ABSTRACT
Post-harvest losses of maize due to storage insect pest such as the maize weevil, have been recognized as an increasingly important problem in the world. A study was conducted with the objectives to determine the performance of Sitophilus zeamais populations originating from the five Brazilian regions in five varieties of maize and to correlate these variables with the enzymatic activity of insects and nutritional components of the grain. The grain of maize hybrids 30F53H, 30K64H, 30F35H, DKB390 and “crioulo” (not commercial) maize produced in the microregion of Alto Médio Gurguéia in Piauí, was infested with 25 unsexed insects of S. zeamais coming from Bom Jesus, PI; Canarana, MT; Volta Redonda, RJ; Cacoal, RO; and Palmeira das Missões, RS. At the end of 120 days enzyme assays of amylase, lipase and protease from the insects, as well as nutritional analyses of the maize grains were performed. The hybrid with the greatest mass of grain consumption was 30F53H, and insects fed on this achieved the highest growth, followed by 30K64H, DKB390, 30F35H and the “crioulo” with the lowest loss of grain mass and hence the least insect growth. For the data of mass consumed by S. zeamais, the populations of Bom Jesus and Canarana were the ones that ate the most and Palmeira das Missões had the lowest consumption. The population from Bom Jesus, PI was the one that consumed the most grain mass, due to its adaptation to maize from the region. The “crioulo” maize was the one that presented the best indicators of resistance to attack by S. zeamais.
Key words: Digestive enzymes, maize weevil, host plant resistance to insects, stored grain.
Maize,
Zea mays L., is an important crop ranking second in world grain production only preceded by wheat. Maize production and distribution is a cosmopolitan (Makate, 2010) and it is an important component of agriculture and food systems all over the world. The crop is versatile in its uses and environmental adaptation. It is consumed all over the world both by human being and animals. Maize grains find themselves in many industries to be processed in to various food and industrial products of multi-purpose functions. The infestation of maize by maize weevil commences in the field but the lion share of the damage to maize grains by maize weevil is done during storage period. Such damaged grains have reduced
nutritional values, low percent germination and reduced weight and market values (Trematerra et al., 2013). Its control is accomplished primarily with insecticides (Miranda et al., 1995; Ferrari-Filho et al., 2011).
Consumers have demanded, from producers and companies, grain and products free of toxic residues. Given this situation, scientists in the search for methods to control insects that are less harmful to the environment and offer less risk to human and animal health have intensified their research in the development of insect-resistant plants, being a more specific and efficient method of control (Keba and sori, 2013).
Grains that express inhibitors of insect digestive enzymes, especially of α-amylases, represent a promising tool for the control of stored product pests because these insects live on a diet rich in polysaccharides and depend on the effectiveness of their amylases for survival (Pueyo et al., 1995; Ishimoto et al., 1996; Mendiola-Olaya et al., 2000).
It is important to compare the development of different populations of the weevil in different maize varieties, because the large differences between populations of the maize weevil are the result of the discontinuous nature of grain storage and also the management of these insect pests within the grain storage units. The distribution of these units together with their management probably accentuates the seasonal cycles of these populations and enables the rapid establishment of new populations from a small number of individuals (Tran and Credland, 1995; Guedes et al., 1997; Keba and Sori, 2013). Within the context of integrated pest management, knowing the behavioral and physiological differences among these populations is an important control tool. The objective of this work was to evaluate the performance of populations of S. zeamais coming from the five Brazilian regions in five varieties of maize and to correlate these variables with the enzymatic activity of the insects and nutritional components of the grain.
Varieties of maize
The maize grain used was produced in the region of Alto Médio Gurguéia, Piauí, in the 2010/2011 harvest. The hybrids 30F53H, 30K64H, 30F35H, DKB390 (not Bt) and the “crioulo” variety from the region of Santa Luz, PI were utilized. These were not subjected to any form of phytosanitary treatment, being previously selected, eliminating impurities and defective grains that could compromise the experiment. Then, they were stored for 10 days under refrigeration at -10°C to eliminate insects in their various stages of development. After this period, 10 kg of grain was thawed and dried naturally in plastic containers, sealed with organza type fabric and kept at 13% humidity.
Initial population of Sitophilus zeamais
The populations of S. zeamais used at all stages of the experiments were collected in different storage units in Brazil: Bom Jesus, IP; Canarana, MT; Volta Redonda, RJ; Cacoal, RO; and Palmeira das Missões, RS. The populations were bred from maize samples collected in the field and kept in 2 L plastic containers covered with an organza-type fabric. The experiment was maintained in a laboratory at room temperature (25 to 27°C) and 40 to 60% RH and photofase of 14 h.
