ABSTRACT
Termites are most pestiferous insects causing damage to crop and buildings. Their control still relies mainly on harmful chemical pesticides to the detriment of eco-friendly pesticides. The aim of this study was to evaluate the effect of three plant extracts on the survival rate of termite species, Macrotermes subhyalinus Smeathman and Trinervitermes geminatus Wasmann, known to cause damage to crops, vegetation and buildings in Togo. Cissus quadrangularis (Vitaceae), Pennisetum purpureum Schumach (Poaceae) and Vetiveria zizanoides Nash (Poaceae) extracts were examined for their termiticidal activity against these termites. Three formulations including acetone and hexane extracts and powder were prepared for each plant species at five different concentrations. Six replicas were made for each tested concentration with 30 workers per replica in the laboratory conditions. All the tested plants showed termiticidal activities by reducing the rate of survival duration of tested termites. The powder of V. zizanoides was very effective on M. subhyalinus; its lowest concentration (5 mg/cm2) reduced the survival rate of this termite by up to 87%. Among the extracts, acetone extract of P. purpureum caused the highest reduction of M. subhyalinus survival rate at higher concentration. Both acetone and hexane extracts of V. zizanoides were very effective on T. geminatus, reducing by more than 90% survival rate of this termite. Although these plants extract seem to bear potential termiticidal activity, however, further studies need to be carried out in order to determine their respective components.
Key words: Botanical insecticides, Cissus quadrangularis, Pennisetum purpureum, Vetivera zizanoides, termite species.
Environmental problems caused by the persistent pesticides have gradually led to an increasing interest in the development of alternative pest control methods. With the prohibition of the chemicals, which were toxic to humans, termite control is currently centered on the use of non-chemical methods including the use of biopesticides (Verma et al., 2009; Rust, 2014).
Thus, plants with insecticidal properties could be regarded as potential alternatives to chemical pesticides. Indeed, various plants or plant extracts are used (under various formulations) to control termites or other insect pests (Isman, 2006; Dhang and Sanjayan, 2014). A broad range of plants are toxic, repellent, or have some antifeeding properties several of which were regarded as insecticides (Bläske and Hertel, 2001; Ganapaty et al., 2004, Boulogne et al. 2012; Raina et al. 2012; Addisu et al. 2014).
Osipitan and Oseyemi (2012) have evaluated the insecticidal effect of three plant extracts including Citrus sinensis, Theobroma cacao, Tithonia diversifolia and Anacardium occidentalis, against Macrotermes bellicosus. They found that the acqueous extract of these plants not only caused the mortality of tested termites, but also showed an important repellency to them. The repellent effect of extracts from few tropical plants on termite has also been reported by Maistrello et al. (2011), Osipitan et al. (2013) and Acda (2014).
The termiticidal proprieties of plants lie mainly on their secondary compounds or organic extractives such as waxes, alkaloids, fat, gums, resins, terpenes and essential oils (Pettersen, 1984). Thus, some flavonoids extracted from the Japanese larch and Larix leptolepis (Lamb) showed strong feeding deterrent activities against Coptotermes formosanus (Shiraki) (Ohmura et al., 2000; Chen et al., 2004). Recently Chowański et al. (2016) reported the toxicity caused by alkaloids from few Solanaceae plants. According to these authors, both formulations (metabolites and water/alcohol extracts) showed lethal and sublethal effects on tested termites and other pestiferous insects.
Locally, plants or plant products are used by farmers to control termites. Some species of living plants are usually left inside the farms in order to deter termites from crops (Sileshi et al., 2008; Mugerwa et al., 2014). In Togo, some farmers believe that keeping plants such as Vetivera zizanoides, Cissus quandrangularis and Pennisetum purpureum within the crop field deter pestiferous termites (especially Macrotermes and Trinervitermes species). However no scientific study has yet been carried regarding this assertion. Hence the aim of this study is to check the termiticidal activity of extracts from these plants through the evaluation of their effect on the survival rate of Macrotermes subhyalinus and Trinervitermes geminatus, termite species known to cause damage to crops, vegetation and buildings in Togo.
