Emphasis on reducing nitrogen fertilizer requirement, better quality forage and cover crops for multiple purpose with perennial plants for living mulch has stimulated interest in white clover (Trifolium repens L.).
White clover is an important perennial forage legume that is grown in Southern Brazil. However, it is not as important in Brazil as it is in other countries with similar climate such as New Zealand and Australia where clover is the most widely-used legume. White clover is also an important forage legume in tropical and subtropical regions of the world (Carvalho et al., 2010).
Despite the fact that herbicide tolerance is the basis for the success of chemical weed control, the results available on white clover tolerance to herbicides are controversial given that some authors have reported that yield and persistence of the legume were markedly reduced (Griffin et al., 1984; Evers et al., 1993), whereas others have shown that although some herbicides can have initial phytotoxic effects, seedlings usually recover without severe damage (Machado et al., 2013)
Selective post emergence herbicides are needed as emerging clover seedlings development is slow and not competitive with faster growing weeds (Schuster et al., 2013). Moreover, on crop livestock farms, corn may be cultivated using clover as living mulch or over its dead biomass, however, herbicide tolerance needs to be known for improved clover management. Furthermore, white clover might become a weed in summer crops such as soybean (Schuster et al., 2015), and thus, herbicide management to control white clover need to be determined.
Once weed competition problems are overcome, white clover adoption might face a positive scenario, greatly due to its advantages such as biological nitrogen fixation, high nutritional value and excellent adaptability to edaphoclimatic conditions in Southern Brazil. In this context, the study aimed to identify white clover tolerance to herbicides and rates at different phenological stages.
Experiments were conducted in greenhouses and designed as randomized complete blocks (RCBD) in a 3x4 factorial scheme with four replications, and aimed at evaluating white clover tolerance to different herbicide at different rates and crop phenological stages. Three herbicides were applied at four rates each onto 40, 105, or 125 day old white clover seedlings, which correspond to seedlings at the three-leaf trifoliate stage, full development, and full bloom, respectively. At the first assay, 40-day old plants (three-leaf trifoliate stage) were sprayed with glyphosate (Zapp Qi), 2,4-D (Aminol), or imazethapyr (Pivot) at the rates of 0, 540, 1080, 1620; 0, 200, 400, 600 g and 0, 100, 150, 200 g i.a ha-1, respectively. This application simulated the time period at which weeds are controlled in white clover fields.
At the second assay, white clover plants at full development (105 days after emergence) were sprayed with glyphosate (Zapp Qi), 2,4-D (Aminol) and Paraquat + Diuron (Gramocil) at the rates of 0, 540, 1080, 1620; 0, 400, 600, 800 and 0, 300, 600 and 900 g i.a ha-1 respectively, in order to simulate herbicide tolerance of white clover at intermediate cycle development.
In the third assay, white clover plants at full bloom (125 days after emergence) were sprayed with glyphosate (Zapp Qi), 2,4-D (Aminol) and Paraquat + Diuron (Gramocil) at the rates of 0, 720, 1440, 2160; 0, 800, 1600, 2400 and 0, 300, 600, 900 g i.a ha-1 respectively. This period simulates a possible desiccation of white clover or its suppression for living mulch cultivation with summer cash crops. White clover cv. Zapican was used in all experiments. Individual experimental units consisted of single 20 cm diameter plastic pots (10 liters capacity) filled with soil with the following chemical properties: pH (CaCl2) 5.0; V% 64; organic matter 63 g dm-3; P-Mehlich 9.5 mg dm³; Ca, Mg, K and Al of 6.6; 1.9; 0.30 and 0.0 cmolcdm-3 respectively and soil cation exchange capacity of 13.81 cmolcdm-3. Soil fertilization was not performed because of the good soil parameters (CQFS, 2004). White clover seeds were hand sowed to the 1 cm top soil layer at a rate of 15 seeds per pot. Pots were watered throughout the course of the experiments to ensure adequate soil moisture.
Herbicides were sprayed with a CO2-pressurized backpack sprayer (XR110.02 spray tips) calibrated to deliver 200 L ha-1 maintained at constant pressure (2.07 bar). At the time of applications, the plant surface was dry, relative humidity was above 60% and temperatures were between 25 and 30°C. The amount of herbicide to be applied was calculated considering the concentration of active ingredient of the herbicide trademarks used.
The assessment of phytotoxicity to white clover plants was carried out weekly at 07, 14, 21, 28, 35, 42 and 49 days after herbicide application (DAA) by assigning percentage grades from 0 (e.g. absence of phytotoxicity) to 100% (complete death of plants), according to the methodology proposed by SBCPD (1995). Phytotoxicity-induced plant symptoms included chlorosis, leaf necrosis and stunted growth.
