ABSTRACT
The monitoring of plants growth constitutes an important activity during the crops formation, including the yellow passion fruit. In this direction, an experiment was carried out in Remigio, Paraiba State, Brazil, to evaluate the effects of saline water irrigation, bovine biofertilizer and potassium on yellow passion fruit growth plants and soil salinity. Treatments were arranged in randomized block with three replications and 12 plants per plot, using factorial design 2 x 2 x 2, relative electrical conductivity of irrigation water of 0.35 to 4.00 dS m-1, in soil with and without biofertilizer and potassium chloride (KCl) conventional and coated with polymers. The variables studied were electrical conductivity of the soil at depths of 0-20 and 21-40 cm, plant height at 30 days after transplanting (DAT), period of the seedlings transplanting to pruning of the main haste and lateral branches, number of productive branches and stem diameter at 30, 90, 150, 210 and 270 DAT. The interaction between water salinity, biofertilizer and potassium increased the soil salinity. The biofertilizer and the slow release of KCl increased the plant growth in height, anticipating the main stem pruning and productive side branches, productive branches number and stem diameter of the plants.
Key words: Passiflora edulis, electrical conductivity, organic input.
The use of saline water for irrigation of foodstuff crops, in general, for a long time is becoming a necessity in the whole world (Rhoades et al., 2000; Malash et al., 2005; Kang et al., 2010). As for the yellow passion fruit, while being sensitive to salinity (Ayers and Westcot, 1999), in semiarid areas of the Paraiba State and Rio Grande do Norte State, in Brazil, it has been irrigated with saline water above 3 dS m-1 (Cavalcante et al., 2005; Soares et al., 2008; Bezerra et al., 2014). According to the questioners, irrigation with saline water of this level or higher affect the growth and production of most commercial value plants, but the culture has produced moderately sensitive plant and up to moderately tolerant to salts (Dias et al. 2011; Diniz et al., 2011).
The irrigation even with water without salt restriction, salt concentration below 0.5 g L-1 or 0.75 dS m-1, ensures the production of food, but increases by at least 50% soil salinity (Ayers and Westcot, 1999). In arid and semiarid areas, the problem is more serious because the waters, in general, have higher salt content and therefore more compromising to the soil and plants (Mesquita et al., 2012). This situation requires the adoption of techniques that leach the salts of the root environment of plants as the leaching fraction (Rhoades et al., 2000) and also of inputs exercising physical improvements expressed by the pore space (Benbouali et al., 2013), chemical by the availability of macro and micronutrients (Patil, 2010) and biological in the increase in population and diversification of soil fauna (Maiti, 2010).
The bovine biofertilizer produced by anaerobic fermentation of fresh cow manure and water, applied to the soil in liquid form, attenuates the aggressive effects of salts during the formation of seedlings of yellow passion fruit– Passiflora edulis (Nascimento et al., 2012) and growth of the plants after transplanting in the field (Dias et al., 2013). Regarding production of crops Silva et al. (2011) and Lima Neto et al. (2013) noted that bovine biofertilizer attenuated the effects of salinity irrigation water from moderate restriction to severe restrictions to the beans of String –Vigna unguiculata and peppers- Capsicum annum. It was also verified that the said organic input has mitigated the negative action of irrigation water salts in the production and quality of yellow passion fruit– P. edulis (Dias et al., 2011; Freire et al., 2014; Nascimento et al., 2015).
In addition to organic fertilizers in solid form (Silva, 2008; Ahmed and Moritani, 2010) or liquid, such as biofertilizers (Mahmoud and Mohamed, 2008) and humic substances (Turkmen et al., 2005; Khaled and Fawy, 2011) employed in reducing the risks of salts, the interaction between salinity and mineral fertilization with nitrogen and potassium should also be evaluated in mitigating the harsh effects of the salts to the plants (Lima et al., 2014; Prazeres et al., 2015). When considering that potassium stimulates the maintenance of ionic balance or osmoregulation of plants, turgidity cell, control the opening and closing of stomata, plant resistance to water stress and soil salinity (Gurgel et al., 2010), the application of a source of slow release of macronutrient should also attenuate the depressive effects of salts in the different phases of phenological plants. The coated or protected fertilizers with polymers release the partially nutrient, but continuously during the crop cycle, also, reduction in losses by leaching, volatilization and result in greater efficiency in soil water absorption, solubility and availability to the plants (Zahrani, 2000). These properties, according to Guareschi et al. (2011) reduce the nutritional imbalance due to nutrients polymerized inside the capsules gradually released into the root zone, meeting the needs of the plants as they age. Another advantage is that they maintain the water content in the cells and, in fact, exerts a diluting effect on the salts absorbed by plants under irrigation with saline water (Ayers and Westcot, 1999); once the concentration of salts decrease with increase in humidity environment (Choi et al., 2005).
For the above, this study aimed to evaluate the effects of irrigation with increasing salinity of water, cattle and potassium biofertilizers, from conventional source and slow release, on growth of yellow passion fruit after transplanting of seedlings and in the soil salinity.
