ABSTRACT
Leaf rust of wheat caused by Puccinia triticina Eriks. is one of the most widespread disease in Egypt. In this study, thirteen Egyptian wheat genotypes were evaluated for leaf rust resistance at seedling stage under greenhouse condition and adult plant stage under field conditions over three growing seasons that are, 2011/2012, 2012/2013 and 2013/2014 and three locations that are, Itay El-Baroud and Nubariya Agricultural Research Stations as well as the Farm of the Faculty of Agriculture, Minufiya University, Shibin El-Kom. The tested wheat genotypes were classified into three groups according to their resistance. The first group, race-specific resistant genotypes including Shandweel 1, Misr 1, Misr 2, Sids 12 and Sids 13, showed the lowest values of final rust severity (FRS %) and area under disease progress curve (AUDPC). The second group, slow-rusting or partially resistant genotypes including Sakha 94, Gemmeiza 9, Giza 168, Sakha 95, Gemmeiza 10 and Gemmeiza 11, displayed low level of FRS and AUDPC. The third one which includes Gemmeiza 7 and Sids 1, showed the highest values of FRS and AUDPC. Postulation of leaf rust resistance genes was differed between the tested genotypes.Results indicated that Sakha 95 and Sids 12 may have seven resistance genes. Moreover, Gemmeiza 10 may has five genes and Misr 1 may has three genes. While, Giza 168, Sids 1, Misr 2 and Shandweel 1 may have two genes. The wheat genotypes Gemmeiza 11 and Gemmeiza 12 may have only single gene. Also, all the tested wheat genotypes may contain some additional genes.In contrast, the wheat genotypes Sakha 94, Gemmeiza 7, Gemmeiza 9 and Sids 13 did not have any of the tested genes.
Key words: Wheat, leaf rust, seedling resistance, adult plant resistance, final rust severity (FRS), area under disease progress curve (AUDPC), gene postulation.
Leaf rust caused by Puccinia triticina Eriks. is a widespread disease of wheat (Triticum aestivum L.) in Egypt and worldwide. Yield losses due to leaf rust disease may be more than 50% for some susceptible wheat genotypes (German et al., 2007). Breeding wheat genotypes with resistance to leaf rust is the most effective control method and environment friendly approach to reduce the yield losses (Winzeler et al., 2000).
At present, more than 80 genes and alleles of leaf rust resistance genes have been identified and described. Among them 33 Lr genes were transferred from other species into Triticum aestivum L. (Herrera-Foessel et al., 2011, 2012; Ingala et al., 2012; McIntosh et al., 2012). Most of the resistance genes are effective at seedling stage and remain effective through the adult plant stage. Some of the leaf rust resistance genes express resistance optimally in adult plants and are known as adult plant resistance (APR) genes, which depends on the genetics of the host pathogen interaction as well as favored environmental conditions.
Rust resistance in wheat has been based on the use of race specific resistance genes. But the short-lived nature of race specific hypersensitive resistance has created the necessity to search for the more durable type of resistance. Several researchers reported that some genotypes showed the ability to retard the rust development even though they had a susceptible reaction type (Caldwell et al., 1970; Singh et al., 1991).This type of resistance known as slow rusting resistance.
Avoiding major rust epidemics in the region is a complex challenge, given that fewer genotypes are being cultivated over large areas, and several of those genotypes are protected by the same resistance genes. To identify those genotypes with resistant sources that are the most fit for the cultivation in the more diseased areas of the country, genotype screening for leaf rust resistance is considered the best and the cheapest method.
The objectives of this research work are to determine the resistance of some Egyptian wheat genotypes at seedling and adult plant stages to leaf rust through postulating and identifying resistance genes in the tested wheat genotypes.
Seedling studies
Genotypes evaluation and resistance genes postulation of the tested wheat monogenic lines were carried out at seedling stage in the greenhouse of Wheat Diseases Res. Dept., Plant Pathology Res. Inst., ARC, Giza, Egypt.
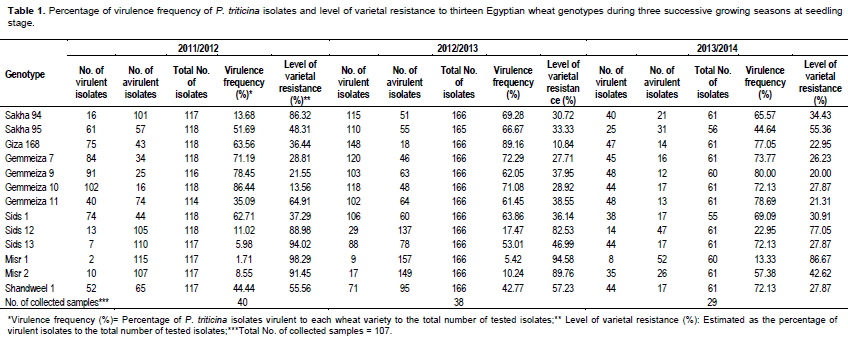
A total of 107 leaf rust samples were collected during three successive growing seasons. Forty samples were collected in 2010/2011, thirty eight samples in 2011/2012 and twenty nine samples in 2012/2013 (Table 1) from the wheat commercial fields and the trap nurseries grown in different locations of Egypt. These locations were Beheira (31 samples), Dakahlia (12), Gharbiya (10), Minufiya (12), Sharqiya (20), Domiata (3), Qalyubia (5) and Bani Swif (14). Sample (2 to 4 infected leaves) was kept at room temperature (18 to 24°C) overnight to be dried off then kept in glycine envelopes (8 × 15 cm) and stored with deiscator in the refrigerator at 2 to 5°C. The infected specimens were transferred through inoculation to the highly susceptible variety; Thatcher for isolation and purification.