White clover plants were harvested 50 days after herbicide application, and oven-dried with forced air at 55°C for five days, after which dry-biomass weights were determined for each replication. The results were analyzed for homogeneity of variance (Bartlett) and normality (Lilliefors) tests. Data were then subjected to ANOVA and means separations performed using the Tukey test at 5% probability with the Statigraphic 4.1 program. No data transformations were required since assumptions for ANOVA were properly met (data not shown).
Results differed among herbicides and rates across different phenological stages. Phytotoxicity occurred at all evaluations periods after application (DAA), and its effects on dry matter yield was also evident.
In the first assay, imazethapyr showed the highest selectivity on white clover among the evaluated herbicides (Table 1). Phytotoxicity levels increased as herbicide rate increased and even though there was an initial phytotoxic effect, such weakened with time and seedlings were thus able to recover without further implications. Accordingly, there was no significant difference on growth at 49 DAA with applications of imazethapyr at 100, 150 and 200 g a.i. ha-1 relative to the untreated control.

Ferrel and Sellers (2014) reported a slight leaf yellowing and a temporary growth reduction on white clover after imazethapyr (Pivot®) application, similar to the symptoms found in this work. However, they did endorse this herbicide as a good option to control broadleaf weeds in post emergence (seedlings older than the two-trifoliate leaf stage), as well as established clovers. They also reported that grasses such as perennial ryegrass in white clover seed fields can be easily controlled with clethodim or sethoxydim using trademarks products such as Select® or Poast® at rates of 120 and 180 g i.a ha-1, respectively. According to Ferrel and Sellers (2014), imazethapyr is already registered and recommended for use in the U.S. as a selective herbicide in white clover. One must, however, follow label guidelines concerning herbicide rates and withholding periods, which indicates white clover should not be used as feed, raze, or harvest until 30 days after application.
Grasses are usually not a problem in white clover pastures since they are grazed by the animals, and thus broadleaf weed species are the most troublesome species in this situation. According to Schuster et al. (2013), sow thistle (Sonchus oleraceus), wild radish (Raphanus raphanistrum), ragweed (Ambrosia elatior), sticky nightshade (Solanum sisymbriifolium), bristly starbur (Acanthospermum hispidum) and arrowleaf sida (Sida rhombifolia) are the most important weed species on white clover fields in southern Brazil. Therefore, 2.4-D stands as a good alternative due to its control efficacy over these weeds. Moreover, in Brazil, Mexican fire plant (Euphorbia heterophylla) is considered resistant to imazethapyr (Xavier et al., 2013) and is still well controlled by 2,4-D.
It was noticed that clover selectivity to 2,4-D decreased as the rate of the herbicide increased, however, plants seemed to be able to detoxify the herbicidemolecule since phytotoxicity was reduced from 45% at 14 DAA to 22.5% at 49 DAA for the highest rate (600 g a.i ha-1) (Table 1). It is noteworthy that label recommendation for broadleaf control at most crops ranges from 400 to 600 g i.a ha-1 and the fact that this rate is effective on broadleaf control and there is no need to use higher rates since they may cause phytotoxicity on the crops. Moreover, the greater the difference between crop and weed tolerance, the greater the security to recommend treatment employing a particular herbicide.
White clover tolerance to 2,4-D and imazethapyr was also suggested in other experiments (Mccurdy et al., 2013). Machado et al. (2013) assessing the selectivity of imazethapyr (100 g a.i ha-1) and 2,4-D (201 g a.i ha-1) applied to white clover seedlings at the two-leaf trifoliate growth stage reported phytotoxic levels of 1.5 and 9.7%, respectively, at 84 DAA, corroborating with the results of this work. Enloe et al. (2013) studying white clover tolerance to multiple formulations of 2,4-D with imazethapyr reported effective weed control in mixed white clover/grass pastures without affecting white clover populations.
Highest phytotoxicity levels occurred when plants were treated with glyphosate at a rate of 1080 g a.i ha-1 (applied as Zapp Qi® herbicide at 1.7 L ha-1), at which plants were dead 21 DAA (Table 1). On the other hand, the lowest glyphosate rate (540 g a.i ha-1) showed intermediate phytotoxicity, reaching values of 35 and 5% at 28 and 49 DAA, respectively. Biomass dry weight showed a slight difference (38% less) as compared to the control. This value was similar to the treatment with 2,4-D at either the 400 and 600 g a.i ha-1.