The experiment was carried out during the period of July/2013 to May/2014, in Macaquinhos Farm Remigio country, Paraiba State Brazil, located by the geographical coordinates of 7° 00 '1.95' 'S, 35° 47' 55'' W and altitude of 562 m, set in Mesoregion Agreste Paraibano and Microregion Western Curimatau (INTERPA, 2008). The climate of the municipality, according to Köppen classification (Brazil, 1972), is the As’ type that means hot, humid and rainy season from March to July with rainfall of 640 mm during the experimental period. The soil of the area, according to the criteria of the Brazilian System of Soil Classification- SiBCS (EMBRAPA, 2013), has been classified as Entisol Dystrophic, non-saline. Before installing the experiment, soil samples from depths of 0-20 and 21- 40 cm were collected for physical and chemical characterization and fertility (Table 1), using the methodologies contained in Donagema et al. (2011). At the same depths, soil was characterized as the salinity of the saturation extract together with the irrigation water and bovine biofertilizer diluted with water in ratio 1:1 (50%), which, by being applied in liquid form, has been evaluated as water for irrigation (Table 2) using the methods of Richards (1954).
The treatments were arranged in a randomized block design with three replications and 12 plants per plot, using the factorial 2 x 2 x 2, referring to the electrical conductivity values of irrigation water of 0.35 and 4.00 dS m-1, the soil without and with bovine biofertilizer at levels of 0 and 50% of the recommended dose of 15 L m2 (Santos, 1992) and fertilization with two sources of potassium (60% K2O), conventional potassium chloride and coated with an organic polymer to slow release potassium during the plant growth.
The holes were opened in dimensions of 40 x 40 x 40 cm and prepared with soil material in the first 20 cm; due to the low content of magnesium and sulfur (Table 1) along with a mixture of 100 g containing 75% calcitic limestone containing 48% CaO, 4.5% MgO and 78% of PRNT mixed with 25% of agricultural gypsum (CaSO4.2H2O) with 24% CaO, 16% S, 0.81% P2O5 and 14% humidity, 10 L of cattle manure with C/N ratio of 1:18 discounted moisture of 6%. Seedlings were formed via seeds of yellow passion fruit genotypes BRS Yellow Giant in substrate containing two parts of the first 20 cm of the same material soil of the experimental area, a part of cattle manure incorporated into the holes and 1% by mass fosmag (18% P2O5, 14.0% Ca, 3.5% Mg, 10.0% S, 0.15% B, 0.18% Cu and 0.65% Zn).
Before the transplant, the second half of July, 2013, when the seedlings were an average height of 25 cm and four pairs of leaves, they were incorporated per hole 3 g of urea (45% N), 5 g of simple superphosphate (18% P2O5, 18,0% Ca and 10,0% S) and 3 g of each source of potassium chloride with 60% of K2O. The plant support system was espalier with a flat wire no. 12, installed on top of the piles to 2.2 m above the ground level. The water of lower salinity irrigation (0.35 dS m-1) was obtained from the surface dam and 4.00 dS m-1 was obtained by diluting the non-iodized sodium chloride, with purity of 92%, with dam water (Rhoades et al., 2000). The biofertilizer was produced by anaerobic fermentation of fresh manure of cattle and water for 30 days (Santos, 1992) provided a blade 6 L m2 after dilution in dam water, at a ratio of 1:1, one day before and every 90 days after transplanting the seedlings- DAT in the area of 0.8 m2 of a circumference of 50 cm radius having the plant with the hole center. In each application of biofertilizer, the plants without the input were irrigated with the same amount of each type of water used for irrigation.
The irrigation of the plants was every 48 h, based on the maximum daily blade crop evapotranspiration of 14 L plant-1 day-1 obtained by the product of pan evaporation class 'A' (ETa x 0.75) installed at the experimental site (ETo = ETc x 0.75) and for each crop coefficient –Kc of 0.4; 0.8 and 1.2 (ETc = ETo x Kc), respectively referring the first 60 days after transplanting-DAT, from 60 to 90 DAT and from of the flowering stage until harvest. In the treatments with water 4.00 dS m-1, despite the sandy texture of the soil, an irrigation blade was applied (10% higher) to reduce the risk of salinity in plants by leaching salts of the root environment (Ayers and Westcot, 1999).
The fertilizer was applied to plants in covering with nitrogen (urea - 45%) and each source of potassium (60% K2O) was made monthly from the transplanting of seedlings and phosphorus (simple superphosphate 18% P2O5, 18.0% Ca and 10.0% S) every two months up to April 2014, as indicated in Table 3. The culture of yellow passion fruit was planted on the spatial arrangement of 3 x 2 m, relative to a 1667 plants density per hectare; the transplant was done in the second half of July, 2013, the support plants was made in simple espalier with a flat wire no. 12 installed to 2.2 m height at the top of the stakes.
The conduction of field seedlings was performed in a single stem to the support wire at the top of the stakes, pruning the apical bud being performed when the plant had 10 to 15 cm above the espalier, for emitting two lateral branches which have been oriented in opposite directions proceeding with pruning of the secondary branches to reach 1.5 m of length.
