ABSTRACT
The aim of this study was to verify the influence of temperature, light, substrate, sowing methods, and salt and water stress on carpetgrass (Axonopus affinis, in the Poaceae family) seed germination. All four trials were performed in a germination chamber under controlled conditions of temperature, moisture and photoperiod. Experimental designs were entirely randomized with 100 seeds per plot. For the study of temperature and light (Experiment 1), treatments were arranged in a 6 x 2 factorial scheme, with six temperature regimes (constant at 20, 25, 30, and 35°C, and alternate at 20 to 30 and 20 to 25°C) combined with two light conditions (light: 8 h of light and 16 h of dark, and dark: 24 h without light) and four replications. For the study of substrate and sowing methods (Experiment 2), there were four treatments (on paper, between paper, on sand, and in sand) with five replications. For the study of salt stress (Experiment 3), there were five treatments, composed of NaCl concentrations (0, 25, 50, 75 and 100 mM), with four replications. For the study of water stress, treatments were arranged in a 3 x 2 factorial scheme (Experiment 4), with three substrate water contents (50, 75 and 100% water retention capacity) and two sowing methods (on sand and in sand), and four replications. Germination percentage and rate were evaluated. It was concluded that seed germination was more effective at the alternate temperatures of 20 to 30 and 20 to 35°C, under light, sown on sand, on a paper substrate or between papers. The NaCl concentrations did not affect germination percentage; however, germination was slower as the NaCl concentration increased. Faster and higher germination occurred when seeds were sown on sand at 100% of its water retention capacity.
Key words: Axonopus affinis, light, salt stress, substrate, temperature, water stress.
Lawns may usually bring many benefits to the surrounding environment. Besides the aesthetic effects, erosions caused by either water or wind are prevented, and problems related to mud and dust are minimized, among others. The increasing demand for quality lawns by the consumer market is the main factor that boots grass producing areas, especially those close to great consumer poles (Godoy et al., 2012).
The grass species Axonopus affinis Chase, in the Poaceae family, is commonly known as carpetgrass. It is still little cultivated in Brazil for ornamental purposes; however, it shows expansion potential for its decorative effect, hardiness and resistance to trampling and cold. Travi et al. (2014) reported that A. affinis is one of the most important grass species in native pastures of Southern Brazil, besides being aggressive and resistant to trampling and cold.
In Brazil, most lawns are still implemented from vegetatively propagated plants; however, the use of seeds is increasing according to the global trend. Batista et al. (2015) highlighted that the lawn formation from seeds, which is a common practice in the United States and Europe, is currently expanding also in Brazil.
However, seed germination depends on several endogenous and exogenous factors, so water, temperature, oxygen, and light are the most important ones (Baskin and Baskin, 1998) among others, such as substrate and salinity (Batista et al., 2015). The influence of these factors varies according to the species; so, knowledge on specific requirements of each plant contributes to guiding research on seed germination and field requirements.
Water is one of the most important factors affecting germination, as it activates the seed metabolism besides being both directly and indirectly involved in all the remaining germination stages (Marco Filho, 2005).
Temperature affects germination percentage and rate, seed water absorption and, also, biochemical reactions that determine all the germination process (Carvalho and Nakagawa, 2000). Furthermore, the substrate used in germination trials greatly influences the germination process due to some factors that may vary according to the material, such as structure, aeration, water retention capacity and pathogen infestation (Popinigis, 1977).
Some species are tolerant to saline environments, what becomes important for their cultivation in areas that are naturally saline or usage of residual water for irrigation. However, salinity may interfere with seed germination and may even inhibit it because of the osmotic effect, which may cause either the physiological drought or seedling toxicity, which is a result of the ion concentration in the cell protoplasm (Tobe et al., 2000).
In this sense, with the aim to endorse and clarify aspects related to seed germination of A. affinis grass species, the objective of this work was to verify the influence of temperature, light, substrate, sowing methods, and saline and water stress on such process.
The experiments were conducted in the first half of 2015 in the Laboratory of Seed Analysis, Department of Crop Production at the College of Agricultural and Veterinary Sciences of the State University of São Paulo (FCAV/UNESP), located in the municipality of Jaboticabal, Brazil (21°15’22" S and 48°18’58" W, at 590 m altitude).
Seeds of A. affinis were bought from a commercial producer, already treated with 0.18% Mayran fungicide [700 g kg-1 Thiram + 0.06% Rovral (500 g L-1 Iprodione)]. Seed water content was determined applying the oven method, at 105 ± 3°C for 24 h, as described by Brasil (2009); it was 14.86%.