Plant materials
The plants used in these experiments include C. quadrangularis (Vitaceae) P. pupureum (Poaceae) and V. zizanoides (Poaceae). The leaves of V. zizanoides and the stems with leaves of C. quadrangularis were collected in the botanical garden of the Faculty of Science of the University of Lomé (Togo). The leaves of P. pupureum were bought in the market at Lomé. In the laboratory, these vegetable materials were cut into small pieces and dried in an air-conditioned room. After drying, they were finely crushed using a blender (OPTIBLEND 2000 TRIO) and sieved. The fine powder obtained was stored at -20°C for further use. The non-fine portion was macerated in acetone and hexane for a week then filtered and evaporated with the rotavapor in cold conditions. The extracts obtained were also stored at - 20°C.
Termites
Macrotermes subhyalinus and Trinervitermes geminatus were used in the biological tests. These termites were collected on the campus of the University of Lomé and acclimatized to laboratory conditions (28°C, Relative Humidity 70%) in total darkness (12:12H DD). During acclimatization, they were fed on fungus comb and filter paper for M. subhyainus and on straw for T. geminatus.
Biological tests
The biological tests were carried out by contact following Raina et al. (2012). Three formulations, powder, acetone and hexane extracts were used. Concentrations were expressed in mg/cm². Each concentration of powder was introduced into a glass Petri dish of 9 cm diameter.
The powder was then distributed uniformly over the surface of Petri dish. The plant extracts (acetone and hexane extracts) were initially dissolved in 5 ml of acetone or hexane respectively. The solution obtained from each concentration was put in a glass Petri dish (9 cm diameter) whose bottom was covered with filter paper. The soaked paper was air dried for 24 h. The different concentrations used are: 0.5, 1, 2, 4 and 6 mg/cm2.
Thirty workers of each termite species were exposed to each concentration and six replications were made. The Petri dishes containing the tested termites were then placed in controlled conditions of 28°C temperature and 78% relative humidity in total darkness.
After each hour and during six hours, all the Petri dishes were checked for dead termites. After the sixth hour, the checking was done at the eighteenth hour then after each 24 h until the death of all termites. The controls were done with filter paper only for tests with plant powder, and filter paper previously soaked with acetone or hexane and air dried.
Determination of survival duration
The survival duration of termites was determined according to the formula below (adapted from Krebs, 1999).
Statistical analysis
The data obtained from calculation of survival duration and the rate of survival reduction were subjected to an analysis of variance (ANOVA) at 5%, and the means were discriminated with the Student-Newman-Keuls (SNK) test using STATISTICA software, version 5.1 (1998).
Effect of plant powders on M. subhyalinus
The powders of P. purpureum, C. quadrangularis and V. zizanoides reduced the survival duration of M. subhyalinus at all the tested concentrations (Table 1). At the highest concentration (6 mg/cm2), the reduction rates were 80, 78 and 90% respectively with P. purpureum, C. quadrangularis, and V. zizanoides. At 0.5 mg/cm2 the powder of V. zizanoides significantly reduced the survival rate of M. subhyalinus (F = 83; df = 5; P < 0.001). Among the plant powders, V. zizanoides caused the highest reduction of survival rate. On the other hand, C. quadrangularis (F = 44.11; df = 5; P < 0.001) was more effective than P. purpureum (F = 33.65; ddf = 5; P < 0.001) at low concentration but they had the same effect at 4 and 6 mg/cm2.
