Full Length Research Paper
ABSTRACT
The objective of this study was to evaluate the induction of genetic variability and plant development of palisade grass (Urochloa brizantha L.) M2 mutants in acclimatization to subtropical climate condition. The mutagenic agent methyl methanesulfonate (MMS), at a dosage of 0.5%, was used to induce mutation in 4,000 seeds of the cultivar Marandu. Thirty five plants survived after the induction of MMS mutagen agent. These plants were isolated transplanted in experimental area to advance the generation. Seeds produced by plants were sown in pots allocated in Biosystems Organized Development incubator and subjected to cold stress at 0°C in seedling stage. Surviving seedlings per family were transplanted to field conditions in Southern Brazil. The plant development was evaluated in the M2 generation during the agricultural years of 2012-2013 and 2013-2014. Genetic variability analyzes were carried out with 21 traits, based on the average Euclidean distance and the relative contribution proposed by Singh (1981). Chemical mutation induction with MMS generates genetic variability in palisade grass, enhancing the selection of superior genotypes in subtropical climate conditions. The chemical induction of mutations with the MMS mutagen provided genetic variability in the population of U. brizantha with formation of 18 divergent groups during the research.
Key words: Urochloa brizantha L., plant breeding, genetic recombination, forage, multivariate analyses.
INTRODUCTION
MATERIALS AND METHODS
RESULTS AND DISCUSSION
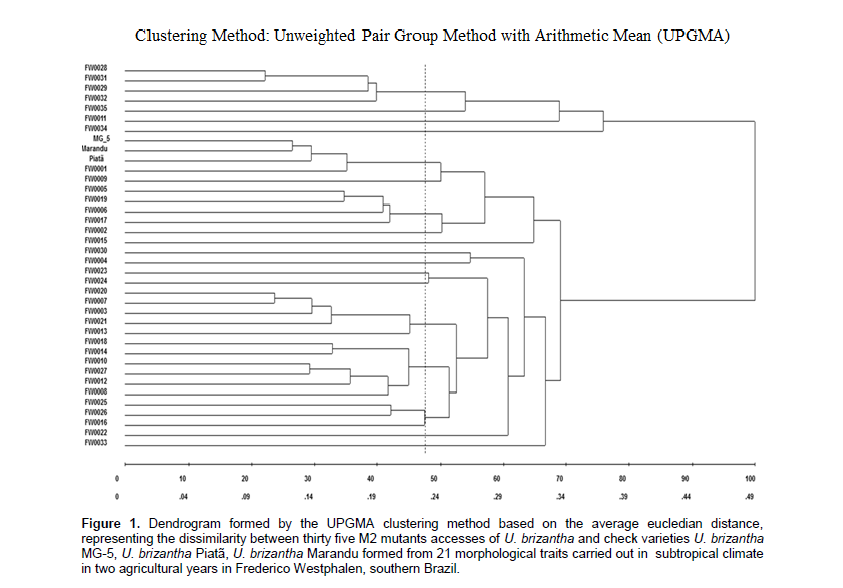
According to Figure 1, there was the formation of 18 distinct groups with average dissimilarity of 23.51% and 17 groups demonstrated dissimilarity in relation to the check varieties. The three check varieties and the genotype FW 0001 were grouped together with unusual characteristics that provide them high similarity. After the measurement of the canopy morphological traits based on 21 studied traits (Table 1), it appears that 97% of the progeny have dissimilarity in relation to check varieties. There was the formation of a group of six genotypes, a group of five genotypes, three groups with four genotypes, one group of three genotypes and 12 groups with one genotype, showing that the UPGMA method was also effective for this research, as also reported in other studies with forage grasses (Totti et al., 2001; Torres et al., 2015).Detected genetic dissimilarity demonstrates that mutation induction generated high genetic variability, which was also found in other studies with Poaceae family species, as these proceeded in Brazil with oat (Coimbra et al., 2004; Souza et al., 2005). This increase in variability provides high importance to Urochloa, mainly due to reproductive difficulties that are provided by the presence of apomixis, present in the genotypes of U. brizantha studied in the Brazilian germplasm bank (Resende et al., 2008; Valle et al., 2009) and chromosomal irregularities (Fuzinatto et al., 2012).