At 30 days after transplanting the height of the plants was measured with a millimeter tape measure. Growth of stem diameter of yellow passion fruit plants fitted to the logistic model, depending on the age of plants.
The model is represented in Equation 1:
Where: Y- Expresses the stem diameter of the plants of passion fruit (mm); A- asymptotic parameter that corresponds to the stem diameter of the plant age; B- corresponds to the location parameter without biological interpretation; K- determines the expansion rate of growth; Exp- refers to the base of neperian logarithm; t- refers to the time of transplanting to reading (day).
Transplanting period of seedlings until plants reach the support wire was recorded for growth assessment up to 2.2 m height for the pruning of the main stem. Transplanting period to the pruning of the side branches and 100 DAT productive branches of the plants were counted and also recorded. When the plants were in full flowering, 115 DAT, simple soil samples were collected in each quadrant of the plants, in the depths of 0-20 and 21-40 cm and made into composite samples for repetition to evaluate the salinity in the root environment of plants (Richards, 1954). The results were submitted to analysis of variance by F test, mean interactions were compared by Tukey at 5% probability and the equation fit for growth assessment by the diameter of the plants was done using the Statistical Analysis System software (SAS / STAT 9.3 (2011).
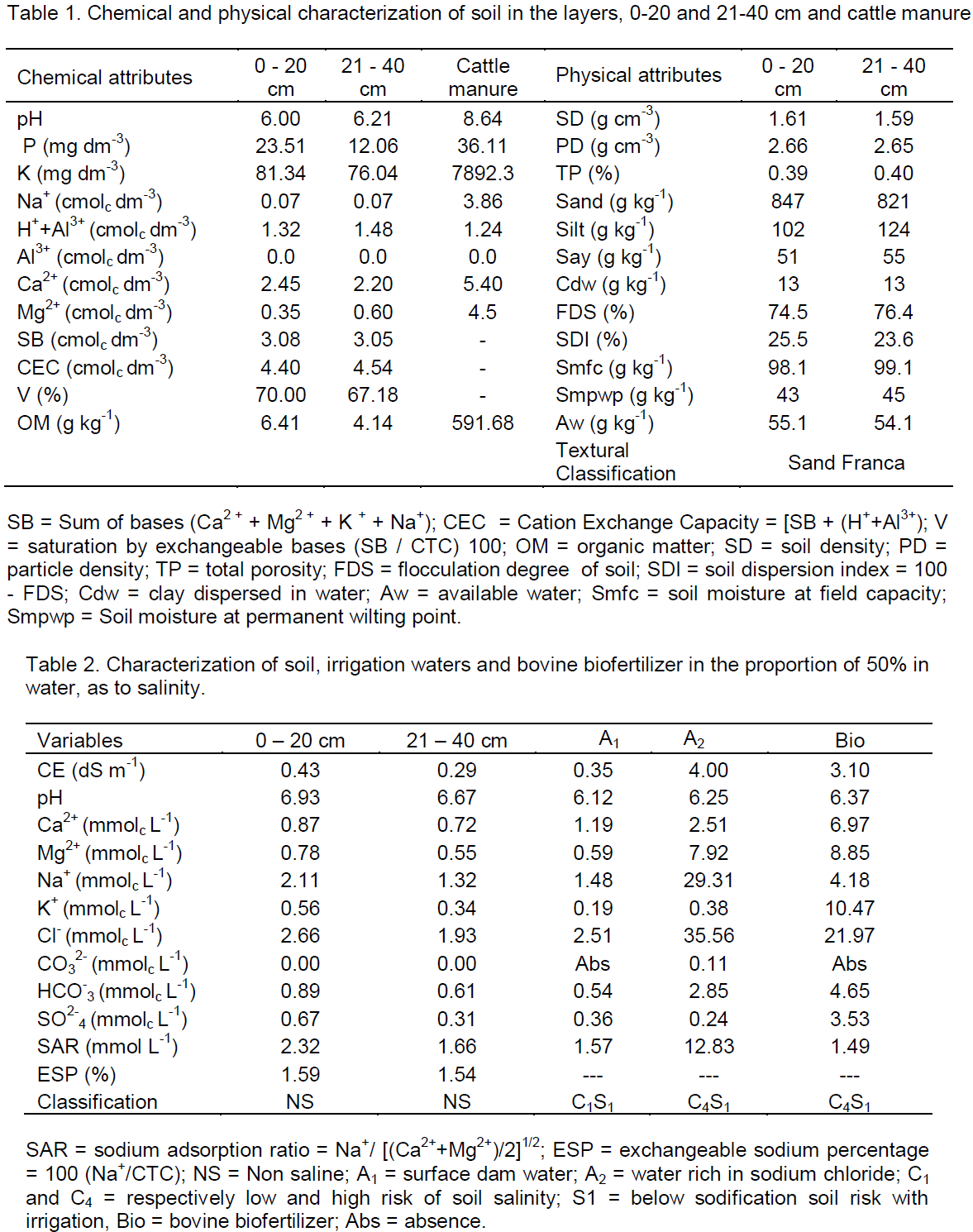
As shown in Table 4, in 0-20 cm, the interaction water × potassium significantly influenced the electrical conductivity of the saturation extract, while from 21-40 cm, the interaction water × biofertilizer led to statistical difference. Regarding the growth of plants, it was observed that the interaction water × potassium exerted a significant difference on the period of transplanting to pruning of the main stem (PTPMS) and the number of productive branches at 100 days after transplanting (NBT), while the biofertilizer interfered significantly with the plant height at 30 days after transplanting (PH 30 days), absolute growth rate (AGR), and the period for pruning the side branches (PPSB).