Population growth and loss of grain mass
The experiment was conducted in a completely randomized design with five varieties of maize, five populations of S. zeamais, of different geographical localities, and three replications. For each treatment 600 ml plastic containers containing 250 g of maize were used, these being infested with 25 unsexed S. zeamais insects aged 1 to 10 days. The number of live and dead insects and grain and insect weight lose was evaluated at 120 days. At the end of each period, the grain was sieved to separate the maize grain from the insects and their droppings. The numbers of live and dead insects and grain mass consumed were recorded. At 120 days, after data collection, live insects from each treatment were frozen at -10°C, until needed for bioassays to perform enzyme bioassays and measure protein.
Enzymatic activity of the homogenate of S. zeamais
Random samples of 90 non-sexed adult insects of each population of S. zeamais fed different maize varieties in the population growth experiment (120 days on feed) were immersed in 1.5% KCl solution (m/v), macerated in a porcelain crucible and mixed with 10.0 ml of water at pH 3.0. The extract was filtered through cheesecloth and centrifuged at 3500 rpm for 30 min. The precipitate was discarded and aliquots of the supernatant were taken for determination of total protein concentration and specific activity of the enzymes amylase, lipase and total protease. The concentration of protein in the enzyme extracts was determined by the Warburg and Christian (1941) method for determination of specific activity.
For amylase assay the BIOCLIN K003 kit (Quibasa - Basic Química Ltda, Belo Horizonte, Minas Gerais, Brazil) was used, containing substrate (starch + 0.4 g L- 1 phosphate buffer (pH 7.0) 100 mmol L- 1 + stabilizers and preservatives) and color reagent (50 mmol iodine L- 1 + stabilizer). This reaction is based on fixed time kinetics (Caraway, 1959) modified. The enzyme extract was incubated with the starch substrate and the absorbance reading was taken at a wavelength of 660 nm.
For the lipase assay the BIOCLIN K025 kit (Basic Química Ltda, Belo Horizonte, Minas Gerais, Brazil Quibasa) was used, containing buffer (methane hydroxymethylamino 100 mmol L- 1) ( pH 8.5), enzyme inhibitor (phenylmethyl sulfonyl fluoride 8 mmol -L- 1 + solubilizer), color reagent (3 mmol ditionitrabenzóico acid L- 1 + sodium acetate 100 mmol L- 1 + stabilizer), substrate (ditiopropanol tributyrate 20 mmol L- 1 + surfactant) and acetone. The absorbance reading was taken at a wavelength of 410 nm.
To obtain the graph of enzyme kinetics, according to Michaelis-Menten for the enzymes amylase and lipase, reactions were performed with aliquots of substrate from 25 to 200 μl and 10 to 80 μl, respectively. The absorbance values ​​from each aliquot were converted into enzyme speed values (amylase unit dL mg protein, for amylase and IU mg -1 protein for lipase).
For the total protease activity azocasein was used as substrate at 2% (w/v) in 0.1 M Tris-HCl buffer, pH 8.0, 37°C according to the method described by Tomarelli et al. (1949). The reaction mixture consists of 250 μl of substrate and 300 μl of enzyme extract, incubated at 37°C for 30 min. Soon after, the reaction was stopped with 1.2 ml of trichloroacetic acid (TCA) at 10% (v/v) and allowed to stand for 15 min on ice. Before reading the absorbance at 440 nm, 1.4 ml of 1.0 M NaOH was added. All assays were performed with four replications.
Analysis of nutritional grains
Samples of 100 g of each grain (free infestation), variety were ground separately, and aliquots of these samples underwent moisture determination by weight loss, in an oven at 105°C, until constant weight; ashes were obtained by incineration of the material in an oven at 550°C. The crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF) and crude fiber (CF) were determined according to the methodology described by Silva and Queiroz (2002). The ether extract was determined by hot extraction with the use of petroleum ether as organic solvent (Silva and Queiroz, 2002). The experimental design was completely randomized with four replications. The results were expressed on a dry basis. Analysis of starch was performed using the methodology of Hendrix (1993) in the same experimental scheme previously adopted.
