).
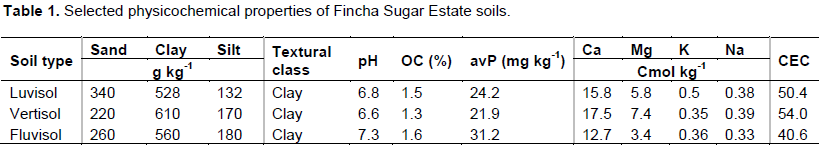
The exchangeable Ca and Mg contents of all samples were in high ranges (Brook, 1983). But the exchangeable K level of the soils was in medium range for sugar cane production.
Chemical forms of phosphorus in Fincha Sugar Estate soils
The total and different chemical forms of phosphorus in surface soils of Fincha Sugar Estate farms are summarized in Table 2. The total P (TP) contents of these soils were in the ranges of 310.2- 544 mg kg-1 soils with mean value of 415.3 mg kg-1 and the amounts are in low range as per the rating of Murphy (1968). However, TP contents of the present soils were similar to the TP contents of soils of Bako and Jimma, western Ethiopia (Piccolo and Hulluka, 1986). The test soils have significantly (P<0.001) varied among each other in their TP contents. It was highest in luvisols followed by fluvisols and the least in vertisols.This shows that the amount of non-active mineral forms of phosphorus is higher in the luvisols than in the remaining two other soils. Considering, the various P fractions studied, organic P (Po) fraction was significantly higher in fluvisols, followed by luvisols and lowest in vertisols. The variation among Po values of these soils is related to soil organic matter content and this can be evidenced by the fact that there was a significant and positive correlation (r = 0.99, P < 0.05) between Po and OC content of the soils (Table 4).
Similar results have been reported by Tekalign et al. (1988) for vertisols of Ethiopia. Among inorganic P fraction, Al-P and Ca-P were significantly highest in vertisols followed by luvisols and they were least in fluvisols. This is in line with Fisseha et al. (2014) who have found higher amount of Ca-P in vertislos and in fluvisol in Tigray, North Western Ethiopia. However, Fe-P fraction was significantly higher in fluvisols than in vertisols and luvisols (Table 2). Ca-P was dominant P fraction in all soils and this could be related to high temperature of Fincha farm where the incidence of leaching loss of Ca is low (Fisseha et al., 2014). On the other hand, total active inorganic P (Pi) fraction (phosphates associated with Ca, Al and Fe) was highest in vertisols followed by fuvisols and lowest in luvisols. However, Po fraction was higher than mean Pi across all the three soils. This is in line with Tekalign et al. (1987) that in most case the organic P fraction accounts for the largest fraction of soil P pool.
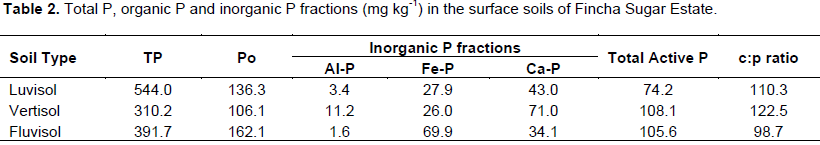
The carbon and Po (C: P) ratio which is an index of mineralization capacity of organic P of soils was in the range of 98.7-110.3 with mean value of 110.5 (Table 2). Accordingly, C: P values of all soils < 200 mg kg-1 indicating rapid turnover and net mineralization of organic P (Tekalign et al., 1987). This may be one of the reasons for high amount of available P (Olsen) in all the soils of Fincha (Table 1). This reasoning can further be substantiated by the fact that there was a significant positive correlation (r = 0.99, P < 0.05) between Po and available P (Table 3).
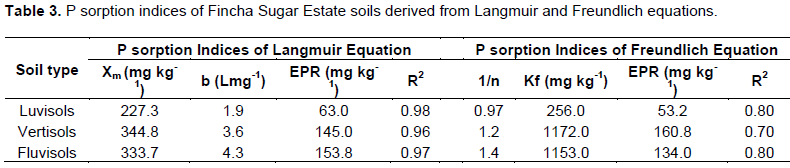
The proportions (%) of the different P-fractions of the three Fincha Sugar Estate soils relative to the respective total P content of each soil are presented in Figure 1. Even if luvisol had the highest TP, the relative proportions of the different P-fractions were generally lower than those in vertisols and fluvisol. This suggests that the degree of weathering is lower in luvisol than that in the latter two soils. Generally, the proportion of Po fraction of the soils was in the range of 25 -41% of total P (TP) andaccounted for the higher proportion of TP than the proportion of total active inorganic P fraction (Pi) which was in the range of 14– 35% indicating that Po isthe major pool of phosphorus in Fincha Sugar Estate soils. However, the proportion of Po of these soils was relatively lower than the percentage of Po reported for some vertisols of Ethiopia which was in the range of 40 -50% of TP (Ahmed and Islam, 1986). This difference could probably be due to rapid turnover rate of soil organic matter which in turn is caused by high humidity and high temperature of Fincha area.