In Figure 1, is observed that the interaction between the salinity of the water, bovine biofertilizers and potassium types interfered significantly in the electrical conductivity of the soil saturation extract (ECse) in the first 20 cm and the interaction between salinity and bovine biofertilizers in the same variable in the layer 21 - 40 cm. At layer 0 - 20 cm, increasing water salinity, regardless of the addition of bovine biofertilizer and potassium type, raised the salt content of the soil, in general, superior in treatments with biofertilizer and conventional potassium chloride, regarding the soil without bovine biofertilizer and with potassium chloride coated with organic polymer (Figure 1A and B). With regards to the effects of the salt water, the results are in agreement with Gonçalves et al. (2011) and Mesquita et al. (2012) when registering the salinity increases with increasing salt content of waters. Larger values of ECse in the soil with conventional potassium chloride and biofertilizer can be due to the high salt content of 116% KCl (Murray and Clapp, 2004) and the high electrical conductivity of biofertilizer (3.1 dS m-1) (Table 2), as also observed by Diniz et al. (2013) in seedlings of neem (Azadirachta indica) and Souto et al. (2015) in cultivated soil with noni (Morinda citrifolia L.), both irrigated with saline water in the soil with bovine biofertilizer. At the highest range (21-40 cm) values of electrical conductivity of the saturation extract, although lower than those of the superficial layer exhibit the same behavior of increased soil salinity, with and without organic inputs applied in liquid form.
By relating the data of Figure 1A with the initial soil ECse (0.43 dS m-1) (Table 2) it can be seen that irrigation, even with non-saline water (0.35 dS m-1) increased salinity at 115 DAT in the range of 0-20 cm in 667 and 737% in soil with and without biofertilizer fertilized with conventional potassium chloride. In the same situation in the soil with a slow release of potassium chloride, the increments were lower with increases of 412 and 667% as compared to the soil with conventional fertilizer. In the same treatments, irrigation with saline water (4.00 dS m-1) significantly raised the salt content in 1086 and 1248% in soil with and without biofertilizer treated with traditional potassium chloride and in 1039 and 1248% in soil with chloride potassium polymer coated. From the results, it is observed that, despite the high percentage increase of electrical conductivity in the soil irrigated with water without salt restriction (0.35 dS m-1), soil in the period studied did not reach the salt level (Richards, 1954) characterized by ECse soil equal to or higher than 4 dS m-1. Moreover, the sandy texture of soil, sand average content in the range 0 - 40 cm higher than 84% (Table 1) and 10% leaching blade added to the blade irrigation with saline water for leaching of salts (Ayers and Westcot., 1999; Rhoades et al, 2000) were not enough to maintain the soil in the non- saline level with ECse below 4 dS m-1.
The electrical conductivity of saturation extract, unlike the surface layer, was not influenced by the kinds of potassium chloride and did not differ among treatments with and without the biofertilizer layer, 21 - 40 cm (Figure 1C). However, considering the low initial value of 0.29 dS m-1 (Table 1) with no difference between the soil in the absence and presence of biofertilizer, the increase was in both cases of 762%, but without raising the value CEes to the saline soil category, that is, electric conductivity >4.00 dS m-1 (Richards, 1954). In a similar form to that observed at the layer of 0-20 cm, irrigation with saline water in these treatments also increased the CEes soil in 1348 and 1624%, thus higher than that in 1086 and 1245%, 1039 and 1248% soil with KCI conventional and slow release, with and without biofertilizer to the saline level. When considering that the ECse soil before application of the treatments, at the layer 0 - 20 cm, was 48.3% higher than the layer 21- 40 cm, it is observed that the entrainment of salts to the deepest layer resulted in higher salt accumulation, was promoted respectively by appropriate physical conditions of the soil to the dynamics of water (Table 1), high electrical conductivity of biofertilizer (Table 2) in 10% leaching fraction added to the irrigation water blade of the saline water and the action of biofertilizer in the physical improvement of the soil (Benbouali et al., 2013).