Statistical analysis
Analysis of variance was performed on the completely randomized factorial design with three replications for the characteristics of population growth and loss of grain mass and four replications for enzymatic activity, for each response variable. The F-test (p ≤ 0.05) was applied and, when a difference was observed for the interaction, an SNK comparison of means test was performed (p ≤ 0.05). For the nutritional analyses of grain we performed analysis of variance for the response variables, with standard procedure for a completely randomized design with three replications. When a significant difference was detected a means test was performed. Subsequently we performed Pearson correlation analysis on all response variables. All analyses were performed with the aid of the SAS computer program (SAS, 2002) by PROC GLM and PROC CORR. The curved non-linear regressions of Michaelis Menten type were plotted using the Sigma Plot program (SPSS®, 2000).
The results demonstrated there is a difference in the growth of populations of S. zeamais on different maize varieties. There was an interaction between populations and varieties, and differentiated behavior of populations was seen on different varieties. The no-choice test was conducted to check for differences in some biological characteristics of S. zeamais between insect populations and tested varieties of maize and their interactions. For the characters population growth, insect mortality and loss of grain mass there was a difference by F-test for all analyzed factors and their interactions.
According to the data presented in Table 1, there was greater population growth of S. zeamais on the 30F53H variety, except for the population from Volta Redonda, RJ (215.33). Among the populations with higher growth, Bom Jesus, PI distinguished itself with an average of 701.66 insects on the 30F53H variety. A similar result was found by Caneppele et al. (2003), who evaluated the increase in infestation by 50 insects of the species S. zeamais in maize grain stored for 120 days, observing an infestation of 899 insects at the end of this timeframe.
According to Toscano et al. (1999), who evaluated the resistance of 30 maize genotypes to attack by S. zeamais, the highest rates of growth are associated with genotype susceptibility to insect attack. Thus, among the varieties of maize subjected to attack by S. zeamais, representing the five regions of Brazil, a difference was observed. The most susceptible variety was the single transgenic hybrid 30F53H, on which S. zeamais collected in Bom Jesus, PI and Canarana, MT, from the Northeast and Midwest regions respectively, had higher population growth. The maize genotype with the greatest resistance was the “crioulo” maize. It deserves mentioning that all the simple hybrids tested in this experiment were more attacked than the landrace cultivar, and that among the hybrids, the 30F35H was the most resistant to damage caused by S. zeamais.
Analyzing mortality data (Table 1), it is observed that the highest cumulative mortality occurred with the population of Canarana, MT on the variety DKB390 (228 individuals). The lowest cumulative mortalities were found in the population from Palmeira das Missões that had the lowest growth and less loss of grain mass. The hybrid 30K64H had a lower average mortality of populations, and 30F35H had high mortality and low insect growth.
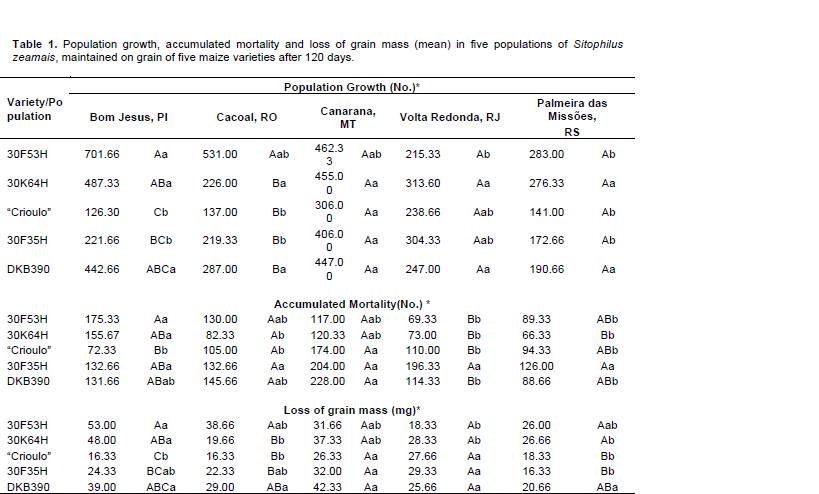
The hybrid that had the greatest loss of grain mass was 30F53H, which was also the one that had the greatest growth, followed by 30K64H, DKB390, 30F35H and the “crioulo” maize with the lowest mass loss and hence lower growth. For the mass consumption data by S. zeamais in this study, it was seen that the populations of Bom Jesus and Canarana were the ones that caused the greatest losses, followed by Volta Redonda and Cacoal, and there were lower losses for Palmeira das Missões (14, 14, 10, 10 and 9%, respectively). These data are similar to those of Caneppele et al. (2003) who studied the correlation between the level of S. zeamais infestation and the storage quality of maize grains. They noted an increase in weight loss of these grains, as time and the number of insects in contact with the maize grains increased. The authors observed weight losses of 15% in the 120 days period, when 50 insects were used in the initial infestation. Antunes et al. (2011) found losses of 17% after 120 days of storage of 600 g of maize with an initial infestation of 150 insects. In agreement with other authors, Marsaro-Junior et al. (2007) found greater weight loss of maize grain in treatments with the greatest number of emerged insects, as was seen in the present study.