The presence of dissimilarity is associated with the efficiency of chemical induction of mutations with the mutagen MMS associated with preselection applied on the progenies. Cold temperatures simulation was applied during preselection and it is characterized as a strategy to eliminate at an early time individuals with low tolerance to cold. At the same time, it allows to select progenies that have cold tolerance, which can provide benefits to the breeding program, selecting promising genotypes for this trait of interest. According to a study performed in Ona, state of Florida, forage selection for cold tolerance was promising as they found herdability of 0.39 to 0.50 for the trait cold tolerance in two crop cycles. These positive results enabled new selections in later generations (Jank et al., 2002).
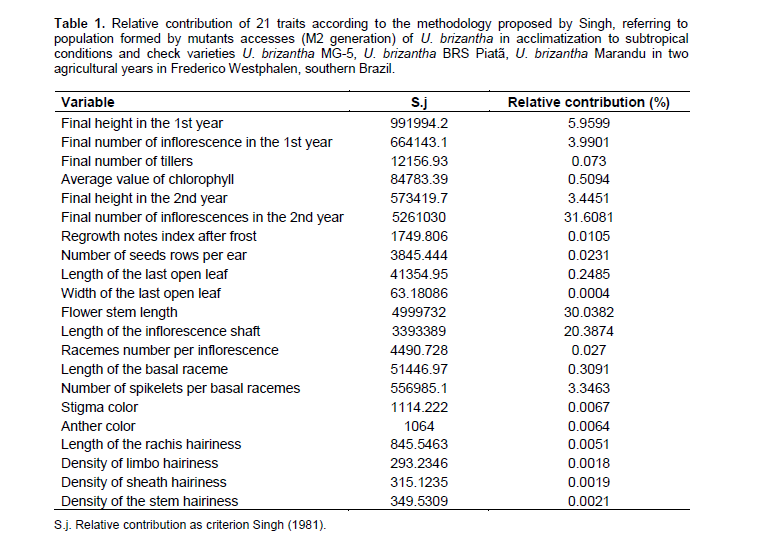
The use of chemicals for mutation induction provides random nucleotide changes over the genome, which can generate individuals with agronomic interest (Allard, 1971). Among the mechanisms developed by plants throughout evolution to increase cold tolerance, it stands out the greater accumulation of amino acids and sugars (Alcázar et al., 2011), action of anti-freeze proteins (Wang et al., 2006), and greater fluidity of the plasma membrane exposed to stress by cold (Taiz and Zeiger, 2013).According to the analysis of the 21 variables (Table 1), there was different inputs depending on the studied trait. Among all studied traits, FNI2 (31.60%), FSL (30.03%), LI (20.38%), FH1 (5.95%), FNI1 (3.99%), FH2 (3.44%), and NSBR (3.34%) were highlighted with greater contributions.
The remaining 14 variables contribute individualy with values ​​below 1%. The study of dissimilarity among genotypes is important to proceed with the screening of individuals with potential to move forward in a breeding program. In order to register a new cultivar, it must have distinctness from other cultivars by a minimum number of descriptors having homogeneity and stability (Brazil, 2011).