Effect of plant extracts on M. subhyalinus
Like the plant powders, acetone and hexane extracts also affected the survival rate of M. subhyalinus. At the lowest concentrations (0.5 and 1 mg/cm2), the acetone extract of V. zizanoides (F = 20.4; df = 6; P < 0.0001) caused more survival reduction (34 and 48% respectively) than C. quadrangularis (16 and 30% respectively) and P. purpureum (3 and 8% respectively) at the same concentrations (Table 1). At these concentrations (for acetone extracts), P. purpureum was less effective than the other two plants. Nevertheless, at the higher concentrations (4 and 6 mg/cm2) P. purpureum (F = 40.26; df = 6; P < 0.0001) and C. quadrangularis (F = 42.23; df = 6; P < 0.0001) became more effective than V. zizanoides with 95% (both two plants) reduction rate against 73% for the former at 6 mg/cm2 (Table 1).
With the hexane extracts, the threshold of 50% reduction rate was observed at 4 mg/cm2 contrary to the acetone extract (Table 1). Nevertheless, like the acetone extract, the hexane extract of V. zizanoides was more effective from 0.5 mg/cm2 to 2 mg/cm2 than the other two plants (F = 12.3; df = 6; P < 0.0001). It was followed respectively by C. quadrangularis (F = 30.1; df = 6; P < 0.0001) and P. purpureum (F = 16.5; df = 6; P < 0.0001). At 6 mg/cm2 C. quadrangularis was the most effective with a reduction rate of 95% while V. zizanoides reduced only 56% of the survival rate (Table 1). But from 1 to 6 mg/cm2 both acetone and hexane extracts of C. quadrangularis were pasty and this considerably reduced the movement of termites.
Effet of plant powders on T. geminatus
The results showed that plant powders caused a significant reduction of T. geminatus’ survival duration (F = 8.48; df = 5; P < 0.0001; F = 40; df = 5; P < 0.0001; and F = 51.4; df = 5; P < 0.0001, respectively for P. purpureum, C. quadrangularis and V. zizanoides). At the lowest concentration (0.5 mg/cm2), all plant powders reduced the survival rate to more than 50% (Table 1). The effect of these powders seemed not to be influenced by concentration (within each plant species) and was also comparable from one plant to another
Effect of plant extracts on T. geminatus
All the tested extracts reduced the survival duration of T. geminatus (P < 0.0001). At 0.5 mg/cm2 the acetone extract of C. quadrangularis (F = 129; df = 6; P < 0.0001) affected termite survival with 53% reduction, and at 6 mg/cm2 it caused the highest reduction rate (97%) (Table 1). V. zizanoides acetone extract was the second most effective on T. geminatus especially from 1 to 6 mg/cm2 (F = 96.6; df = 6; P < 0.0001). The dose 4 mg/cm2 of P. purpureum was more effective (F = 44.5; df = 6; P < 0.0001) than that of 6 mg/cm2 of the same plant. In the latter concentration, the extract was highly stuck to the filter paper. The lower concentration (0.5 mg/cm2) of hexane extracts of these plants reduced the survival rate to more than 50% (Table 1). It was respectively 63, 54 and 59% for P. purpureum, C. quadrangularis and V. zizanoides (Table 1). The highest reduction was recorded at 6 mg/cm2 with C. quadrangularis (96%) followed by V. zizanoides (93%) and P. purpureum (89%). Here also, from 1 to 6 mg/cm2 both acetone and hexane extracts of C. quadrangularis were pasty.