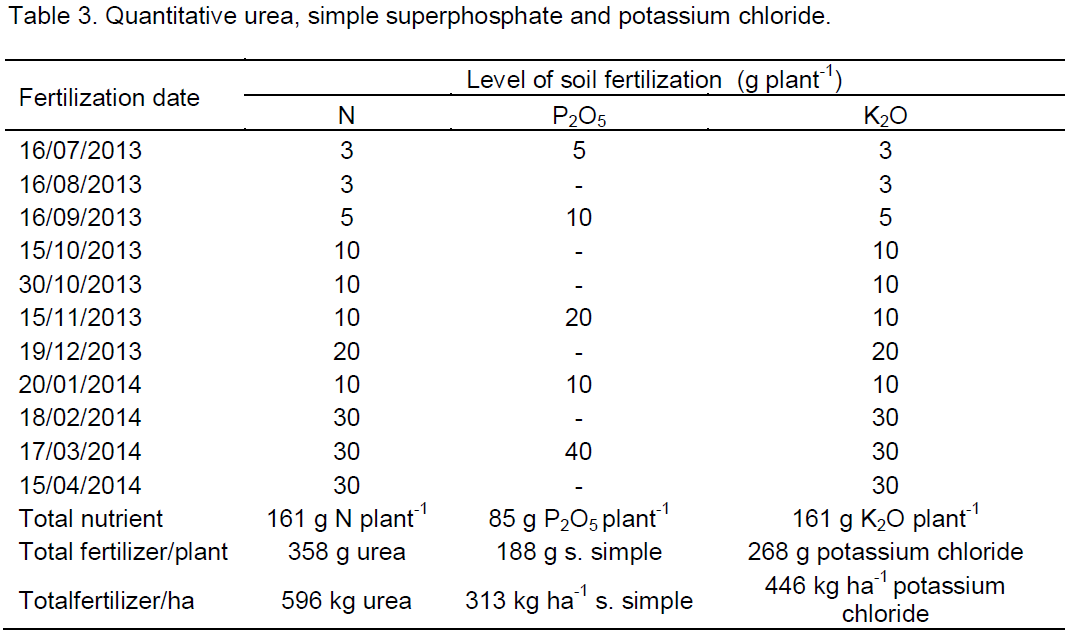
The initial growth of the plant height at 30 DAT was influenced only by the addition of biofertilizer supplied to the soil (Figure 2). At this age, the plants reached 96.6 and 106.6 cm respectively in soil with and without organic inputs. When considering that the seedlings were transplanted with an average height of 25 cm, 71.6 and 81.6 cm values with superiority of 14% was afforded by the bovine biofertilizer. These results, although lower than 92 cm recorded by Dias et al. (2013) in yellow passion fruit plants irrigated with water of lower salt concentration (2.5 dS m-1) indicates that the high salt level of the irrigation water (4.00 dS m-1) did not affect the initial growth of plants during the initial phase.
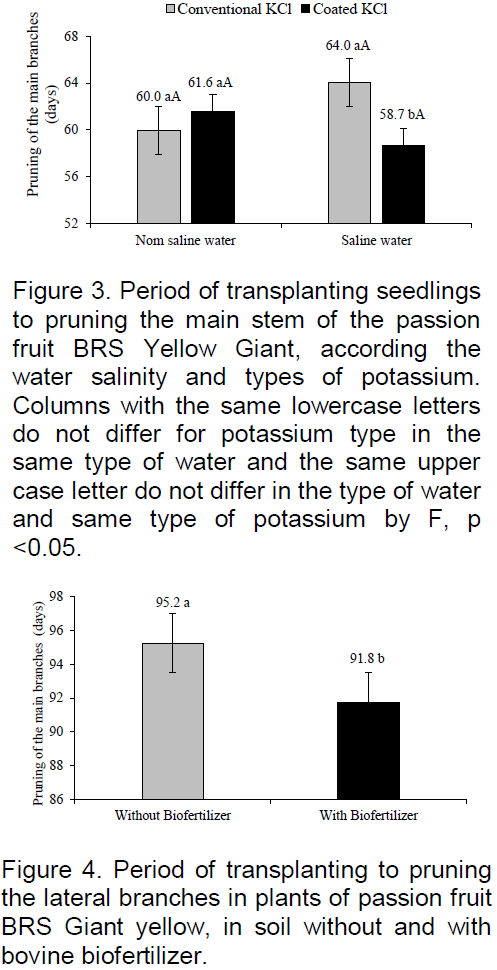
The plants of yellow passion fruit in the field grow tutors made by a string or thin stake, support wire installed on the stakes or fence posts at a certain height. Thus, the growth in height between the transplanting period of seedlings and pruning of the main stem can be assessed. In Figure 3, it is observed that the plants irrigated with non-saline water was brought from 60 to 61.6 days after transplanting to reach the wire, but showed no significant difference with the conventional potassium effect and slow release to growth at 230 cm (220 cm to the wire + 10 cm above) and at that weight, the main stem pruned to the lateral branches. The values are similar to the variation from 59 to 63 days recorded by Cavalcante et al. (2007) in yellow passion fruit in Nova Floresta, Paraíba, as Guinezinho under irrigation with non-saline water (0.5 dS m-1) in the soil without biofertilizer.