The proportion of inorganic P fractions, in luvisols and vertisols were in the order of Ca-P> Fe-P>Al-P. This is in agreement with the report of Desta (1982). But the trend for fluvisol was in order of Fe-P>Ca-P>Al-P. The present finding for fluvisol is in line with Piccolo and Huluka (1986) who found that the inorganic P fractions of seven different soils types of Ethiopia including calcic fluvisol to be in the order of Fe-P >Ca-P > Al-P. In all the three soils, Al-P accounted for the lowest proportions of TP compared to the other inorganic P forms and this is in agreement with Piccolo and Huluka (1986). There is a high degree of weathering in soils where Fe and Al bound P is dominant (Wakene and Heluf, 2003). This implies that Ca-P is dominant in the luvisol and vertisolof Fincha sugar cane farm indicating low degree of weathering in these soils whereas Fe-P makes dominant fraction of inorganic P in fluvisol suggesting that it is advanced stage of weathering (Tekalign et al., 1988).
Phosphorus sorption isotherms
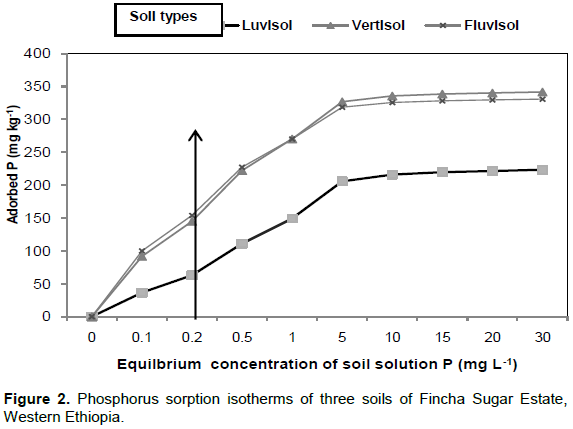
The P sorption isotherms of the three Fincha Sugar Estate soils are represented in the graphs of Figure 2
The isotherms of these soils are similar and took more or less L-shaped curve. This indicates that the affinity of P was higher to the soil than to the soil solution at lower equilibrium concentration of P. However, as the soil solution concentration increased, the situation is reversed and the affinity of P towards the soil solution became higher than to the soils (Sparks, 2003). But, the soils greatly varied in the steepness of the curve where by the isotherm of fluvisol was stepper than those of vertisols and fluvisol. This is evidenced by the fact that amount of adsorbed P by fluvisolat standard soil solution concentration of P (0.2 mg L-1) was > 150 mgkg-1 (vertical arrow in Figure 2) of soil whereas the amount adsorbed by luvisol and vertisol was <150 mg kg-1.
Phosphorus sorption indices of Fincha Sugar Estate soils
The P sorption data of all the three soils of Fincha Sugar
Estate were fitted well with Langmuir equation with mean r2 value of 0.97. However, the sorption data of these soils failed to obey well with Freundlich equation (Table 4). It is natural that the P sorption data of different soils fit with different equations to different degrees and there are occasions where the P-sorption data of particular soils may not obey with one, two or none of such sorption equations (Chaudhary et al., 2003; Hussain et al., 2003). For instance, Moazed et al. (2010) studied the P sorption characteristics of five soils in Iran and they found that Langmuir equation was best fit than Freundlich equation. But, contrary to these, Khan et al. (2010) reported that Freundlich equation was best in describing the P sorption data of three Pakistan soils. Such variations are related to P sorption mechanisms on the surfaces of different soils. For example, Langmuir equation fits best with soil having homogenous sorption sites throughout their surfaces but for soils with heterogeneous sorption sites, Freundlich equation suits better in describing the P sorption data of such soils (Sparks, 2003). Thus, it seems that the present soils had homogenous P sorption site on their surfaces.