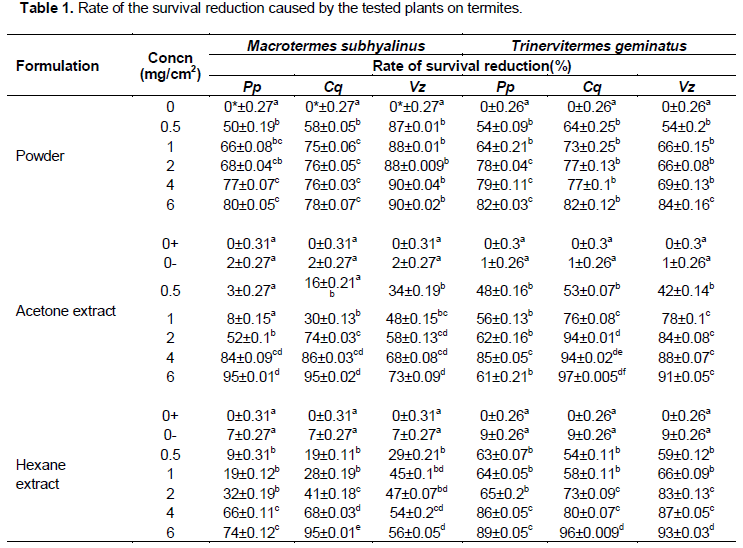
The powders of these tested plants were effective against M. subhyalinus. The effect of V. zizanoides was very spectacular even at the lower concentration. This is likely due to the higher amount of silica (SiO2) present in the leaves of Vetiver grass.
According to Methacanon et al. (2003), silica content of V. zizanoides leaves is about 50%. This inorganic component not only affects positively plants growth but also confers to them a certain resistance against phytophagous insects and other wood eating microorganisms (Djamin and Pathak, 1967; Massey et al., 2007). It is also known that the essential oil extracted from the root of Vetiver grass contains more than 300 compounds belonging to the sesquiterpene group of natural products including α and β vetivones (Jain et al., 1982; Zhu et al., 2001a), and nootktanone (Zhu et al., 2001b) which were all reported as insect repellents.
However, leaves but not roots were used in our study. So we could not sustain the presence of these repellent components within the leaves, since there is no reliable data on the essential oil extracted from leaves of Veriter grass. Acetone and hexane extracts also affected the survival rate of M. subhyalinus. Among the plant species, V. zizanoides extracts (acetone and hexane) were not effective as was the case with other plant species on the survival rate of M. subhyalinus at higher concentrations (contrary to the two lowest concentrations).
This suggested that the most active compounds which had an insecticidal property in V. zizanoides against M. subhyalinus may be volatiles. These volatiles are abundantly present in V. zizanoides essential oil extracted from roots (Zhu et al., 2001a, b). The survival reduction rate observed in acetone and hexane extracts of C. quadrangularis was likely due essentially to the physical aspect of the extracts than their toxicity. Indeed all the extracts were pasty (except at the lower concentration) which considerably reduced the movement of termites. On the other hand, the acetone and hexane extracts of P. purpureum were not pasty. These extracts were very effective especially at the highest concentration of acetone extract.
T. geminatus’ survival rate was also affected by plant powders and plant extracts. Like the test with M. subhyalinus, the threshold of 50% survival rate reduction was observed with all the plant powders. But here, V. zizanoides (like the other plants) powder seemed to be less effective on T. geminatus than on M. subhyalinus. The lowest concentration of V. zizanoides caused more survival rate reduction of M. subhyalinus than did the highest concentration on T. geminatus. This could be explained by the feeding habit of T. geminatus. Indeed T. geminatus like other Trinervitermes feed essentially on herbaceous plants (Ohiagu and Wood, 1976; Wood, 1978). They may have developed a certain resistance to some defensive extracts of these grasses (Harborne, 1982) than did M. subhyalinus. Thus T. geminatus was less affected by plant powders than M. subhyalinus which seemed to be polyphagous, preferentially eating bark, wood or living plants (Anani Kotoklo et al., 2009) rather than grasses. Nevertheless, T. geminatus could not overcome the defensive extracts of these plants as there was a significant difference between the controls and all concentrations of plant powders.
Acetone and hexane extracts of P. purpureum seemed to have almost the same effect on T. geminatus at the highest concentrations. In the test with acetone extract, more reduction of survival rate occurred with 4 mg/cm2 than 6 mg/cm2 (actually the highest). Because, extract with 6 mg/cm2 concentration was highly stuck to the filter paper, and could not be removed by the leg of T. geminatus (contrary to M. subhyalinus which is stronger). Thus there was less contact between the extract and the termite. The acetone and hexane extracts of V. zizanoides were very effective on T. geminatus especially at higher concentration unlike M. subhyalinus (more susceptible to powder than extracts). Therefore the active components of V. zizanoides against T. geminatus might be less volatile.