In the soil irrigated with saline water (4.0 dS m-1) the plants reached higher growth when fertilized with potassium chloride coated by vegetable polymers and achieved the support wire 5.3 days before those of the soil with conventional potassium chloride. When considering the same growth of the main stem of 230 cm, in both situations, and to relate the period of 58.7 to 64.0 it appears that the slow-release potassium chloride 8.3% attenuated degenerative action of irrigation water salinity on the growth in height of passion fruit BRS yellow Giant. The later pruning of plants treated with conventional KCl may be due to rapid release of chloride ion, which, despite being micronutrient in high level, exerts a toxic action of plants and increases the saline water phytoxicity. The behavior of the data in the treatments with KCl coated with vegetable polymers is similar to yellow passion fruit plants irrigated with saline water of 0.5 to 4.5 dS m-1, the soil with bovine biofertilizer supplied a week before and every 90 DAT (Day et al., 2013), in mitigating the depressive effects of salts on plants.
The organic polymers used in the KCl coating, as well as biofertilizer contain humic substances that exert attenuation on water salinity and soil to plants as reported by Mahmoud and Mohamed (2010) on wheat seedlings (Triticum aestivum) under irrigation with saline water and biofertilizers. The respective mineral fertilizer with organic coated stimulated more plant growth in saline soil (CEes> 4.0 dS m-1) caused by irrigation with saline water, as observed in Figure 1. This situation was similar to that of Guareschi et al. (2011) in non-saline soil, they found that application of KCl coated polymers 15 days before seeding, provided higher dry matter yield, number of pods and productivity soya beans, relative to plants soil with conventional KCl.
The period of transplanting of seedlings to pruning the lateral branches from where the productive branches arise was significantly reduced from 95.2 to 91.8 DAT, between the soil plants without and with biofertilizers and shows a growth 3.57% faster to plants promoted by organic input (Figure 4). Despite modest average reduction of 3.4 days in plants irrigated with saline and good quality water, the results are promising as compared to 100 and 91 DAT recorded for the growth of lateral branches of passion fruit irrigated with non-saline water in the soil without and with bovine biofertilizer (Cavalcante et al., 2007).
The issuance of productive branches at the 100 DAT responded to biofertilizer effects, and the interaction between irrigation water and the types of potassium chloride (Figure 5). The addition of biofertilizer (Figure 5A) gave 13.2% more productive tertiary branches that plants in the soil without said organic input and the value of 15.4 branches at 100 DAT, is in the range of 8-23 branches plant -1 registered by Dias et al. (2013), from 113 to 153 DAT in yellow passion fruit irrigated with saline water in the soil with biofertilizer, but lower than variation from 27 to 30 branches plant-1 of the same culture in the soil with and without bovine biofertilizer irrigated with non-saline water (Choi et al., 2007).
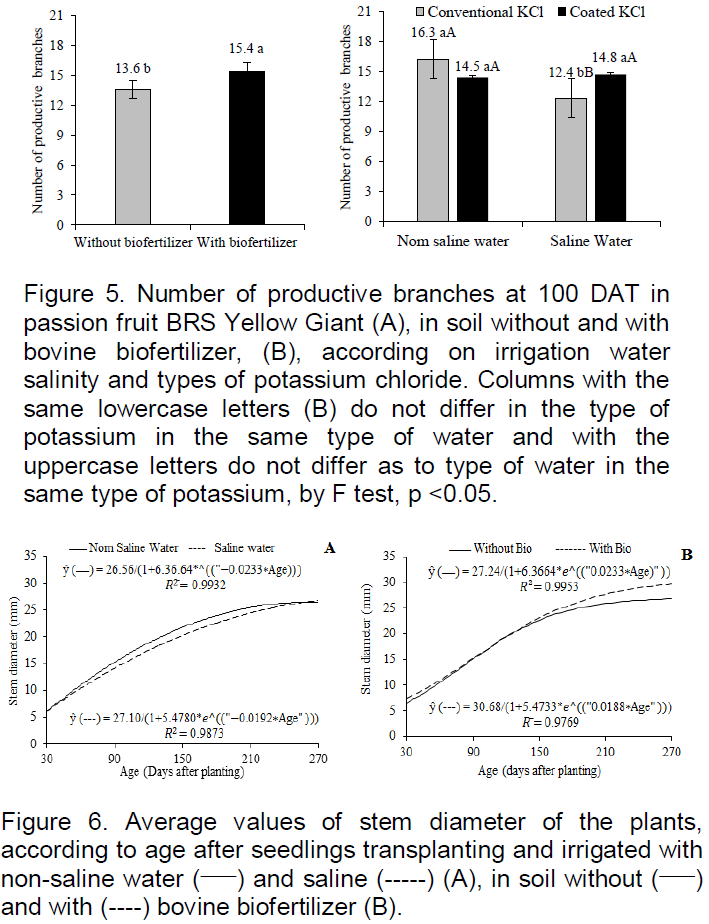
The different types of potassium did not interfere with productive branches of plants irrigated with non-saline water (0.35 dS m-1), but the type coated with polymers provided greater issue of productive branches by passion fruit BRS yellow Giant irrigated with saline water of electrical conductivity, 4.00 dS m-1 (Figure 5B). The data express coherence with the growth in height of the main stem evaluated by transplanting the pruning period (Figure 3) in which the pruning was more delayed or more precocious and the number of productive branches is respectively lower or higher. The different types of potassium did not differ with regards to the issue of productive branches in plants irrigated with lower salinity water, but potassium chloride coated with vegetable polymers increased by 19.4%, the issue of productive branches of plants irrigated with higher saline water content. The slow release provides potassium for a longer time in the soil and absorption by plants resulting in cell osmotic regulation, keeping turgor (Taiz and Zeiger, 2013), also, contributing to the nutritional balance (Guareshi et al., 2011) and, indeed, to the phyto growth and the issue of productive branches, including in saline environment.