Studies on amylase inhibitors of maize hybrids in resistance to attack by S. zeamais concluded that the greatest amounts of weight loss are associated with lower grain resistance to insect attack and smaller amounts of substances that inhibit insect feeding (Marsaro-Junior et al., 2005). Thus, it is noted again that the “crioulo” maize and the 30F35H hybrid were the least damaged by insects in studies; that is, the most resistant in the conditions under which the work was performed in terms of kernel hardness or substances that inhibit feeding or development of S. zeamais are more efficient (Keba and Sori, 2013).
S. zeamais population from Bom Jesus, PI was the one that caused the greatest losses in hybrids planted in the region of Bom Jesus, PI, cited here (Table 1), probably because they are more adapted to the physical and chemical characteristics of the maize, so landrace maize is the most planted by family farmers in the region, however unrepresentative in a region dominated by corporate agriculture. Among the landrace maize and hybrids planted in the region there was a preference by the Bom Jesus population for the hybrids over the “crioulo” maize (Keba and Sori, 2013).
In developing the hybrid as the best conditions for the production and nutritional characteristics, chemical and physical characteristics which suppressed the attack and the development of S. zeamais were lost (Keba and Sori, 2013).
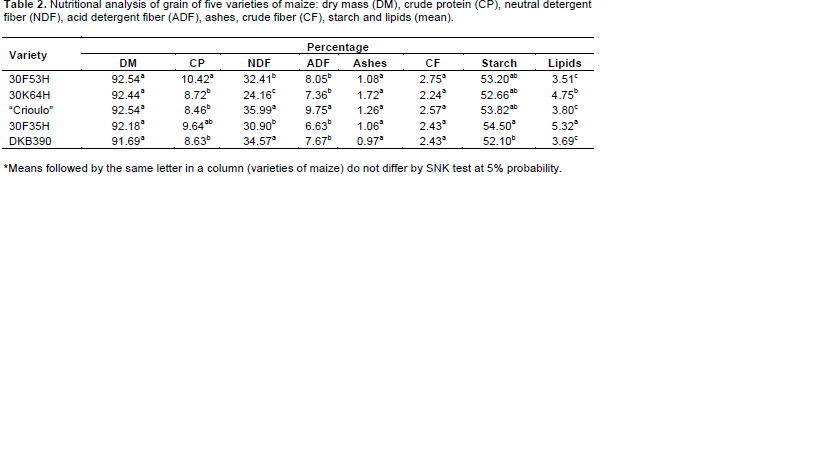
The “crioulo” maize on nutritional analysis of the grains showed a higher content of acid detergent fiber (ADF) (Table 2) and this was negatively correlated to growth and loss of grain mass. Similarly, higher crude protein (CP) may be positively related to growth, due to increased availability of organic constituents in grains. Insect mortality may also be related to the lipid content in the grains, where the highest mortality was observed in the 30F35H variety which showed the highest lipid content.