All the three tested plants showed antitermite activity by reducing the survival rate of M. subhyalinus and T. geminatus. The powder of V. zizanoides was very effective against M. subhyalinus at lower concentration. It was also very vulnerable to the higher concentration of the acetone extract of P. purpureum. Both acetone and hexane extracts of V. zizanoides were highly effective against T. geminatus. All the extracts of C. quadrangularis were pasty, thus unsuitable for these experiments.
The authors have not declared any conflict of interests.
REFERENCES
|
Acda MN (2014). Repellent effects of Annona crude extract on the Asian Subterranean Termite Coptotermes gestroi Wasman (Isoptera: Rhinotermitidae). Sociobiology 61(3):322-337.
Crossref
|
|
|
|
Addisu S, Mohamed D, Waktole S (2014). Efficacy of botanical extracts against termites, Macrotermes spp (Isoptera: Termitidae) under laboratory conditions. Int. J. Agric. Res. 9(2):60-73.
Crossref
|
|
|
|
|
Anani Kotoklo E, Kasseney BD, Nyamador W, Ketoh GK, Glitho AI (2010). Attaques des arbres par les termites sur le campus de l'Université de Lomé (Togo). Int. J. Biol. Chem. Sci. 4(1):61-68.
Crossref
|
|
|
|
|
Bläske VU, Hertel H (2001). Repellent and toxic effects of plant extracts on Subterranean Termites. J. Econ. Entomol. 94(5):1200-1208.
Crossref
|
|
|
|
|
Boulogne I, Petit P, Ozier-Lafontaine H, Desfontaines L, Loranger-Merciris G. (2012) Insecticidal and antifungal chemicals produced by plants: a review. Environ. Chem. Lett. 10(4):325-347.
Crossref
|
|
|
|
|
Chen K, Ohmoura W, Doi S, Aoyama M (2004). Termite feeding deterrent from Japanese larch wood. Bioresour. Technol. 95(2):129-134.
Crossref
|
|
|
|
|
ChowaÅ„ski S, Adamskí Z, Marciniak P, RosiÅ„ski G, Büyükgüzel E, Büyükgüzel K, Falabella P, Scrano L, Ventrella E, Lelario F, Bufo SA (2016). A review of bioinsecticidal activity of Solaceae Alkaoids. Toxins 8(60):1-28.
|
|
|
|
|
Djamin A, Phatak MD (1967). Role of silica in resistance to asiatic rice borer Chilo suppressalis (Walker), in rice varieties. J. Econ. Entomol. 60(2):347-351.
Crossref
|
|
|
|
|
Dhang P, Sanjayan KP (2014). Plants with pest control properties against Urban pest. In: Dhang P (ed), Urban insect Pest sustainable management strategies. CABI, Cryodon, United Kingdom. pp 216-238.
Crossref
|
|
|
|
|
Ganapaty S, Thomas PS, Fotso S, Laatsch H (2004). Antitermitic quinones from Diospyros sylvatica. Phytochem. 65(9):1265-1271.
Crossref
|
|
|
|
|
Harborne JB (1982). Introduction to ecological biochemistry. 2nd ed. London, New York: Academic Press. pp. 278.
|
|
|
|
|
Isman MB (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 51:45-66.
Crossref
|
|
|
|
|
Jain SC, Nowicki S, Eisner T, Meinnald J (1982). Insect repellents from vetiver oil: 1. Zizanal and epizanal. Tetrahedron. Lett. 23(45):4639-4642.
Crossref
|
|
|
|
|
Krebs CJ (1999). Ecological Methodology. 2nd Edition. Addison Wesley Longman, Menlo Park, California, USA. pp. 745.
|
|
|
|
|
Maistrello L, Martin L,Macías-Pavon I, Bortolini S, Marchrttini N. (2011). Evaluation of polyphenols-rich natural compounds as treatments to prevent attacks by subterranean and drywood termites: preliminary results. J. Entomol. Acarol. Res. 43(2):261-267.