In cultivation period of 30 at 270 DAT plant growth by diameter, there was significant response to the effects of interaction between saline water and plants age and bovine biofertilizer and plants age (Figure 6). The stem diameter of plants irrigated with non-saline water, obtained an increase of 1.6 mm in the range of 115 at 145 DAT (Figure 6A), exceeding the same period by 8%, the diameter of the plants irrigated with saline water. From that age, despite the increase in respective values, the data between the plants treated with the different waters were basically the same and the increments between the ages for each type of water, was nil or almost nil. The similarity between the results, especially from 210 at 270 DAT, may be due to sandy texture of the soil (Table 1) and blades of 10% more applied to the plants irrigated with water of greater salt concentration for the leaching of the salts root environment (Ayers and Westcot, 1999), stimulating the growth of plants.
The plants of with biofertilizers reached 270 DAT, the largest stem diameter of 29.7 mm, thus 10.4% higher than the 26.9 mm of plants grown in soil without organic inputs. The supremacy of the data from the 190 DAT is compatible with Dias et al. (2011), Nascimento et al. (2012), Bezerra et al. (2015) to conclude that the bovine biofertilizer reduces the intensity of the harmful effects of water salinity to yellow passion fruit during the growth of seedlings in the greenhouse and the plants in the field.
The passion fruit BRS yellow giant diameters at DAT 270 (Figure 6) exceeded the values of 12.31 mm from 30 to 210 DAT under irrigated crop with non-saline water obtained by Cavalcante al. (2007), and the average value of 17.8 mm at DAT 150 in irrigated crop with increasing salinity water from 0.5 to 4.5 dS m-1 (Dias et al., 2013).
Increased salinity of the water increases the soil condition of non-saline to saline. The biofertilizers, despite raising the salinity of the soil, stimulates the growth of passion fruit BRS Yellow Giant in soil irrigated with saline water. The high water salinity of 4.00 dS m-1 did not affect the growth of plants in sandy soil. The slow-release potassium chloride stimulates the growth of plants irrigated with high salinity water in sandy soil.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed BA, Moritani MIS (2010). Effect of saline water irrigation and manure application on the available water content, soil salinity, and growth of wheat. Agric. Water. Manage. 97(1):165-170.
Crossref
|
|
|
|
Ayers RS, Westcot WD (1999).The quality of water in agriculture.UFPB.218 p.
|
|
|
|
Bezerra MAF, Pereira WE, Bezerra FTC, Cavalcante LF, Medeiros ASS (2014). Saline water and nitrogen in the emergence and biomass of yellow passion fruit seedlings. Agric. Technol. J. 35(1):150-160.
|
|
|
|
BRAZIL (1972). Exploratory survey and acknowledgment of the State of Paraiba soils. MA / CONTAP / USAID-SUDENE, 670 p.
|
|
|
|
Cavalcante LF, Dantas TAG, Andrade R, Sa JR Macedo JP S, Gondim SC, Cavalcante IHL (2005). Response of yellow passion fruit to water salinity in different forms of planting. Braz. J. Agric. Environ. Eng. 9(2):314-317.
|
|
|
|
Dias TJ, Cavalcante LF, Leon MJ, JLO Freire, FO Mosque, GP Santos, Albuquerque RPF(2011). Production of passion fruit and soil mechanical resistance with biofertilizers under irrigation with saline water. Agron. Sci. J. 42(3):644-651.
|
|
|
|
Dias TJ, Cavalcante LF, Pereira WE, Freire JLO, Souto AGL (2013). Irrigation with saline water on soil with bovine biofertilizer on the growth of yellow passion fruit. Sem. Agric. Sci. 34(4):1639-1652.
|
|
|
|
Diniz AA, Cavalcante LF, Rebequi AM, JC Nunes, Brehm MA S (2011). Liquid bovine manure and urea on growth and biomass production of yellow passion fruit. Agron. Sci. J. 42(3):597-604.
|
|
|
|
Donagema GK, Campos VDB, Calderano SB, Teixeira WG, Viana JHM (2011). Manual of Soil Analysis Methods. 2ed. Rio de Janeiro: Embrapa Solos 230 p. Freire JLO, Cavalcante LF, Rebequi AM, Dias Vieira TJ, MS. Growth of yellow passion fruit under salt stress and Biofertilization in a protected environment against hydric losses. Holos. J. 28(4):55-68.
|
|
|
|
Galvão DC (2013). Strategy of saline water use in corn irrigation AG 1051 UFERSA, 62 p.
|
|
|
|
Gonçalves CVI, Freire MBGS, Santos MA, Santos ER, Freire FJ (2011) Chemical changes of an irrigated soil Fluvent Salinas with water. Agron. Sci. Magaz. 42(3):589-596.