Moreover, it is important to emphasize that this growth reduction and lower dry matter accumulation may over time be recovered once weeds are controlled. On the other hand, if weed control is not performed, losses can reach as high as 96%, practically making it impossible for grower to cultivate this species (Schuster et al., 2013).
It is also important to observe the herbicide tolerance increases as plant age increases. Machado et al. (2013) evaluating 2,4-D applied at the first (16 days after emergence) and second-leaf trifoliate (27 days after emergence) growth stage reported lower toxicity for older plants. Young plants are more susceptible to herbicides than older plants, mainly because they have more meristematic tissues (Evers et al., 1993).
Schuster et al. (2013) while evaluating weed interference in white clover, reported that the critical period of interference occurred between 20th and 62nd days after emergence. Rolston and Archie (1999) also reported that phenological stage affected cultivar sensitivity to the herbicide diflufenican. According to the results, clover growth suppression can occur, but is thought to be due to the clover growth stage at treatment time, rather than reflecting a difference in cultivar tolerance. Plants at the four-trifoliate leaf stage were more tolerant than younger plants. Furthermore, white clover seed crops showed good seed yield tolerance to diflufenican at rates ranging from 25 to 75 g a.i ha-1.
In the second assay, it was noticed, a higher tolerance to herbicides glyphosate and 2,4-D. Phytotoxicity level of glyphosate-treated plants ranged from 28 to 95% at 49 DAA between the lower and highest rate. By day 50, the relative dry weights were 100, 39, 24 and 1.4% for the control, low, middle and high glyphosate rates, respectively (Table 2).
As for the 2,4-D herbicide, phytotoxicity variation between the lowest and highest rate at 49 DAA was lower, ranging from 0 to 37.5% at the rate of 400 and 1200 g a.i ha-1. It caused some phytotoxicity, but the plants were generally not severely damaged and recovered. By the end of experimentation, 2,4-D at the 1X rate did not affect biomass dry weight, but the two (2X) and three times (3X) rates reduced shoot dry weight by 53 and 75%, respectively, as compared to the untreated control treatment.
In general, herbicides affected plant development and caused a delay in plant growth. At the end of the 2nd trial, control plants had already flowered while other treatments were still at vegetative growth stage. It shows that beyond dry matter, these herbicides applied at later stages can affect white clover seed yield.
At both 2nd and 3rd assay (Tables 2 and 3), white clover plants treated with Paraquat + Diuron showed rapid wilting and desiccation of sprayed leaves which, according to Martins (2013) is a consequence of complete loss of photosynthetic electron transport in treated plant material.
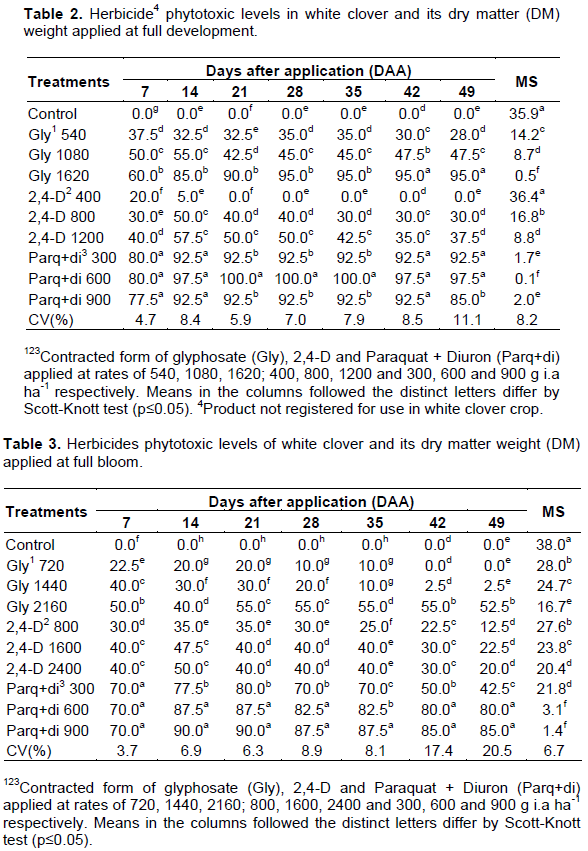
Moreover, Paraquat + Diuron caused the highest levels of white clover phytotoxicity throughout the experiment with up to 92 and 97% of white clover plants showing severe foliar injury with the recommended and double rates, respectively at 14 DAA. By the end of evaluation period, treated plant biomass weighed 96 and 99% less than the control for the 1X and 2X rates, respectively. Clark and Mahanty (1991) also reported that paraquat was extremely toxic to white clover plants.