The method of inoculation was carried out as described by Stakman et al. (1962), in which wheat seedling leaves (7 days old) were rubbed gently between moisted fingers with tap water, sprayed with water in the incubation chambers.Then inoculated by shaking or brushing rusted materials from collected samples over the plant leaves and sprayed gently again with water in order to form initial film of free water on the plants which is essential for spore germination and establishment of infection. The inoculated seedlings were incubated in humid chambers for 24 h to allow spore germination and cause infection. The inoculated plants were moved onto the benches in the greenhouse with daily temperature 10 to 25°C. After approximately 12 to 15 days, three single pustules were isolated separately from each sample for rust reproduction on seedlings of the highly susceptible wheat variety Thacher to obtain enough urediniospores for inoculation.
Race designations were assigned as described by Long and Kolmer (1989) including 16 differential lines, each with a single leaf rust resistance gene from one of four subsets, first (Lr 1, Lr 2a, Lr 2c and Lr 3a), second (Lr 9, Lr 16, Lr 24 and Lr 26), third (Lr 3ka, Lr 11, Lr 17 and Lr 30), fourth (Lr 10, Lr 18, Lr 21 and Lr 2b). Supplemental near-isogenic lines containing Lr 14b, Lr 15, Lr 36 and Lr 42 were also inoculated at the same time as additional differential sub-set from Egypt suggested by McVey et al. (2004).
At the same time, seeds of the tested wheat genotypes i.e. Sakha 94, Sakha 95, Giza 168, Gemmeiza 7, Gemmeiza 9, Gemmeiza 10, Gemmeiza 11, Sids 1, Sids 12, Sids 13, Misr 1, Misr 2 and Shandweel 1 were sown in 6 cm square plastic pots. Five seeds of each tested wheat material were sown in each corner in a clockwise order. Seven days old seedlings of the tested wheat materials, when first leaf full emerged, were inoculated with single pustule isolates of P. triticina which was previously propagated. Inoculation was carried out by shaking with propagated urediniospores over the seedling leaves of the tested materials. The inoculated seedlings were transferred onto the greenhouse benches(18 to 20°C and 100% RH).
Seedling disease assessment
Infection type (IT) data for each tested wheat genotype were recorded 12 days after inoculation using standard infection type scoring scale 0 to 4 (Stakman et al., 1962). Genotypes which showed low infection types (scores = 0, 0; 1, and 2) were considered as resistant or low infection types (LITs). While, those with scores = 3 and 4 were susceptible or high infection types (HITs) (Stakman et al., 1962).
Virulence frequency
Virulence frequency was calculated as percentage of virulent isolates to the total number of the tested isolates.
Postulation of leaf rust resistance genes
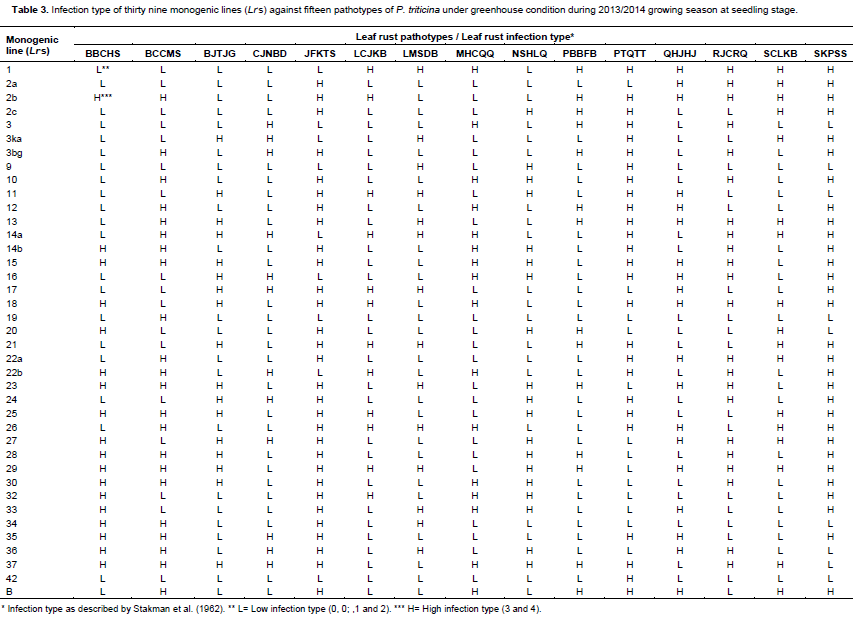
Thirteen Egyptian wheat genotypesand 39 monogenic lines carrying single gene for leaf rust resistance were tested in seedling stage using 15pathotypes of leaf rust i.e. BBCHS, BCCMS, BJTJG, CJNBD, JFKTS, LCJKB, LMSDB, MHCQQ, NSHLQ, PBBFB, PTQTT, QHJHJ, RJCRQ, SCLKB and SKPSS. All plant materials were grown in plastic pots.Seedlings were inoculated by urediniospores of the selcted identified pathotypes during 2013/2014 growing season. The inoculated seedlings were incubated also as previously mentioned and transferred onto benches of the greenhouse. Both inoculation and incubation procedures were done according to the method described by Tervet and Cassel (1951). Leaf rust disease infection type (IT) datawere recorded for the wheat testedmaterials as mentioned before using disease assessmentapproaches previously suggested by Stakman et al. (1962).