Temperature and light
The experimental design was entirely randomized and treatments were arranged in a 6 x 2 factorial scheme: six temperature conditions (constant at 20, 25, 30, and 35°C, and alternate at 20-30 and 20-35°C) and two light regimes (light: 8 h of light and 16 h of dark; and dark: 24 h without light), and four replications of 100 seeds each.
Experimental plots were composed of plastic boxes (11 x 11 x 3.5 cm) of “gerbox” type, which were placed in tied low-density polyethylene bags to avoid moisture loss. Both transparent (light treatment) and black (dark treatment) plastic boxes were used. Seeds were sown on two sheets of filter paper moistened with distilled water, whose mass applied was 2.5 times the dry paper mass (Brasil, 2009).
The experiment was conducted in BOD germinators set at different temperatures according to the proposed treatments.
Substrate and sowing methods
The experimental design was entirely randomized with four treatments (on paper, between paper, on sand and in sand) and five replications of 100 seeds each. Experimental plots were composed of plastic boxes (11 x 11 x 3.5 cm) of “gerbox” type, which were placed in tied low-density polyethylene bags to avoid moisture loss. Seeds were sown either on filter paper or between two sheets of filter paper moistened with distilled water, and mass applied was 2.5 times the dry paper mass (Brasil, 2009). For the sand treatments, there was 250 g washed sand per plot, which was sterilized at 200°C for 2 h. For the ‘in sand’ treatment, seeds were sown at 4 mm depth. The experiment was conducted in a BOD germinator at the alternate temperature of 20-35°C and photoperiod of 8 h of light and 16 h of dark.
Saline stress
The experimental design was entirely randomized with five treatments (five NaCl concentrations: 0, 25, 50, 75, and 100 mM) and four replications of 100 seeds each. The electrical conductivity (EC) of the solutions was, respectively, 2.16, 2.90, 5.80, 8.46, and 11.37 μS cm-1. For the 0 mM concentration, only distilled water was used. Experimental plots were composed of plastic boxes (11 x 11 x 3.5 cm) of “gerbox” type, which were placed in tied low-density polyethylene bags to avoid moisture loss. Seeds were sown between two sheets of filter paper, moistened with distilled water, and mass applied was 2.5 times the dry paper mass (Brasil, 2009).
The experiment was conducted in a BOD germinator at the alternate temperature of 20-35°C and photoperiod of 8 h of light and 16 h of dark.
Water stress and sowing methods
The experimental design was entirely randomized and treatments were arranged in a 3 x 2 factorial scheme: three substrate water contents (50, 75, and 100% substrate water retention capacity) and two sowing methods (on sand and in sand), with four replications of 100 seeds each. Experimental plots were composed of plastic boxes (11 x 11 x 3.5 cm) of “gerbox” type, which were placed in tied low-density polyethylene bags to avoid moisture loss. For the sand treatments, there was 250 g washed sand per plot, which was sterilized at 200°C for 2 h. For the ‘in sand’ treatment, seeds were sown at 4 mm depth.
The experiment was conducted in a BOD germinator at the alternate temperature of 20-35°C and photoperiod of 8 h light and 16 h dark. Sand maximum water retention capacity was calculated prior to definition of the water volume to be provided to the other treatments. For 100% water retention capacity, 218 mL water kg-1 sand was provided. Plastic boxes with sand were daily weighted so water replacement was performed whenever necessary to maintain the calculated water retention capacity for each treatment.
Assessment and statistical analysis
Seed germination was daily observed and noted for 28 days. Seeds were considered germinated when presented normal seedlings of at least 5 mm. Analyzed variables were germination percentage and rate, which were calculated according to Maguire (1962).
For statistical analysis, values of germination percentage were transformed into arcsine (x/100)1/2 for normalization. Data of both variables were submitted to variance analysis and means were compared by the Tukey test at 5% significance level. Polynomial regression analysis was also performed to evaluate variable behavior according to increasing salinity.
Temperature and light
The interaction between temperature and light was significant for both germination percentage and rate. Higher percentage and faster germination occurred under light, reaching 88.00 and 81.25% germination at the alternate temperatures of 20-30 and 20-35°C, respectively. Germination under dark was null or very low at the constant temperatures; however, there was 60.75% germination when seeds were submitted to the alternate temperature of 20-35°C (Table 1). It was also this temperature regime, in the dark, that promoted faster germination (Table 1). Therefore, in general, great germination percentages of A. affinis were observed at alternate temperatures (Table 1). Similar results with other Poaceae species were obtained by Carmona et al. (1998), who studied several grass species, and by Ever and Parsons (2009) and Batista et al. (2015) for Cynodon dactylon.