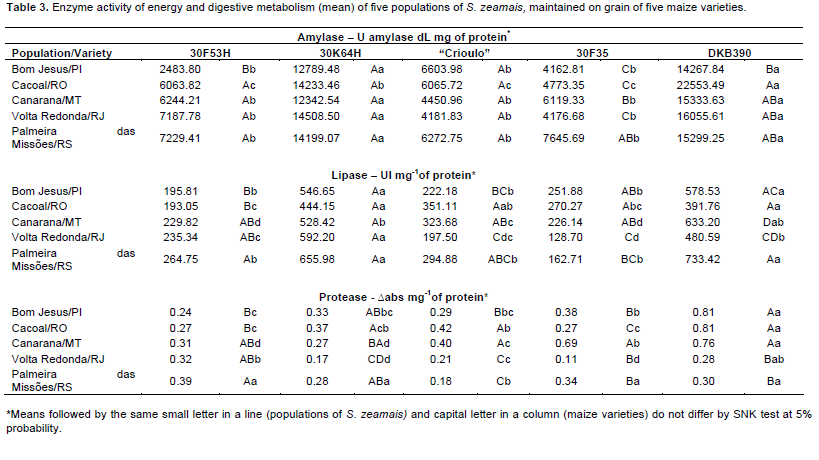
There were certain specific activities of the enzymes of energy metabolism, amylase and lipase, and digestive metabolism, protease, in the homogenate of five populations of S. zeamais, fed on the maize varieties: 30F53H, 30K64H, landrace, 30F35H and DKB390. The varieties with the lowest averages of amylase and lipase were 30F35, landrace and 30F53H, and for protease the 30K64, landrace and 30F53H varieties had the lowest averages. According to analysis of variance and subsequent mean test, it appears there is not a pattern of responses among populations of S. zeamais collected in five regions of Brazil fed the five maize varieties (Table 3).
The Michaelis-Menten equation model (Figures 1 and 2) was derived to consider the kinetic properties of the enzymes. The Michaelis-Menten constant (KM) and the maximum reaction rate (Vmax) are the kinetic parameters determined by the hyperbola. The first, for example. KM, represents the substrate concentration at which the velocity reaches half of the maximum speed and in its simplest form and is a measure of the affinity of the enzyme for the substrate. The kinetic parameter Vmax is reached when all the active sites are filled with substrate molecules. Both lipase and amylase for all treatments followed the Michaelis-Menten curves, but for lipase, in the 30F53H, landrace and DKB390 varieties, except in the Canarana, MT population which showed greater lipase activity, the remaining populations showed lower speed of the enzyme reaction due to a lower amount of lipase among the insect homogenate protein (Figure 2).
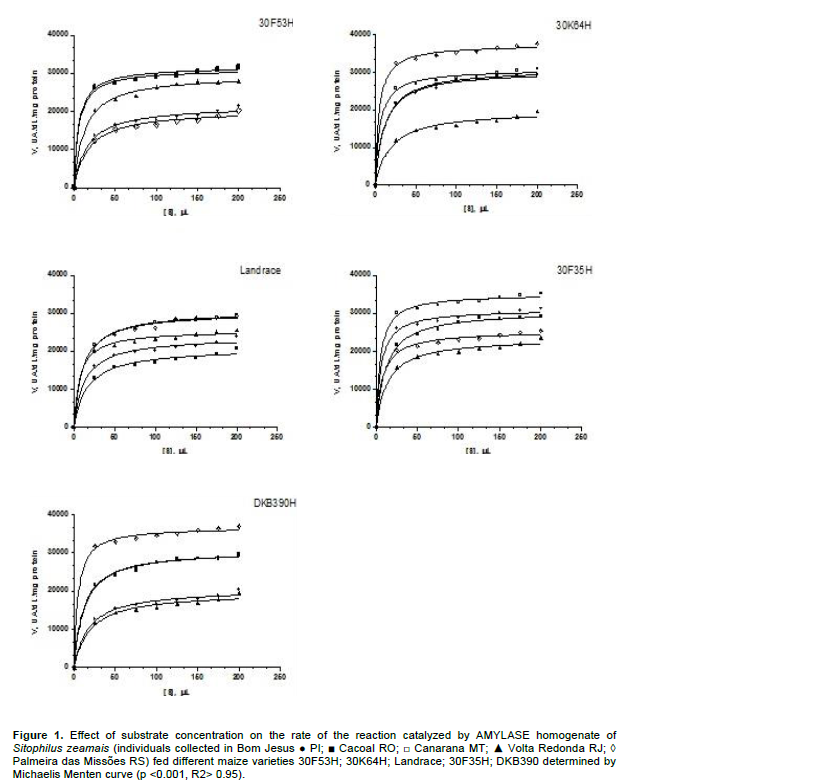
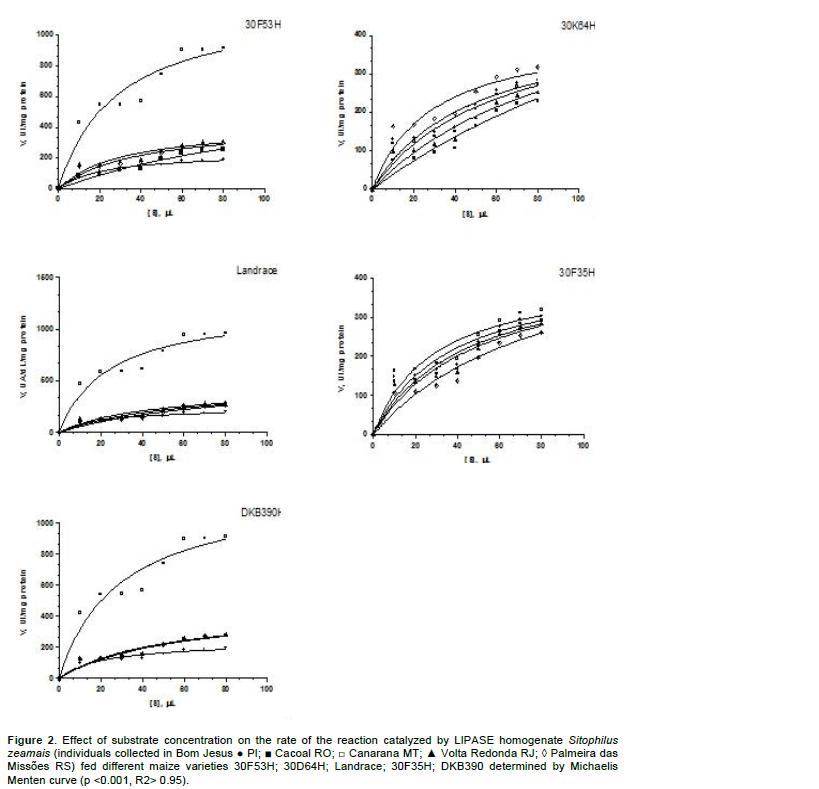