Field studies
Field work was carried out at three locations that are, Itay El-Baroud and Nubariya Agricultural Research Stations as well as the Farm of the Faculty of Agriculture, Minufiya University, Shibin El-Kom during 2011/2012, 2012/2013 and 2013/2014. Each of the previously mentioned thirteen wheat genotypes were planted in aplot (3 m X 3.5 m= 10.5 m2) consisting of seven rows, and each row was 3 m long and 40 cm apart. Whole experimental plots were surrounded by spreader plants of one meter width sown with a mixture of the highly susceptible wheat genotypes to leaf rust that is, Thatcher and Morocco. The spreader plants were artificially inoculated with a mixture of urediniospores and talcum powder(1:20 v/v) of the most prevalent and aggressive fifteen leaf rust physiologic pathotypes previously mentioned. The methods of inoculation were described by Tervet and Cassel (1951).
Adult plant disease assessment
Percentage of final rust severity (FRS %)
Percentage of leaf rust severity was recorded for the thirteen wheat genotypes using the modified Cobb’s scale described by Peterson et al. (1948). Rust severity data were scored after the appearance of the first symptoms (appear of the first pustule on any of the tested wheat genotypes) at seven days intervals. The percentage final rust severity (FRS %) was assessed according to Das et al. (1993), as the percentage disease severity for each tested genotypes when the highly susceptible check genotype(Sids 1) was severely rusted and the disease rate reached the highest level of leaf rust severity.
Area under disease progress curve (AUDPC)
AUDPC was also calculated for each genotype under field conditions. The values of AUDPC were calculated by using the following equation of Pandey et al. (1989).
AUDPC = D [1/2 (Y1 + Yk) + (Y2 + Y3+ - - - - - + Yk-1)]
Where: D = Days between two consecutive recording (time intervals)
Y1 + Yk = Sum of the first and last scores.
Y2 + Y3 + - - - - - + Yk-1 = Sum of all in between disease scores.
Statistical analysis
Least significant difference (LSD at 5 %) test was performed to determine the significant differences between means according to Steel and Torrie (1980).
Evaluation of the tested wheat genotype sat seedling stage under greenhouse condition
Percentage frequency of virulence to the tested wheat genotypes
Wheat genotypes Misr 1, Sids 13, Misr 2, Sids 12 and Sakha 94 showed low virulence frequencies that is, 1.71, 5.98, 8.55, 11.02 and 13.68%, respectively. While, Gemmeiza 7, Gemmeiza 9 and Gemmeiza 10 showed the highest percentage of virulence frequencies that is 71.19, 78.45 and 86.44%, respectively. In comparison, the other tested genotypes were intermediate (Table 1) in 2011/2012 growing season.
During the second growing season 2012/2013, the wheat genotypes Misr 1, Misr 2 and Sids 12 showed the lowest percentage ofvirulence frequencies that is, 5.42, 10.24 and 17.47%, respectively. While, Gemmeiza 10, Gemmeiza 7 and Giza 168 showed the highest percentage of virulence frequency that is, 71.08, 72.29 and 89.16, respectively. The other tested genotypes were extremely low to intermediate (Table 1).
In 2013/2014 growing season, both ofMisr 1 and Sids 12 wheat genotypes showed low percentage of virulence frequency i.e. 13.33 and 22.95, respectively. While, Gemmeiza 9 and Gemmeiza 12 (each with 80.00% frequency) as well as Gemmeiza 11 (78.69%) and Giza 168 (77.05) showed the highest virulence frequencies. Comparatively, the other tested wheat genotypes showed low to intermediate percentage of virulence frequencies (Table 1).
Level of varietal resistance to Puccinia triticina isolates
Data in Table 1 also revealed that five wheat genotypes Sakha 94 (86.32%), Sids 12 (88.98%), Misr 2 (91.45%), Sids 13(94.02%) and Misr 1 (98.29%) gave the highest levels of resistance (more than 80%) against the tested isolates during 2011/2012 growing season. While, Sids 12 (82.53%), Misr 2 (89.76%), Misr 1 (94.58%) and Gemmeiza 12 (100%) showed high level of resistance against 166 tested isolates of P. triticina collected in 2012/2013 growing season. In 2013/2014 growing season, only genotype Misr 1 gave the highest resistant reaction and exhibited 86.67%.