In the 2nd assay, plants treated with Paraquat + Diuron at 600 g a.i ha-1 (applied as Gramocil® at 3 liters ha-1) were dead at 21 DAA. The differences among rates might be explained by the mode of action of the herbicide. Due to its contact action, control is achieved only where the herbicide reaches the target and a possible protection of older leaves to the younger ones resulted in differences in phytotoxicity and biomass accumulation among herbicide levels. Non-selective, ystemic herbicides might avoid this problem. Moreover, unfolded leaves which emerged after the herbicide application were undamaged and continued to grow and through that, treatment with 300 g a.i ha-1 showed some dry matter accumulation at the end of evaluated period.
In order to develop efficient herbicide use and prevent crop yield loss, information of critical period of weed control is essential. Moreover, the effect of herbicide on white clover across time is important to determine the right moment to spray it before corn seeding, especially in living mulch production systems.
According to Hall et al. (1992), the critical period of weed control in grain corn starts at the 3 and ends at the 14 leaf stage of development. In accumulated thermal units (ATU), it represents 222 to 416 ATU or approximately 15 to 30 DAE (Bedmar et al, 1999). Following this idea, it is noticed that at 14 DAA, the phytotoxicity levels ranged from 20 to 90% for the lowest and highest rate of Zapp Qi® and Gramocil®, respectively.
It is difficult to estimate the optimal percentage of phytotoxicity or white clover suppression to allow a good corn intercrop development while allowing at the same time a good pasture regrowth in the next growing season. Based on this work, it was estimated, however, that phytotoxicity levels from 20 to 50%, presented by glyphosate and 2,4-D are not sufficient to suppress white clover growth and prevent its competition with corn, which could lead to corn yield losses. Moreover, in spring, days are
longer and temperatures are higher than winter, resulting in higher growth rates and more competition potential in white clover.
Differences on biomass accumulation may be explained by leaf injury and also by root injury. Ceballos et al. (2004) detected 2,4-D root injury at the beginning of the experiment. Moreover, Garcia and Jordon (1969) found that nodulation and nitrogen fixation in birdsfoot trefoil (Lotus corniculatus L.) were reduced by 2,4-D. Martensson (1992) reported that, despite the ability of certain Rhizobium trifolii strains to detoxify glyphosate, its use inhibited root nodulation in red clover (Trifolium pratense).
Furthermore, Rolston et al. (1976) reported that paraquat caused a marked decrease in the white clover nitrogen-fixing activity. The equivalent of 70 and 280 g a.i ha-1 of paraquat reduced nitrogen-fixing activity per plant by 35 and 70%, respectively. The change was detectable 24 h after spraying and no recovery was detected by day 16. The reduction in nitrogen-fixing activity after paraquat treatment is probably an indirect response, reflecting a decrease in growth and possibly a decline in the supply of photosynthetic assimilate to the nodule. Clark and Mahanty (1991) also reported that paraquat was toxic to Rhizobium trifolii.
Phytotoxicity levels decreased with time and increased as herbicide rates changed from low to high for both glyphosate and 2,4-D treated plants. Plants showed good recovery when glyphosate (720 g a.i ha-1) and 2,4-D (800 g a.i ha-1) were applied showing phytotoxicity levels of 0 and 12% at 49 DAA, respectively, and dry matter weight of 73% as compared to the control.
Schuster (2015), studying glyphosate on clover control reported high tolerance to this herbicide since beyond desiccation, two more glyphosate applications (720 g a.i ha-1) were necessary to reach a percentage of control above 90%. Chakwizira et al. (2011) showed than the higher concentrations of glyphosate (360 g a.i) can reduce seed production and total dry matter yield for white clover.
The herbicides studied show potential to be used on white clover depending on theirrates, phenological stages of spraying, and on the specificity of existing weeds. Furthermore, according to the normative no. 1 of February 23th, 2010 from MAPA, launched in order to regulate the registration of pesticides to cultures with insufficient phytosanitary support, also called minor crops and considering that some of these herbicides are already used in related broadleaf species such as soybean, it is possible according to the results found in this experiment, to start the herbicides study directing to its use in white clover.
Moreover, this research was conducted under green-house conditions and further evaluation under field conditions are needed to verify these findings, since environmental factors may influence the herbicide effects on white clover. Furthermore, other herbicides should also be studied in order to avoid plant resistance.