|
|
|
|
Guareschi RF, Gazolla PR, Perin A, Santini JMK (2011). Early fertilization in soybean with triple superphosphate and potassium chloride coated with polymers. Sci. Agrotechnol. 35(2):643-648.
|
|
|
|
Gurgel MT, Gheyi HR, Oliveira FHT (2010) Accumulation of dry matter and nutrients in melon crop produced under salt stress and potassium. Agron. Sci. Mag. 41(2):18-28.
|
|
|
|
Kang CK, Tsai HJ, Liu CC, Lee TH, Hwang PP (2010) Salinity-dependent expression of the Na+, K+ and Cl- co-transporter in gills of the brackish medaka Oryzias dancena: the molecular correlate for hyposmoregulatory endurance. Comp. Biochem. Phys. 157(1):7-18.
Crossref
|
|
|
|
Land institute of agricultural planning of paraíba state (2008). Report: Mesoregion of Agreste Paraibano; Microgregion of Western Curimataú. INTERPA 142 p.
|
|
|
|
Lima GS, RG Noble, Gheyi HR, Smith LAA, Lawrence GS, Silva SS (2014).Growth and production aspects of irrigated castor beans with salinewater and nitrogen fertilizer. J. Agric. Engineer. Amb. 18(6):615-622.
|
|
|
|
Mahmoud AA, Mohamed HF (2008). Impact of Biofertilizers Application on Improving Wheat (Triticum aestivum L.) Resistance to Salinity. Res. J. Agric. Biol. Sci. 4(5):520-528.
|
|
|
|
Malash NM, Flowers TJ, Ragab R (2010). Effect of irrigation methods, management and salinity of irrigation water on tomato yield, soil moisture and salinity distribution. Irrig. Scien. New York 26(4):313-323.
|
|
|
|
Mellek JE, Dieckow J Silva VL, Favaretto N, Pauletti V, Vezzani FM, Souza JLM (2010). Dairy liquid manure and no-tillage: Physical and hydraulic properties and carbon stocks in a Cambisol of Southern Brazil. Soil Till. Res. 110(2):69-76.
Crossref
|
|
|
|
Mosque FO, Cavalcante LF, Pereira WE, Rebequi AM, Lima Neto AJ, Nunes JC (2012). Production of yellow passion fruit seedlings submitted to salinity in soil with bovine biofertilizer. Soil Sci. 30(1):31-41.
|
|
|
|
Murray TP, Clapp JG (2004) Current fertilizer salt index tables are misleading. Communications in soil science and plant analysis 35(19-20):2867-2873.
Crossref
|
|
|
|
Nascimento JAM, Cavalcante LF, Dantas SAG Dias TJ, Medeiros SAM (2015). Biofertilizers and mineral fertilization on fruit quality of irrigated passionfruit with saline water. Irrig. 20(2):220-232.
|
|
|
|
Nascimento JAM, Cavalcante LF, Lima Neto AJ, Beckmann-Cavalcante MZ, FO Mosque, Rebequi AM, RM Rodrigues, Santos JB (2012). Seedling formation. In: CAVALCANTE LF (Ed). The passion fruit and water salinity.2:32-49.
|
|
|
|
Patil NM (2010) Biofertilizer effect on growth, protein and carbohydrate content in stevia rebaudiana var. bertoni. Recen. Res. Sci. Technol. 2(2):42-44.
|
|
|
|
Prazeres SS, CF Lacerda, Barbosa FEL, Amorim AV, Araujo ICSA, Cavalcante LF (2015). Growth and gas exchange of cowpea plants under saline irrigation and potassium. Magaz. Agro@e. 9(2):111-118.
|
|
|
|
Rhoades JD, Kandiah A Mashali AM (2000). Use of saline water for agricultural production.UFPB 117 p.
|
|
|
|
Richards LA (1954). Diagnosis and rehabilitation of saline and sodic soils. United States Department of Agriculture of America 172 p.
|
|
|
|
Santos ACV (1992). Liquid biofertilizers: defensive nature. EMATER-RIO 16 p.
|
|
|
|
Silva CA (2008). Use of organic waste in agriculture, in: Santos GA, LS Silva, Canellas LP, Camargo FAO, Fundamentals of soil organic matter: tropical and subtropical ecosystems. P. 621.
|
|
|
|
Silva JLA, Alves SSV, Nascimento IB, Silva MVT, Medeiros JF (2011). Evolution of salinity in representative soils of Mossoró-assu agropolo cultivated with melon crop with deferent salinity water. Sci. Agric. Semia. Region 7(4):26-31.
|
|
|
|
Soares FAL, Ram PT, Gomes IN, Gheyi HR, Fernandes PD (2008). Growth and production of yellow passion fruit under supplementary irrigation with saline water. J. Agric. Sci. 3(2):151-156.
|
|
|
|
Taiz L, Zeiger E (2013) Plant physiology. Artmed Publishing 820 p.
|
|
|
|
Zahrani S (2000) Utilization of polyethylene and paraffin waxes as controlled delivery systems for different fertilizers. Ind. Eng. Chem. Res. 39(1):367-371.
Crossref
|