Postulation of leaf rust resistance genes (Lr,s)
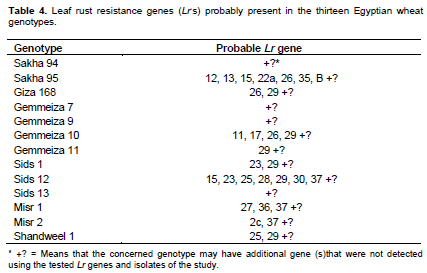
Low and high infection types displayed by the thirteen tested wheat genotypes (Table 1) compared with the infection types of 39 known Lr genes (Table 2) against fifteen identified tested pathotypes of P. triticina under greenhouse condition. Data obtained in Tables 3 and 4 were summarized in Table 3 in which different genes could be postulated as follows: The wheat genotype Sakha 95 probably possessed Lr 12, Lr 13, Lr 15, Lr 22a, Lr 26, Lr 35, LrB. Wheat genotype Sids 12 may have seven genes that is, Lr 15, Lr 23, Lr 25, Lr 28, Lr 29, Lr30 and Lr 37. Moreover, the wheat genotype Gemmeiza 10 probably has four genes that is, Lr 11, Lr 17, Lr26 and Lr 29. The wheat genotypeMisr 1 may have three genes that is, Lr 27, Lr36 and Lr 37. While, the wheat genotypes Giza 168, Sids 1, Misr 2 and Shandweel 1 may have two genes. These genes were Lr26 and Lr29 in Giza 168, Lr23 and Lr29 in Sids 1 and Lr 2c and Lr 29 in Misr 2. While, wheat genotype Gemmeiza 11 may hasone gene that is, Lr 29. The wheat genotypes Sakha 94, Gemmeiza 7, Gemmeiza 9 and Sids 13 did not have any of the tested Lr genes under study using the fifteen tested pathotypes of P. triticina but it may carry some additional genes. Moreover, all of the tested wheat genotypes may be carries some additional gene (s).
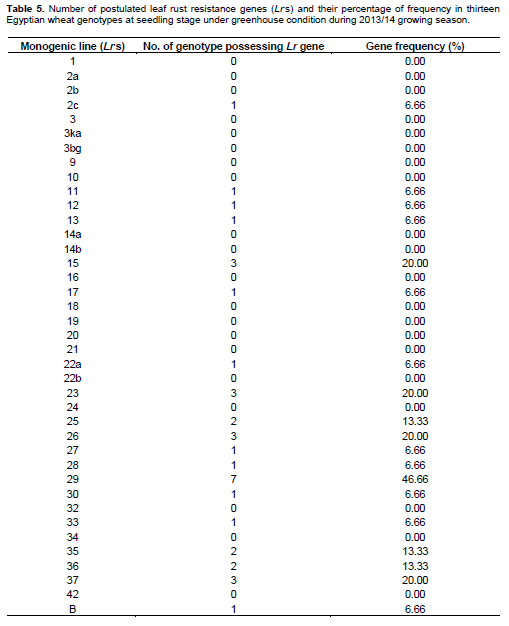
Data in Table 4 indicated that only 19 resistance genes that are, Lr 2c, Lr 11, Lr 12, Lr 13, Lr 15, Lr 17, Lr 22a, Lr 23, Lr 25, Lr 26, Lr 27, Lr 28, Lr 29, Lr 30, Lr33, Lr 35, Lr 36, Lr 37 and Lr B out of the total 39 tested genes proved to be the most common genes that were probably detected and showed percentage of gene frequency from 6.66 to 46.66% of the tested wheat genotypes. Moreover, Lr 29 was the most frequent gene that was probably present in seven genotypes (represented 46.66% frequency), followed by Lr15, Lr 23, Lr 26 and Lr 37 (each with 20.00% frequency). While, the 20 leaf remaining rust resistance genes Lr 1, Lr 2a, Lr 2b, Lr 3, Lr 3ka, Lr 3bg, Lr 9, Lr 10, Lr 14a, Lr 14b, Lr 16, Lr 18, Lr 19, Lr 20, Lr 21, Lr 22b, Lr 24, Lr 32, Lr 34 and Lr 42 were not detected or postulated in any of the tested wheat genotypes.
Evaluation of the tested wheat genotypes under field conditions
Percentage of final rust severity (FRS %)
Data in Table 5 showed that there are significant differences in FRS means among the tested wheat genotypes. Meanwhile, there are no significant differences in means of FRS of environments as well as the interaction between genotypes and environments.
Data in Table 6 showed that percentage final rust severity of the tested wheat genotypes compared with the check genotype Sids 1 at adult stage under field conditions during the three tested growing seasons. In 2011/2012 growing season, the wheat genotypes Shandweel 1 (2.00%), Misr 1 (3.00%), Sakha 94 and Sids 13 (each with 4.33%), Giza 168 (5.00%), Misr 2 (6.67%), Sids 12 (8.33%), Sakha 95 (10.00%), Gemmeiza 9 and Gemmeiza 10 (each with 13.33%) were showed the lowest values of final rust severity (did not exceed up to 20%). While, Gemmeiza 11 (26.67%), Gemmeiza 7 (30.00%) and Sids 1 (76.67%) showed higher percentage values of FRS at Nubariya location.