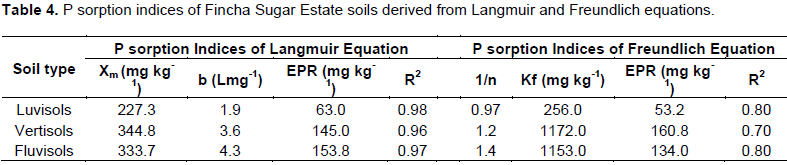
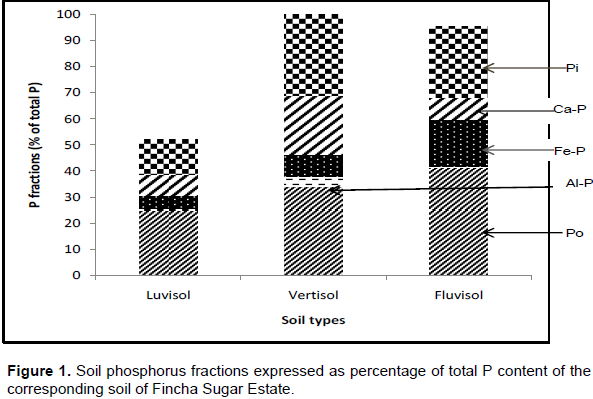
The Langmuir adsorption maxima (Xm) values of the study soils ranged between 227.3-344.8 mg kg-1 with mean value of 302 mg kg-1. It was highest for vertisol and lowest for luvisols. However, these values are quit lower than that we have reported for seven highland acidic soils of southern Ethiopia (Sahlemedhin and Ahmed, 1983) and also very much lower than that reported for tropical soils (Hartono et al., 2005). The relatively smaller Xm values of the present soils could be due to better management of these soils including yearly application P fertilizer as Fincha sugar cane farm is state owned sugar producing enterprise, better management is practiced relative to that done in subsistent farmers’ field. The other probable reason could be related to soil type where the present soils are belongs to luvisol and vertislols which are relatively low P fixing soils than other soil types (Sanchez et al., 1997). This is also in agreement with Birru et al. (2003) who studied the P sorption isotherms of representative soils of north western Ethiopia and reported that vertisols were in low P fixing categories against nitisols and cambisols which were high P fixing soils due to the fact that they were at their advanced stages of weathering.
The Langmuir boding energy (b) values which show the tenacity with which P isadsorbed by soils was in the ranges of 1.9–4.3 L mg-1 with mean value of 3.3 L mg-1. The value of b was highest in fluvisol followed by vertisol and list in luvisol (Table 4). However, the values of b for all the three soils were > 0.07 Lmg-1 suggesting that there is no risk of loss of P in to water (Mcdowell et al., 2002).
The external P requirements (EPR) derived from Langmuir equation of experimental soils varied from 63.0–153.8 mg kg-1 with mean value of 120.6 mg kg-1. It has significantly and positively correlated with Xm and b indices of the soils (Table 5). This means that as Xm and b values of the soils increase, the corresponding values of EPR increases. In luvisol and vertisol the EPR was < 150 mg kg-1 of soil and consequently classified as low P-fixing soils whereas fluvisol had EPR > 150 mg kg-1 and hence classified as high P sorbing soil (Fox, 1981). As luvisols and vertisols are low P sorbing soil and at the same time these soils account for more than 95% of the farm, limitation of P due to high P sorption is not a major problem in Fincha Sugar Estate soils.
At present, Fincha Sugar Estate is practicing application of P fertilizer at 30 kg P ha-1 across all soil types which is equivalent to 15 mg P kg-1 of soil (Damte et al., 2012) and this rate was developed based on the result of crop response study. However, compared to the EPR estimated from the Langmuir equation for each soil in this study, this rate of P being applied currently on the farm is very low.