In insects, the fat body is the major site of synthesis and storage of carbohydrates, lipids and proteins, which are readily used as an energy source for many different activities (Chown and Nicolson, 2004). The enzyme amylase is responsible for hydrolysis of α-1.4 glycosidic linkages within starch and related polysaccharides (Lehninger et al., 2000; Zeng and Cohen, 2000). Many organisms, including insects that are serious pests in stored grains, live on a diet rich in polysaccharides and depend on the effectiveness of their amylases to survive in metabolic terms (Mendiola-Olaya et al., 2000). Moreover, this enzyme plays a central role in carbohydrate metabolism causing organisms that have a diet rich in starch to depend on the effectiveness of their amylases for survival (Titarenko and Chrispeels, 2000). This is the case with insects that are serious agricultural pests and consume plant organs rich in starch such as seeds and roots (Titarenko and Chrispeels, 2000).
The populations studied showed differences in the mobilization of starch in the different varieties studied. The high amylase activity in the Palmeira das Missões and Cacoal populations suggests greater importance of this enzyme in digestive metabolism as a source of large amounts of carbohydrate for accumulation in fat bodies of this same population. The digested starch appears to be used only for storage and not to supply sugar for energy metabolism.
The levels of lipids in the grains showed a positive correlation with amylase and lipase in insects and a negative correlation with crude fiber, crude protein and mortality, but did not correlate with mass consumed and emergence (Table 4). In Marsaro-Junior et al. (2005), of the nutritional parameters evaluated, lipids were the ones that most influenced the resistance of hybrids to attack by S. zeamais, since lipid content was positively correlated with the life cycle of the pest (r = 0:46, p <0.05), indicating that increasing the lipid content in the grain leads to an increase of the biological cycle, that consequently provides a lower number of generations produced by S. zeamais, resulting in less weight loss of grain dry matter.