Moreover, the wheat genotype Shandweel 1 showed complete resistance and no visible infection occurred. Also, the wheat genotypes Sids 12, Misr 1, Sids 13, Misr2, Sakha 94, Gemmeiza 10, Giza 168, Gemmeiza 9 and Sakha 95 showed low percentage levels of FRS that is, 3.00, 3.00%, 3.67, 4.33, 4.78, 6.00, 8.33, 10.00 and 13.33%, respectively at Italy El-Baroud location during this season. At Shibin El-Kom location, the wheat genotypes Sids 12, Misr 1, Misr 2 and Shandweel 1 were very resistant and showed no visible infection. Also, the wheat genotypes Sids 13 (2.00%), Sakha 94 (3.67%), Sakha 95, Gemmeiza 9 and Gemmeiza 10 (each with 6.67%), Giza 168 (8.33%) and Gemmeiza11(11.67%) showed low percentage levels of FRS. While, the wheat genotypes Gemmeiza 7 (26.67%) and Sids 1 (73.33%) showed high percentage levels of FRS.
Data of percentage final leaf rust severity in the second season (2012/2013) showed that the wheat genotypes Shandweel 1, Sids 13, Sids 12, Misr 1, Misr 2, Sakha 94, Gemmeiza 11, Gemmeiza 9, Giza 168, Gemmeiza 10 and Sakha 95 showed the lowest percentage values of FRS (less than 20.00%). While the wheat genotypes Gemmeiza 7 and Sids 1 showed the highest percentage values of FRS during this season at the three locations that is, Nubariya, Itay El-Baroud and
Shibin El-Kom.
In 2013/2014 growing season, the wheat genotypes Misr 1, Misr 2 and Shandweel 1 showed complete resistance and no visible infection occurred. Also, the wheat genotypes Sids 12 (2.00%), Gemmeiza 12 (4.33%), Sids 13 (6.00%), Sakha 94, Giza 168 and Gemmeiza 9 (each with 6.67%) showed the lowest percentage values of FRS at Nubariya location. While, the wheat genotypes Sakha 95 (23.33%), Gemmeiza 11 (36.67%), Gemmeiza 10 (43.33%), Gemmeiza 7 and Sids 1 (each with 76.67%) showed the highest percentage values of FRS at Nubariya location. The wheat genotypes Shandweel 1, Misr 1, Sids 12, Sids 13, Misr 2, Sakha 94, Gemmeiza 9, Sakha 95, Giza 168 and Gemmeiza 10 showed the lowest percentage values of FRS at the other two tested locations (Itay El-Baroud and Shibin El-Kom) during this season. While, the wheat genotypes Gemmeiza 11, Gemmeiza 9 and Sids 1 showed the highest percentage values of FRS at the two locations Itay El-Baroud and Shibin El-Kom.
Data of mean percentage values of FRS during three seasons and at three locations indicated that the wheat genotypes Shandweel 1 (0.22%), Misr 1 (2.33%), Sids 12 (3.22%), Sids 13 and Misr 2 (each with 3.41%), Sakha 94 (5.36%), Gemmeiza 9 (7.11%) and Giza 168 (8.26%) showed the highest resistance response with the lowest mean percentage values of FRS, followed by Sakha 95 (12.41%), Gemmeiza 10 (14.37%) and Gemmeiza 11 (18.74%). While, the wheat genotypes Gemmeiza 7 (41.48%) and Sids 1 (72.96%) were susceptible and showed the highest mean percentage values of FRS.
The wheat genotype Shandweel 1 showed resistance infection type. While, the wheat genotypes Sids 12, Sids 13, Misr 1 and Misr 2 showed moderately resistance infection type and the reminder genotypes showed susceptible infection type at the three tested locations during the three seasons.
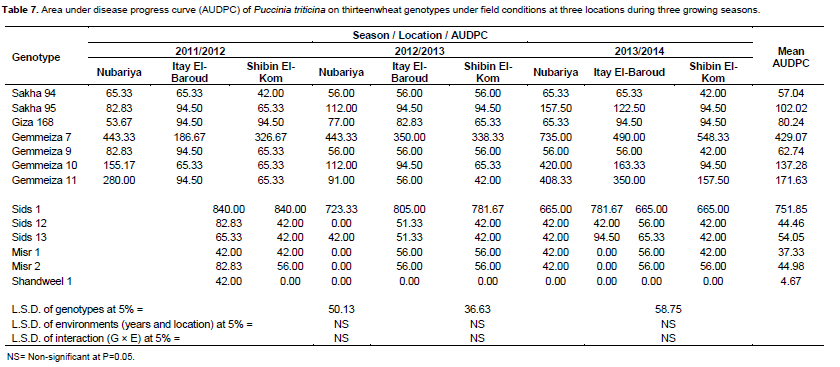
Area under disease progress curve (AUDPC)
Data in Table 7 estimated the mean values of AUDPC over the three years and three locations.There are significant differences in AUDPC means among the tested wheat genotype sat P = 0.05. Meanwhile, no significant differences in means of AUDPC of environments (years and locations) as well as the interaction between genotypes and environments. Moreover, the tested wheat genotypes can be classified into three groups. The first group race-specific resistant includes wheat genotypes of Shandweel 1 (4.67), Misr 1 (37.33), Sids 12 (44.46), Misr 2 (44.98) and Sids 13 (54.05). These genotypes displayed the highest levels of adult plant resistance and showed the lowest values of AUDPC. Also, they showed resistant and moderately resistant infection types.