Relation between soil properties, P fractions andsorption indices
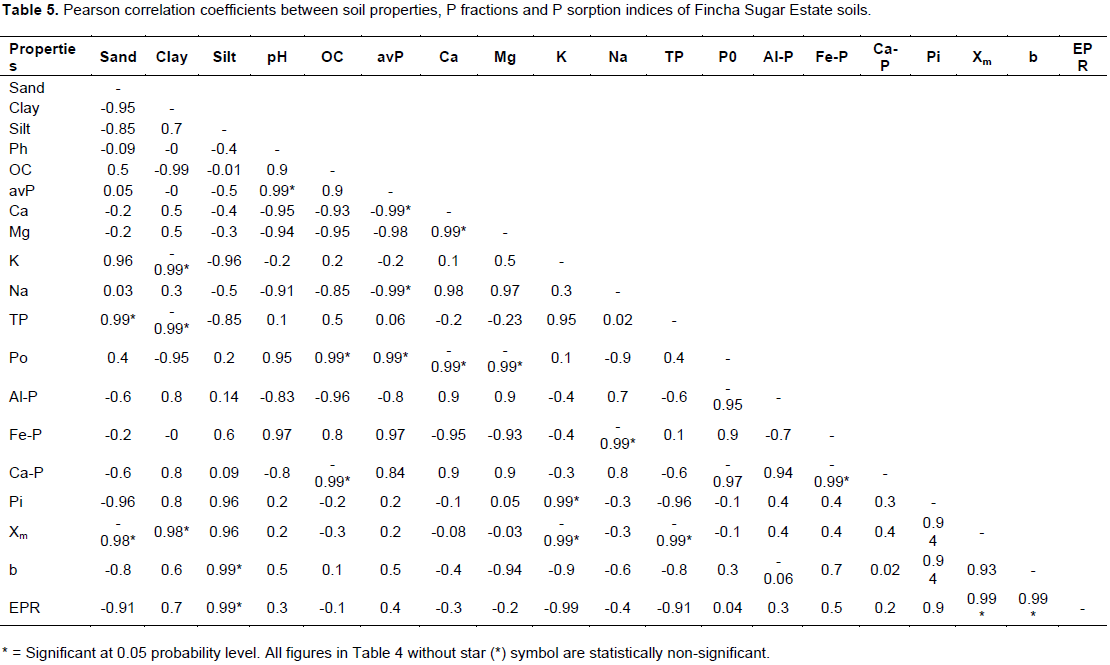
The Pearson correlation coefficients between some soil properties, P fractions and P sorption indices of Fincha Sugar Estate soils are presented in Table 4. Organic P (Po) fraction has significantly (P< 0.05) and positively correlated with OC (r = 0.99) but negatively correlated with exchangeable Ca and Mg contents. On the contrary, the Pi fractions of Al-P and Ca-P were found to significantly and negatively correlate with OC content of the soils. Positive and significant correlation between Po and OC is expected because the source of organic P is soil organic matter and thus as the OC content of soil increase there will be an increase in Po content of soils and vice versa. Ca-P and Fe-P have significantly and negatively correlated with each other indicating that in soils where Ca-P is dominant Fe-P is low and vice versa.
The P sorption index of Xm was found to significantly and negatively correlate with sand, K and TP but positively correlated with clay, silt content and Pi. The apparent negative correlation observed between Xm and TP is in agreement with the finding of Bastounopoulou et al. (2011) who found negative correlationsbetween P sorption parameters and soil TP and Pi in Greek inceptisols. On the other hand, the bonding energy (b) has significantly and negatively correlated with sand and K but positively correlated with silt content. Similarly, EPR correlated negatively with sand and K content but positively correlated with silt, Pi, Xm and b (Table 4).
The results of P fractionation study of Fincha Sugar Estate soils showed that the organic P accounted for the largest proportion of total P (TP) in luvisol and fluvisoil. But in vertisols,Po and Pi accounted for 50:50 proportions of TP. The C:P ratio was <200 in all three soil types suggesting that there is net minerlization of soil Po. Among inorganic P fractions, Ca-P accounted for the highest proportion in luvisols and vertisols whereas Fe-P accounted for highest proportion in fluvisols. This implies that the incidence of weathering is relatively high in fluvisol than in the former two soils.
On the other hand, the results of sorption experiment revealed that the P-sorption data of all the three soil fitted best with Langmuir equation but failed to fit with Freundlich equation. The study soils varied in their adsorption maxima (Xm), bonding energy (b) and EPR. Based on these indices the P-sorption capacities of experimental soils were in increasing order of luvisols < vertisols < fluvisols. Sand, clay, TP and Pi were found to be major soil properties responsible for variations in P sorption capacities of Fincha Sugar Estate soils. Luvisols and verisols which account for more than 95 % of the ESTATE had EPR < 150 mg kg-1 suggesting that P limitation due to high P sorption is not a problem in the farm. This could be due to low P sorbing nature of luvisol and vertisol; and the prevailing good management practices in the farm are the two most likely reasons for this to happen. However, the current P fertilizer rate of 30 kg ha-1which is roughly equivalent to 15 mg kg-1 being practiced across all soil types in the farm is far less than that estimated for the three soils using Langmuir equation in this study. Thus, it is recommended that this dose of P fertilizer should be revised after validation of the EPR values estimated for each soil through real time field or greenhouse experiment.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed B, Islam A (1986). Extractable phosphate in relation to the forms of phosphate fractions in some humid tropical soils. Trop. Agric. 52:113-118.