Seeds of some species do present higher germination percentage, germination rate, and vigor when subjected to alternate temperatures, which corresponds to natural fluctuations found in the environment (Copeland and McDonald, 1995). Although, germination percentage and rate were significantly higher when A. affinis seeds were sown under light, there was also germination in the dark, which indicates that the species was insensitive to light. Therefore, light did stimulate A. affinis seed germination, but it did not limit the process. Similar behavior of other grass species have also been reported by Opeña et al. (2014), Bastiani et al. (2015) and Batista et al. (2015), corroborating our results.
Different temperatures, combined with presence or absence of light, are also important environmental factors acting as germination triggering agents (Carvalho and Nakagawa, 2000). According to these results, the combination of alternate temperatures and light stimulated seed germination of A. affinis. Such combination also promoted germination of Melinis minutiflora grass species (Carmona and Martins, 2010), bermudagrass ‘Riviera’ (Batista et al., 2015), and other grass species native to the Brazilian cerrado (Carmona et al., 1998).
Substrate and sowing methods
For both germination percentage and rate, there were no significant differences among ‘on paper’, ‘between paper’ and ‘on sand’ treatments, which were superior to sowing in sand (Table 1). These results corroborate recommendations from the Rules for Seed Analysis (Brasil, 2009), which indicates that ideal substrates and sowing methods for germination trials are on either paper or sand, so we may extend it to seed sowing and also between paper.
Lower germination percentage observed for seed sowing in sand may be related to seed size. For instance, Evers and Parsons (2009), when studying bermudagrass seeds, recommended sowing on a lightly compacted surface because of seed minute size.
Saline stress
For germination percentage, there were no significant differences among treatments, resulting in the mean of 84.10%; germination was slower as the saline concentration increased, shown by the adjustment of the negative linear regression (Figure 1).
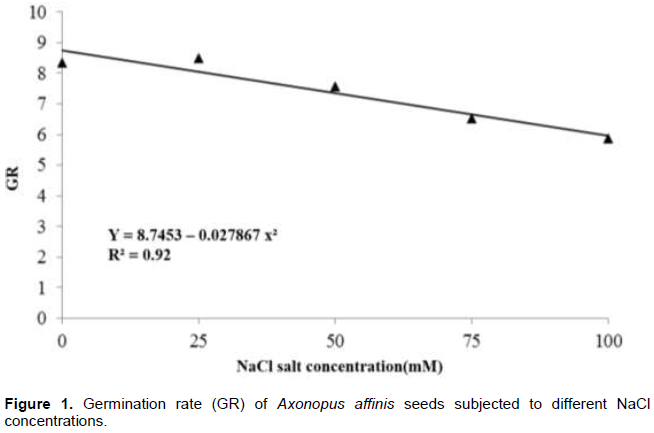
Even with the slower germination with salt increase, A. affinis was considered tolerant to salinity as germination percentage was high regardless the electrical conductivity, which ranged from 2.16 to 11.37 μS cm-1. Therefore, seeds of this species may be selected for lawn implementation in saline soils or when using brackish or residual water for irrigation.
Some plant species; however, do benefit from salinity during germination, which denotes greater adaptation capacity to such condition along their life cycle (Viana et al., 2004). Nevertheless, salt effects depend on factors such as species, cultivar, phenological stage, salt kind, intensity and duration of saline stress, crop management, irrigation, and soil and weather conditions (Tester and Davénport, 2003).
In comparison with other grasses, Myers and Couper (1989) reported that, after Puccinellia ciliata and Lolium perenne seed germination has started and, also, at seven days after sowing, there were germination losses from substrates irrigated with a saline solution. Also, Coan et al. (2008) observed that irrigation with saline waters of up to 6.0 dS m-1 electrical conductivity did not inhibit seed germination of Lolium perenne and bermudagrass ‘Mirage’. In addition, Batista et al. (2015), when studying the effects of NaCl concentrations on germination of bermudagrass ‘Riviera’ and ‘Princess 77’, noted that germination of both cultivars was more effective under NaCl absence.
Water stress and sowing methods
The interaction among substrate water contents and sowing methods was significant for germination percentage and rate. Both variables presented superior results when seeds were sown on sand maintained at 100% water retention capacity (Table 1).
The most favorable substrate water content for seed germination of many species ranges from 40 to 60% substrate water retention capacity (Piana et al., 1994). However, it varies with species and cultivar, substrate composition, and sowing method, as reported by Batista et al. (2015). These authors observed that bermudagrass ‘Princess 77’ seeds presented an even germination when sown either in or on sand maintained at 50% water retention capacity; for ‘Riviera’ cultivar, germination was more effective when seeds were sown on sand at 100% water retention capacity, similarly to what was found in this study.