The second group included the wheat genotypes Sakha 94, Gemmeiza 9, Giza 168, Sakha 95, Gemmeiza 10 and Gemmeiza 11, which they displayed acceptable levels of adult plant resistance. However, results showed low values of AUDPC for these genotypes that is, 57.04, 62.74, 80.24, 102.02, 137.28 and 171.63, respectively. Also, this group showed susceptible infection type. Therefore, they have been classified as slow-rusting or partially resistant genotypes. Whereas, the third group included Gemmeiza 7 (429.07) and Sids 1 (751.85), which they showed the highest values of AUDPC to leaf rust infection, Also, displayed the lowest levels of adult plant resistance and these genotype sclassified as fast-rusting genotypes.
One hundred eighteen, one hundred sixty-six and sixty-one single isolates of leaf rust were evaluated for virulence during three growing seasons that is, 2011/2012, 2012/2013 and 2013/2014, respectively against the thirteen tested wheat genotypes. During 2011/12 growing season low virulence frequencies of leaf rust have been found on Misr 1, Sids 13, Misr 2, Sids 12 and Sakha 94 wheat genotypes.
In 2012/2013 growing season low virulence frequencies of leaf rust have been found on the wheat genotypes Misr 1, Misr 2 and Sids 12. While, in 2013/2014 growing season the wheat genotype Misr 1 showed low virulence frequency. Occurrence of virulence was stable on the wheat genotype Misr 1 during the three seasons of this study, while, virulence frequencies of Misr 2 and Sids 12 which were almost stable during the two growing seasons 2011/2012 and 2012/2013. Moreover, little changes in varietal resistance against the tested isolates in the growing seasons of study were recorded; this is might be due to the differences in the genetic make-up of the tested wheat genotypes (Negm et al., 2013).
Based on results of gene postulation, five wheat genotypes that is, Sakha 94, Gemmeiza 7, Gemmeiza 9, Gemmeiza 11 and Sids 13 of the thirteen tested genotypes appeared to have either none or only one known Lr gene. While, all of the tested wheat genotypes appear to have one or more unidentified leaf rust resistance genes.The wheat genotypes Sakha 95 and genotypes Lr 12, Lr 13, Lr 15, Lr 22a, Lr 26, Lr 35 and Lr B in the wheat genotype Sakha 95.Lr 15, Lr 23, Lr25, Lr28, Lr29, Lr 30 and Lr37 were detected in the wheat genotype Sids 12. Similar results were recorded by McVey (1989), Youssef (2006), Boulot (2007), Shynbolat and Aralbek (2010) and Abdelbacki et al. (2013).
Changing in race pathogenesis led the breeders to involving alternative forms of resistance that would be more durable such as slow rusting or partial resistance (Broers, 1989; Singh et al., 2000a, b). It has been demonstrated that durable rust resistance is more likely to be of adult plant type rather than of seedling infection type and is not linked with the genes producing hypersensitive reaction (McIntosh, 1992; Bariana et al., 2001). Durable rust resistance is a mechanism conferring resistance to a genotype for long period of time during its widespread cultivation in a favorable environment for a disease (Johnson, 1978, 1988). This type of resistance is mainly associated with the minorgenes, which are also known as slow rusting genes. The concept of slow rusting in wheat was previously recommended by Caldwell (1968).
Many researchers have emphasized the need to identify and exploit durable resistance. Johnson and Law (1975) defined durable resistance as a resistance source that remained effective after widespread deployment over a considerable period. A general concept of a durable resistance source for cereal rusts is that it is polygenic, likely to express at adult plant stage, non-race-specific and produce non-hypersensitive response to infection.
The tested wheat genotypes were evaluated at adult plant stage at three locations that is, Itay El-Baroud and Nubariya Agricultural Research Stations as well as the Farm of the Faculty of Agriculture, Minufiya University, Shibin El-Kom during three successive growing seasons that is, 2011/2012, 2012/2013 and 2013/2014. Percentage of FRS was recorded for each of the tested genotypes. However, the wheat genotype Shandweel 1 was highly resistant and showed lowest percentage level of FRS and resistance infection type. Moreover, the wheat genotypes Sids 12, Sids 13, Misr 1 and Misr 2 were also highly resistant and showed low percentage level of FRS and moderately resistance infection type. Resistance to leaf rust in these wheat genotypes mainly due to race-specific resistance gene (s), which were showed infection type resistance (R) to moderately resistance (MR). German and Kolmer (1992) found that individual major genes for adult plant resistance to leaf rust can show enhancement effectiveness when combined in wheat genotypes. However, the wheat genotypes Sakha 94, Gemmeiza 9, Giza 168, Sakha 95, Gemmeiza 10 and Gemmeiza 11 showed low percentage levels of FRS (did not exceeded up to 20%) also, these genotypes showed susceptible infection type (S). These genotypes displayed an adequate level of partial resistance to leaf rust infection, in comparison with the two genotypes Gemmeiza 7 and Sids 1 (fast rusting or highly susceptible genotypes). These results are previously supported by Bassiony (1979) and Nazim et al. (1983, 1990).
AUDPC is a good indicator of adult plant resistance under field condition (Wang et al., 2005). They added that genotypes which had low AUDPC and terminal severity values may have good level of adult plant resistance (Wang et al., 2005). Furthermore, AUDPC, in particular, is the result of all factors that influence disease development such as differences in environmental conditions, varieties and population of the pathogen (Pandey et al., 1989; Lal Ahmed et al., 2004; Singh et al., 2005; Boulot, 2007).