|
|
|
|
Anderson JM, Ingram SJ (1996). Tropical soil biology and fertility: A handbook of methods, 2nd edition, CAB International, Oxford.
|
|
|
|
|
Bastounopoulou M, Gasparatos D, Haidouti C, Massas L (2011). Chemical fractionation and sorption of phosphorus in Greek inceptisols. J. Agric. Sci. Technol. 1:33-38.
|
|
|
|
|
Birru Y, Heluf G, Gupta VP (2003). Phosphorus sorption characteristics of some soils of north western highlands Ethiopia. EJNR 5:1-16.
|
|
|
|
|
Brady NC, Weil RR (2002). The Nature and properties of soils, 13th edition, Prentice Hall, New Jersey.
|
|
|
|
|
Brook RH (1983). International course on soil and plant analysis: Lecture notes. Service Laboratory for Soil, Plant and Water Analysis, Soil Science Department, Faculty of Agriculture, Minia University, Egypt.
|
|
|
|
|
Chaudhary EH, Ranjha AM, Gill MA, Mehdi SM (2003). Phosphorus requirement of maize in relation to soil characteristics. Int. J. Agric. Biol. 5:625-629.
|
|
|
|
|
Damte A, Fantaye A, Teshome Z (2012). Correlation of foliar nutrient status with yield of sugarcane varieties at different crop stages and nitrogen levels at Wonji-Shoa and Finchaa sugarcane plantations of Ethiopia. J Appl. Sci. Technol. 3:9-22.
|
|
|
|
|
Desta B (1982). Diagnosis of phosphorus deficiency in Ethiopian soils.Soil Science Bulletin No. 3. Institute of agricultural research, Addis Ababa, Ethiopia.
|
|
|
|
|
Dodor DE, Oya K (2000). Phosphate sorption characteristics of major soils in Okinawa, Japan. Comm. Soil Sci. Plant Anal. 31:277-288.
Crossref
|
|
|
|
|
Fisseha H, Heluf G, Kibebew K, Birru Y (2014). Study of phosphorus adsorption and its relationship with soil properties, analyzed with Langmuir and Freundlich models. Agric. For. Fish. 3:4-5.
|
|
|
|
|
Fox RL (1981). External phosphorus requirements of crops. In: Chemistry in the soil Environment, Shelly M (eds). ASA Special Publication No. 40:223-240.
|
|
|
|
|
Getahun K, Heluf G, Tenna A, Oulumana M, Hurni H (2013). Land use changes induced by irrigation development in the Fincha'a sugar estate, Blue Nile basin, Ethiopia. J. Biodiv. Environ. Sci. 3:31-47.
|
|
|
|
|
Gichangi EM (2007). Enhancing phosphorus availability in some phosphate fixing soils of the transkei region of South Africa using goat manure. PhD Thesis, University of Fort Hare, South Africa.
|
|
|
|
|
Graetz DA, Nair VD (2000). Phosphorus sorption isotherm determination. In: Methods of P analysis for soils, sediments, residuals and water, (Kovar JL and Pierzynski GM., eds.). SERA-IEG 17. So. Coop. Ser. Bull. 396:29-32.
|
|
|
|
|
Hartono A, Funakawa S, Kosaki T (2005). Phosphorus sorption-desorption characteristics of selected acid upland soils in Indonesia. Soil Sci. Plant Nutr. 51:787-799.
Crossref
|
|
|
|
|
Hedley MJ, Stewart JWB, Chauhan BS (1982). Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 46: 970–976.