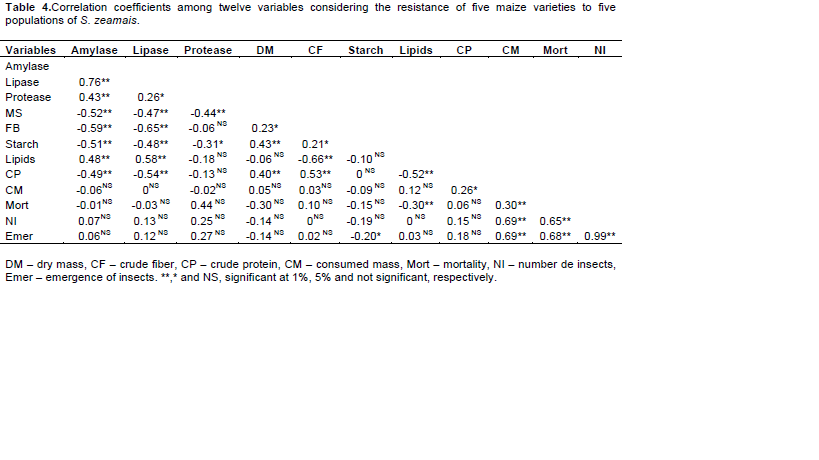
Canepelle et al. (2003) also found a positive correlation between the number of insects and loss of grain weight (r = 0.95, p < 0.001) on evaluating the correlation between level of infestation of S. zeamais and the quality of stored grain, similar to that found in the present study (Table 4). Regarding access and utilization of food, it was verified by enzyme activity (amylase and lipase), that after the food is ingested, there was no difference in responses to a given variety, as indicative of resistance associated factors. There are different answers regarding growth in relation to maize variety, this can be considered in handling maize varieties resistant to attack by S. zeamais. These results allow us to conclude that the factors of resistance of maize are substances that will be assimilated subsequently, inhibiting population growth as was observed in the present work with the grain of landrace maize, without any genetic manipulation.
According to the S. zeamais population growth data and enzymatic activities, it can be inferred that the resistance or susceptibility of grain to pest attack is related to physical and chemical constituents of the grain than the assimilation of carbohydrates and subsequent storage in fat tissue, as can be verified by analyzing the results of enzyme activity (amylase, lipase and protease) of insects fed on different maize varieties.
Populations from different regions show different behavior on the maize varieties planted in the region, with the 30F53H variety being most susceptible and the landrace variety most resistant to S. zeamais attack. Among the populations, the one originating in Palmeira das Missões had the least development on the varieties and Bom Jesus the best development. The analyses of insect enzymes and grain nutrition are tools that aid in understanding the mechanisms of resistance to define best management tactics for S. zeamais.
According to the results presented here it can be concluded that there are differences in the responses of populations as to geographic origin and the different maize varieties studied, data that are useful for understanding failures in controlling this important pest of stored maize. It can be concluded that there exists differential reaction of different maize varieties currently under production in Brazil. From the present study, the most resistant variety among the varieties tested is “crioulo” maize. This may be due to the differences of this variety from the other varieties in its morphological and biochemical constituents that confer resistance and reduced the successful utilization of itself by maize weevil, S. zeamais.
The authors have not declared any conflict of interests.
Authors appreciate the financial support provided by CNPq (National Counsel of Technological and Scientific Development), CAPES Foundation (Coordination of Improvement of Higher Education Personnel - Ministry of Education), FAPEPI (Foundation for Research Support of the State of Piauí) and UFPI (Federal University of Piauí).
REFERENCES
|
Antunes LEG, Viebrantz PC, Gottardi R, Dionello RG (2011). Características físico-químicas de grãos de milho atacados por Sitophilus zeamais durante o armazenamento. Rev. Bras. Eng. Agric. Ambient 15:615-620.
Crossref
|
|
|
|
Canepelle MAB, Caneppele C, Lázzari FA, Lázzari SM (2003). Correlation between the infestation level of Sitophilus zeamais Motschulsky, 1855 (Coleoptera, Curculionidae) and the quality factors of stored corn, Zea mays L. (Poaceae). Rev. Bras. Entomol. 47:625-630.
Crossref
|
|
|
|
|
Caraway WT (1959). A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Pathol. 32:97-99.
|
|
|
|
|
Chown SL, Nicolson SW (2004). Insect Physiological Ecology: mechanisms and patterns. Oxford University Press, Oxford, UK.
Crossref
|
|
|
|
|
Ferrari-Filho E, Antunes LEG, Tiecker A, Dionello RG, Spolt P (2011). Controle de gorgulho-do-milho submetido ao tratamento térmico. Rev. Bras. Milho Sorgo10:196-204.
|
|
|
|
|
Guedes RNC, Kambhampati S, Dover BA, Zhu KY (1997). Biochemical mechanisms of organophosphate resistance in Rhyzopertha dominica (Coleoptera: Bostrichidae) from the United States and Brazil. Bull. Entomol. Res. 87:581-586.
Crossref
|
|
|
|
|
Hendrix DL (1993). Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop Sci. 33:1306-1311.
Crossref
|
|
|
|
|
Ishimoto M, Sato T, Chrispeels MJ, Kitamura K (1996). Bruchid resistance of transgenic azuki bean expressing seed alpha-amylase inhibitor of common bean. Entomol. Exp. Appl. 79:309-315.