Seed germination of A. affinis was more effective at the alternate temperatures of 20-30 and 20-35°C, under light, when sown on paper, between paper and on sand. The NaCl concentrations did not affect the germination percentage; however, its increment gradually decreased the germination rate. Greater germination percentage and rate were obtained when seeds were sown on sand at 100% water retention capacity.
The authors have not declared any conflict of interests.
REFERENCES
|
Baskin CC, Baskin JM (1998). Seeds: ecology, biogeography and evolution of dormancy and germination. New York: Academic Press.
|
|
|
|
Bastiani MO, Lamego FP, Nunes JP, Moura DS, Wickert RJ, Oliveira JI (2015). Germinação de sementes de capim-arroz submetidas a condições de luz e temperatura. Planta Daninha 33(3):395-404.
Crossref
|
|
|
|
|
Batista GS, Mazzini-Guedes RB, Scaldelai VR, Pivetta KFL (2015). Controlled environmental conditions on germination of bermudagrass seeds. Afr. J. Agric. Res. (11):1184-119.
|
|
|
|
|
Brasil, Ministério da Agricultura e Reforma Agrária (2009). Regras para análise de sementes. Brasília, Brasil: Secretaria Nacional de Defesa Agropecuária.
|
|
|
|
|
Carmona R, Martins CR, Fávero AP (1998). Fatores que afetam a germinação de sementes de gramíneas nativas do cerrado. Rev. Bras. Sementes 20(1):16-22.
Crossref
|
|
|
|
|
Carmona R, Martins CR (2010). Qualidade física, viabilidade e dormência de sementes recém-colhidas de capim-gordura (Melinis minutiflora P. Beauv.). Rev. Bras. Sementes 32(1):77-82.
Crossref
|
|
|
|
|
Carvalho NM, Nakagawa J (2000). Sementes: ciência, tecnologia e produção. Jaboticabal: Funep 4:588.
|
|
|
|
|
Coan RM, Cavalcante MZB, Cavalcante IHL, Pivetta KFL (2008). Salinidade na emergência de plântulas de duas espécies de gramas ornamentais. Rev. Biologia Cienc. Terra 8(2):86-92.
|
|
|
|
|
Copeland LO, McDonald MB (1995). Principle of seed science and technology. New York: Chapman & Hall P 409.
|
|
|
|
|
Evers GW, Parsons MJ (2009). Temperature influence on seeded Bermudagrass germination. Texas J. Agric. Nat. Resour. 22:74-80.
|
|
|
|
|
Godoy LJG, Villas Bôas RL, Backers C (2012). Produção de tapetes de grama Santo Agostinho submetida a doses de nitrogênio. Semina Ciênc. Agrárias 33(5):1703-1716.
|
|
|
|
|
Maguire JD (1962). Speed of germination - aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 2(2):176-177.
Crossref
|
|
|
|
|
Marcos Filho J (2005). Fisiologia de sementes de plantas cultivadas. Piracicaba: FEAlQ. P 495.
|
|
|
|
|
Myers BA, Couper DI (1989). Effects of temperature and salinity on the germination of Puccinellia ciliata (Bor) cv. Menemen. Austr. J. Agric. Res. 40(3):561-571.
Crossref
|
|
|
|
|
Ope-a JL, Chauhan BS, Baltazar AM (2014). Seed germination ecology of Echinochloa glabrescens and its implication for management in rice (Oryza sativa L.). Plos One 9:3.
|
|
|
|
|
Piana Z, Cavariani C, Tillmann MAA, Minami K (1994). Disponibilidade hídrica e germinação de sementes de cebola (Allium cepa L.). Sci. Agric. 5(3):486-489.
Crossref
|
|
|
|
|
Popinigis F (1977). Fisiologia da semente. Brasília: Agiplan P. 209.
|
|
|
|
|
Tester M, Davénport R (2003). Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91(5):503-527.
Crossref
|
|
|
|
|
Tobe KLIX, Omasa K (2000). Seed germination and radicle growth of a halophyte Kalidium capsicum (Chenopodiaceae). Ann. Bot. 85(1):391-396.
Crossref
|
|
|
|
|
Travi MRL, Scheffer-Basso SM, Escosteguy PAV, Brustolin KD, Zabot V, Miranda M (2014). Morfogênese da grama-tapete em resposta à adubação com dejeto líquido de suínos. Cienc. Rural 44(3):461-466.
Crossref
|
|
|
|
|
Viana SBA, Fernandes PD, Gheyi HR, Soares FAL, Carneiro PT (2004). Índices morfofisiológicos e de produção de alface sob estresse salino. Rev. Bras. Eng. Agric. Ambiental 8(1):23-30.
Crossref
|
|