Crossref
|
|
|
|
|
Massey FP, Ennos AR, Hartley SE (2007). Herbivore specific induction of silica based plant defenses. Oecologia 152(4):677-683.
Crossref
|
|
|
|
|
Methacanon P, Chaikumpollert O, Thavorniti P, Suchiva K (2003). Hemicellulosic polymer from Vetiver grass and its Physicochemical properties. Carbohyd. Polym. 54:335-342.
Crossref
|
|
|
|
|
Mugerwa S, Mpairwe D, Zziwa E, Swaans K, Peden D (2014). Integrated termite management for improved rainwater management: A synthesis of selected African experiences. NBDC Technical Report 9. Nairobi, Kenya: ILRI. P 33.
|
|
|
|
|
Ohiagu CE, Wood TG (1976). A method for measuring rate of grass harvesting by Trinervitermes geminatus (Wasman) (Isoptera, Nasutitermitinae) and observation on its foraging behaviour in southern guinea savanna, Nigeria. J. Appl. Ecol. 13(3):705-713.
Crossref
|
|
|
|
|
Ohmura W, Doi S, Aoyama M, Ohara S (2000). Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. J. Wood. Sci. 46:149-153.
Crossref
|
|
|
|
|
Osipitan AA, Jegede TO, Adekanmbi DI, Ogunbanwo IA (2013). Assessment of Datura metel, local soap and garlic (Allium sativum) in the management of Termite (Termitidae: Isoptera). Mun. Ent. Zool. 8(1):407-414.
|
|
|
|
|
Osipitan AA, Oseyemi AE (2012). Evaluation of the bio-insecticidal potential of some tropical plant extracts against termite (Termitidae: Isoptera) in Ogun State, Nigeria. J. Ent. 9(5):257-265.
Crossref
|
|
|
|
|
Pettersen RC (1984). The Chemical Composition of Wood. In: R M Rowell (ed), The Chemistry of Solid Wood. Madison: American Chemical Society Washington DC, USA. pp. 57-126.
Crossref
|
|
|
|
|
Raina A, Bedoukian R, Florane C, Lax A (2012). Potential of natural products and their derivatives to control Formosan Subterranean Termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 105:1746-1750.
Crossref
|
|
|
|
|
Rust MK (2014). Management strategies for Subterranean Termites. In: Dhang P (Ed), Urban insect Pest sustainable management strategies. CABI, Cryodon, United Kingdom. pp. 114-129.
Crossref
|
|
|
|
|
Sileshi G, Kuntashula E, Matakala P, Nkunika PO (2008). Farmers' perceptions of pests and pest management practices in agroforestry in Malawi, Mozambique and Zambia. Agrofor. Syst. 72(2):87-101.
Crossref
|
|
|
|
|
Verma M, Sharma S, Prasad R (2009). Biological alternatives for termite control: a review. Int. Biodeterior. Biodegrad. 63(8):959-972.
Crossref
|
|
|
|
|
Wood TG, Sands WA (1978). Food and feeding habit of termites. In: Brian MV (Ed.), Production ecology of ants and termites. Cambridge University Press, London, United Kingdom. pp. 55-80.
|
|
|
|
|
Zhu BCR, Henderson G, Chen F, Fei H, Laine RA (2001a). Evaluation of vetiver oil and seven insect-active essential oils against the Formosan Subterranean Termite. J. Chem. Ecol. 27(8):1617-1625.
Crossref
|
|
|
|
|
Zhu BCR, Henderson G, Chen F, Maistrello L, Laine RA (2001b). Nooktanone is a repellent for Formosan Subterranean Termite (Coptotermes formosanus). J. Chem. Ecol. 27(3):523-531.
Crossref
|
|