Crossref
|
|
|
|
|
Keba T, Sori W (2013). Diffential resistance of maize varieties to maize weevil (Sitophilus zeamais Motschulsky) (Coleoptera: Curculionidae) under laboratory conditions. J. Entomol. 10:1-12.
Crossref
|
|
|
|
|
Lehninger AI, Nelson DI, Cox MM (2000). Principles of Biochemistry. Worth Publishers Inc., New York, NY, USA.
|
|
|
|
|
Makate N (2010). The susceptibility of different maize varieties to postharvest infestation by Sitophilus zeamais (motsch) (Coleoptera: Cuculionidae). Sci. Res. Essay 5:30-34.
|
|
|
|
|
Marsaro-Júnior AL, Lazzari SMN, Souza JL, Lazzari FA, Cândido LMB (2007). Influência de diferentes sistemas de adubação na composição nutricional do milho Zea mays L.(Poaceae) e seus efeitos no ataque de Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) no produto armazenado. Semina: Ciências Agrárias 28:51-64.
Crossref
|
|
|
|
|
Marsaro-Júnior AL, Lazzari SMN, Figueira ELZ, Hirooka EY (2005). Inibidores de amilase em híbridos de milho como fator de resistência a Sitophilus zeamais (Coleoptera: Curculionidae). Neotrop. Entomol. 34:443-450.
Crossref
|
|
|
|
|
Mendiola-Olaya E, Valencia-Jiménez A, Valdés-Rodríguez S, Délano-Frier J, Blanco-Labra A (2000). Digestive amylase from the larger grain borer, Prostephanus truncates Horn. Comp. Biochem. Physiol. 126:425-433.
Crossref
|
|
|
|
|
Miranda MMM, Araújo JM, Picanço M, Faleiro FG, Machado AT (1995). Detecção de não preferência a Sitophilus zeamais Mots. em espigas e grãos de 49 populações de milho. Rev. Bras. Armaz. 20:21-25.
|
|
|
|
|
Pueyo JJ, Morgan TD, Ameenuddin N, Liang C, Reeck GR, Chrispeels MJ, Kramer KJ (1995). Effects of bean and wheat α-amylase inhibitors on α-amylase activity and growth of stored product insect pests. Entomol. Exp. Appl. 74:237-244.
Crossref
|
|
|
|
|
SAS Institute Inc. (2002). Statistical Analysis System user's guide. Version 9.0 ed. Cary.
|
|
|
|
|
Silva DJ, Queiroz AC (2002). Análises de alimentos (métodos químicos e biológicos). Editora UFV, Viçosa, MG, Brazil.
|
|
|
|
|
SPSS (2000). Science Software Products. Chicago. Version 8.0.
|
|
|
|
|
Titarenko E, Chrispeel MJ (2000). DNA cloning, biochemical characterization and inhibition by plant inhibitors of the α-amylases of the western corn rootworm, Diabrotica virgifera virgifera. Insect Biochem. Mol. Biol. 30:979-990.
Crossref
|
|
|
|
|
Tomarelli RM, Charney J, Harding ML (1949). The use of azoalbumin as a substrate in the colorimetric determination of peptic and tryptic activity. Lab. Clin. Med. 34:428-433.
|
|
|
|
|
Toscano LC, Boiça-Júnior AL, Lara FM, Waquil JM (1999). Resistência e mecanismos envolvidos em genótipos de milho em relação ao ataque do gorgulho, Sitophilus zeamais Mots. (Coleoptera: Curculionidae). An. Soc. Entomol. Bras. 28:141-146.
Crossref
|
|
|
|
|
Tran BMD, Credland PF (1995). Consequences of inbreeding for the cowpea seed beetle, Callosobruchus maculatus. Biol. J. Linn. Soc. Lond. 56:483-503.
Crossref
|
|
|
|
|
Trematerra P, Ianiro R, Athanassiou CG, Kavallieratos NG (2013). Behavioral responses of Sitophilus zeamais Motschulsky adults to conditioned grain kernels. J. Stored Prod. Res. 53:77-81.
Crossref
|
|
|
|
|
Warburg O, Christian W (1941). Isolierung und kristallisation des garingsferments enolase. Biochem. Z. 310:384-421.
|
|
|
|
|
Zeng F, Cohen AC (2000). Partial characterization of α-amylase in the salivary glands of Lygus hesperus and L. lineolari. Comp. Biochem. Physiol. 126:9-16.
Crossref
|
|