According to the obtained results and depending on the mean values of AUDPC, the wheat genotypes Sakha 94, Gemmeiza 9, Giza 168, Sakha 95, Gemmeiza 10 and Gemmeiza 11 showed lowest values of AUDPC. These results indicated that such genotypeshave good level of adult plant resistance under field conditions in three locations through three growing seasons to leaf rust and can be used as resistance sources. Therefore, this group of genotypes characterized as partially or slow rusting resistant group. While, the two wheat genotypes Gemmeiza 7 and Sids 1 showed the highest AUDPC values. These genotypes classified as the highly susceptible or fast rusting genotypes group, similarly to those reported by Nazim et al. (1990); Denissen (1993) and Singh et al. (2005).
According to the data obtained, nineteen known leaf rust resistance genes and one or more unknown genes were postulated in the thirteen tested wheat genotypes. These findings should be useful in the Egyptian wheat breeding programs in order to improve leaf rust resistance. Also, the present study revealed that most of the tested wheat genotypes were having enough resistance, ranging from complete resistance to partial resistance. Also, most of the tested wheat genotypes exhibited better performance under high disease pressure shown by susceptible varieties. Further studies for testing stability of the tested wheat genotypes over growing seasons and locations against leaf rust along with other desirable characters must be studied.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdelbacki A, Soliman N, Najeeb M,Omara R (2013). Postulation and identification of resistance genes against Puccinia triticina in new wheat cultivars in Egypt using molecular markers. Int. J. Chem. Environ. Biol. Sci. 1:104-109.
|
|
|
|
Bariana HS, Hayden MJ, Ahmad NU, Bell JA, Sharp PJ, McIntosh RA (2001). Mapping of durable adult plant and seedling resistance to stripe and stem rust disease in wheat. Aust. J. Agric. Res. 52:1247-1255.
Crossref
|
|
|
|
|
Bassiony AA (1979). Comparative study on the nature of resistance in some wheat varieties to stem and leaf rusts. Ph.D. Thesis, Fac. Agric., Tanta Univ. (Kafr El-Sheikh Branch).
|
|
|
|
|
Boulot OA (2007). Durable resistance for leaf rust in twelve Egyptian wheat varieties. Egypt. J. Appl. Sci. 22:40-60.
|
|
|
|
|
Broers LHM (1989). Partial resistance to wheat leaf rust in 18 spring wheat cultivars.Euphytica 44:247-258.
Crossref
|
|
|
|
|
Browder LE, Eversmeyer MG (1980). Sorting of Puccinia recondita: Triticum infection-type data sets toward the gene-for-gene model. Phytopathology 70:666-670.
Crossref
|
|
|
|
|
Caldwell RM (1968). Breeding for general and/or specific plant disease resistance. In: Proc. 3rd Int. Wheat Genetics Symp., Canberra, Australia. pp. 263-272.
|
|
|
|
|
Caldwell RM, Roperts JJ, Eyal Z (1970). General (slow rusting) resistance to Puccinia recondita f. sp. tritici in winter and spring wheats. Phytopathology 60:1287.
|
|
|
|
|
Das MK, Rajaram S, Ktonstad WK, Mundt CC, Singh RP (1993). Association and genetics of three components of slow rusting in leaf rust of wheat. Euphytica 68:99-109.
Crossref
|
|
|
|
|
Denissen CJM (1993). Components of adult plant resistance to leaf rust in wheat. Euphytica 70:134-140.
Crossref
|
|
|
|
|
German SE, Kolmer JA (1992). Effect of gene Lr 34 in the enhancement of resistance to leaf rust of wheat. Theor. Appl. Genet. 84:97-105.
Crossref
|
|
|
|
|
German SE, Barcellos A, Chaves M, Kohli M, Campos P, Viedma L (2007). The situation of common wheat rusts in the Southern Cone of America and perspectives for control. Aust. J. Agric. Res. 58:620-630.
Crossref
|
|
|
|
|
Herrera-Foessel SA, Lagudah ES, Huerta-Espino J, Hayden MJ, Bariana HS, Singh D, Singh RP (2011). New slow-rusting leaf rust and stripe rust resistance genes Lr 67 and Yr 46 in wheat are pleiotropic or closely linked. Theor. Appl. Genet. 122:239-249.
Crossref
|
|
|
|
|
Herrera-Foessel SA, Singh RP, Huerta-Espino J, Rosewarne GM, Periyannan SK, Viccars L, Calvo-Salazar V, Lan C, Lagudah ES (2012). Lr 68- a new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 124:1475-1486.
Crossref
|
|
|
|
|
Ingala L, López M, Darino M, Pergolesi MF, Diéguez MJ, Sacco F (2012). Genetic analysis of leaf rust resistance genes and associated markers in the durable resistant wheat cultivar Sinvalocho MA. Theor. Appl. Genet. 124:1305-13014.
Crossref
|
|
|
|
|
Johnson R (1978). Practical breeding for durable resistance to rust diseases in self-pollinating cereals. Euphytica 27:529-540
Crossref
|
|
|
|
|
Johnson R (1988). Durable resistance to yellow (stripe) rust in wheat and its implications in plant breeding. In: Breeding Strategies for Resistance to the Rusts of Wheat, Simmonds, N.W. and S. Rajaram (eds.), CIMMYT, Mexico. pp. 63-75.
|
|
|
|
|
Johnson R, Law CN (1975). Genetic control of durable resistance to yellow rust (Puccinia striiformis) in the wheat cultivar Hybride de Bersee. Ann. Appl. Biol. 81:385-391.