Crossref
|
|
|
|
|
Hussain AA, Ghafoor M, Ul-haq J, Nawaz M (2003). Application of the Langmuir and Freundlich equations for P adsorption phenomenon in saline-sodic soils. Int. J. Agric. Biol. 3:349-356.
|
|
|
|
|
Jackson ML (1964). Soil chemical analysis. Prentice Hall, New York.
|
|
|
|
|
Jones BJJ (2001). Laboratory guides for conducting soil tests and plant analysis. CRC press, London.
|
|
|
|
|
Khan QU, Khan MJ, Saif-ur-Rehman, Ullah S (2010).Comparison of different models for phosphate adsorption in salt inherent soil series of Dera Ismail. Soil Environ. 29:11-14.
|
|
|
|
|
Mcdowell R, Sharpley A, Withers P (2002). Indicator to predict the movement of phosphorus from soil to subsurface flow. Environ. Sci. Technol. 36:1505-1509.
Crossref
|
|
|
|
|
Duffera M, Robarage WP (1999). Soil characteristics and management effect on phosphorus sorption by highland plateau soils of Ethiopia. Soil Sci. Soc. Am. J. 63:1455-1462.
Crossref
|
|
|
|
|
Moazed H, Hoseini Y, Naseri AA, Abbasi F (2010). Determining phosphorus adsorption isotherm in soil and its relation to soil characteristics. J. Food Agric. Environ. 8:1153-1157.
Crossref
|
|
|
|
|
Murphy HF (1968). A report on fertility status and other data on some soils of Ethiopia. Experimental Station Bulletin 44, Collage of Agriculture HSIU, 1968.
|
|
|
|
|
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA, USA.
|
|
|
|
|
Piccolo A, Huluka G (1986). Phosphorus status of some Ethiopian soils. Trop. Agric. 63:137-142.
|
|
|
|
|
Ravikovitch S (1986). Anion exchange: I. sorption of phosphoric acid ions by soil. In: Harter RD (Ed.), Sorption phenomena. Soil Science Series. NewYork: Van Nostr and Reinhold Co.
|
|
|
|
|
Rubio L, Mcgrath D, Culleton N, Glennon J (1998). A preliminary comparative investigation of phosphorus in Irish grassland and in Spanish soils. Pastor 28:97-105.
|
|
|
|
|
Sahlemedhin S, Ahmed A (1983). Phosphorous sorption characteristics of some Ethiopia soils. Ethiop. J. Agric. Sci. 5:1-12.
|
|
|
|
|
Samadi A (2006). Phosphorus sorption characteristics in relation to soil properties in some calcareous soils of western Azarbaijan province. J. Agric. Sci. Technol. 8:251-264.
|
|
|
|
|
Sanchez PA, Shepherded KD, Soule MJ, Place FM, Buresh RJ, Izac A, Mokwunye AU, Kwesiga FR, Ndiritu CG,d Woomer PL (1997). Soil fertilityreplenishment in Africa: An investment in natural resource capital. In: Replenishing soil fertility in Africa, (Buresh, R. J., Sanchez, P. A. and Calhounn, F., eds.), SSSA special publication number 51. Madison. pp. 1-46.
|
|
|
|
|
SAS (2000). User's guide statistics, version 8.1. SAS Inst, Inc., Cary, NorthCarolina, USA.
|
|
|
|
|
Shayan A, Davey BG (1978). A universal dimensional phosphate adsorption isotherm for soil. Soil Sci. Soc. Am. J. 42:878-882.
Crossref
|
|
|
|
|
Siradz SA (2008). Phosphorus sorption characteristics red soils from Lanpung, West and Central java. J. Tanah Trop. 14:25-31.
|
|
|
|
|
Sparks DL (2003). Environmental Soil Chemistry, 2nd edition. Academic Press, Amsterdam.
|
|
|
|
|
Tekalign M, Haque I (1991). Phosphorus status of some Ethiopian soils. III: Evaluation of soil test methods for available phosphorus. Trop. Agric. 68:51-56.
|
|
|
|
|
Tekalign M, Haque I, Kamara CS (1988). Phosphorus status of some Ethiopian highland vertisols. In: Proceedings of a conference of Management of vertisols in sub-Saharan Africa, Addis Ababa, Ethiopia.
|
|
|
|
|
Wakene N, Heluf G (2003). Form of phosphorus and status of available micronutrients under different land use system of Alfisols in Bako area of Ethiopia. Ethiop. J. Nat. Resour. 5:17-37.
|
|
|
|
|
Zhang H, Schroder JL, Fuhrman JK, Basta NT, Storm DE, Payton ME (2005). Path and multiple regression analyses of phosphorus sorption capacity. Soil Sci. Soc. Am. J. 69:96-106.
Crossref
|
|