Crossref
|
|
|
|
|
Lal Ahamed M, Singh SS, Sharma JB, Ram RB (2004). Evaluation of inheritance to leaf rust in wheat using area under disease progress curve. Hereditas 141:323-327.
Crossref
|
|
|
|
|
Long DL, Kolmer JA (1989). A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology. 79:525-529.
Crossref
|
|
|
|
|
McIntosh RA (1992). Close genetic linkage of genes conferring adult-plant resistance to leaf rust and stripe rust in wheat. Plant Pathol. 41:523-527.
Crossref
|
|
|
|
|
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris C, Somers DJ, Appels R, Devos KM (2012). Catalogue of gene symbols for Wheat.
|
|
|
|
|
McVey DV (1989). Verification of infection type data for identification of genes for resistance to leaf rust in some hard red spring wheats. Crop Sci. 29:304-307.
Crossref
|
|
|
|
|
McVey DV, Nazim M, Leonard KJ, Long DL (2004). Patterns of virulence diversity in Puccinia triticinaon wheat in Egypt and the United States in 1998-2000. Plant Dis. 88:271-279.
Crossref
|
|
|
|
|
Nazim M, El-Shanawani MZ, El-Shennawy Z, Boulot, OA (1990). Partial resistance to leaf rust in some Egyptian wheat varieties. Proc. 6th Congress of the Egyptian Phytopathological Society, Part 1. pp. 77- 97.
|
|
|
|
|
Nazim M, El-Shehidi AA, Abdou YA, El-Daoudi YH (1983). Yield loss caused by leaf rust on four wheat cultivars under epiphytotic levels. Proc. 5th Confer. Microbiol., Cairo, Egypt. pp. 17-27.
|
|
|
|
|
Negm SS, Boulot OA, Hermas GA (2013). Virulence dynamics and diversity in wheat leaf rust (Puccinia triticina) populations in Egypt during 2009/2010 and 2010/2011 growing seasons. Egypt. J. Appl. Sci. 28:183-212.
|
|
|
|
|
Pandey HN, Menon TCM, Rao MV (1989). A simple formula for calculating area under disease progress curve. AGRIS 8:38-39.
|
|
|
|
|
Peterson RF, Campbell AB,Hannah AE (1948). A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 26:496-500.
Crossref
|
|
|
|
|
Shynbolat R, Aralbek R (2010). Evaluation of leaf rust resistance genes in durum wheat varieties in Kazakhstan. Asian Aust. J. Plant Sci. Boitechnol. 4:77-80.
|
|
|
|
|
Singh RP, Huerta-Espino J, Rajaram S (2000a). Achieving near immunity to leaf and stripe rusts in wheat combining slow rust resistance genes. Acta Phytopathalogica et Entomolgica Hungarica 35:133-139.
|
|
|
|
|
Singh RP, Huerta-Espino J, William HM (2005). Genetics and breeding for durable resistance to leaf and strip rusts in wheat. Turk. J. Agric. 29:121-127.
|
|
|
|
|
Singh RP, Nelson JC,Sorrells ME (2000b). Mapping Yr28 and other genes for resistance to stripe rust in wheat. Crop Sci. 40:1148-1155.
Crossref
|
|
|
|
|
Singh RP, Payne TS, Rajaram S (1991). Characterization of variability and relationships among the components of partial resistance to leaf rust in CIMMYT bread wheats. Theor. Appl. Genet. 82: 674-680.
Crossref
|
|
|
|
|
Stakman EC, Stewart DM, Loegering WQ (1962). Identification of physiologic races of Puccinia graminis var. tritici. USDA Agric. Res. Service E 617:1-53.
|
|
|
|
|
Statler GD (1984). Probable genes for leaf rust resistance in several hard red spring wheats. Crop Sci. 14:883-886.
Crossref
|
|
|
|
|
Steel RGD, Torrie JH (1980). Principles and Procedures of Statistics. McGraw-Hill Book Company, New York. pp. 187-188.
|
|
|
|
|
Tervet I, Cassell RC (1951). The use of cyclone separation in race identification of cereal rusts. Phytopathology 41:282-285.
|
|
|
|
|
Wang ZL, Li LH, He ZH, Duan X, Zhou YL, Chen XM, Lillemo M, Singh RP, Wang H, Xia XC (2005). Seedling and adult plant resistance to powdery mildew in Chinese bread wheat cultivars and lines. Plant Dis. 89:457-463.
Crossref
|
|
|
|
|
Winzeler M, Mesterhazy A, Park RF, Bartos P, Csosz M, Goyeau H, Ittu M, Jones E, Loschenberger F, Manninger K, Pasquini M, Richter K, rubiales D, Schachermayr G, Strzembicka A, Trottet M, Unger O, Vida G, Walther U (2000). Resistance of European winter wheat germplasm to leaf rust. Agronomie 20:783-792.
Crossref
|
|
|
|
|
Youssef IAM (2006). Physiological specialization in Puccinia triticina and genes conditioning resistance to wheat leaf rust disease in Egypt. J. Agric. Sci. 31:2057-2071.
|
|