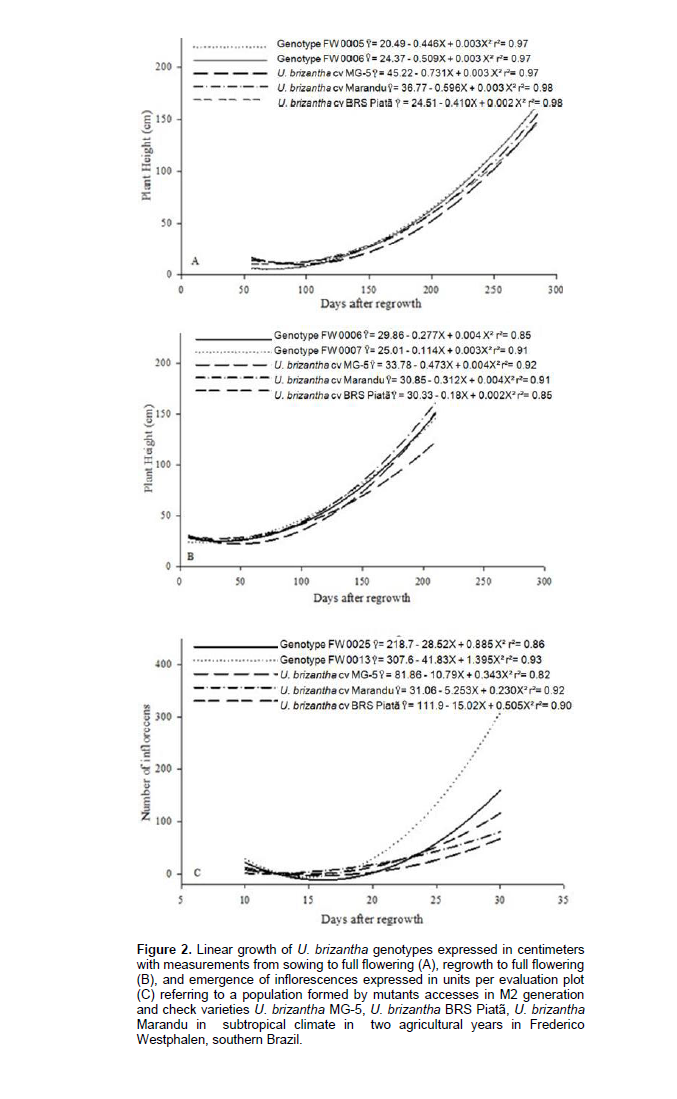
Regarding the analysis of genotypes acclimatization, the greater development (Figure 2) regarding the linear growth of check varieties and genotypes more acclimated to the study conditions was explained by the polynomial regression curve of second degree order, with emphasis on access FW 0005 and FW 0006. The proper initial growth before the flowering is important as the initial flowering moment is characterized as the optimal resting point on forage species. At this time, there is a high assimilates translocation from the aerial part to the roots, keeping the reserves available for the forage regrowth. Thereby, grazing performed before or after the optimum point of rest may degrade the pasture (Lenzi, 2012). The experimental precision during the study was considered appropriate due to the crop development from sowing to flowering presented r² above 0.97, regrowth to flowering showed r² above 0.85, and appearance of inflorescences time exhibited r² above 0.82.
The beginning of flowering corresponded to 208 DAS, as the estimated value for growth rate of U. brizantha MG-5 was 22.96 cm, U. brizantha BRS Piata was 25.75 cm, and for U. brizantha Marandu was 42.59 cm. These values are low compared to the better mutant genotypes that expressed 48.29 cm for the FW 0006 genotype and 57.51 cm for the FW 0005 genotype. The estimated values ​​expressed influence of increased cold tolerance in the initial period. Therefore, the main characteristics required for cold tolerance in subtropical regions are the use of more vigorous seedlings, tillering capacity, presence of axillary tillers, and fast regrowth after periods of low temperatures (Souza et al., 2013).
The growth in the second year of evaluation corresponds to regrowth (Figure 2). It is explained by polynomial regression curve of second degree order, where the beginning of flowering was used to estimate the regrowth height with estimated height values ​​of 34.14 cm for U. brizantha MG-5, 37.23 cm for U. brizantha BRS Piata, 50.36 cm for U. brizantha Marandu, 53.54 cm for FW0006, and 53.92 cm for FW0007, that were the better accesses. The mutant genotypes presented ​​greater values than U. brizantha Marandu, that was the best check variety for this trait. It is necessary reliable phenotypic expressions that can form the basis for evaluation under field conditions in order to have proper selection and discard of genotypes (Cruz, 2005). This statement can be attributed to the FW0006 genotype, which was expressed among the best individuals related to its growth in two years. This access has potential for further applications in a breeding program.
Regarding the emergence of inflorescences in the second year of evaluation (Figure 2), it showed development explained by the polynomial regression curve of second degree order, with trend line estimated in the full flowering of 66 inflorescences for U. brizantha MG-5, 115 inflorescences for U. brizantha BRS Piata, 80 inflorescences for U. brizantha Marandu, 308 inflorescences for access FW0013, and 159 inflorescences for access FW0025. The planning and selection decision is a vital moment for a breeding program. However, the topic of associating high matter production and seed production of the genotypes should present a balance because genotypes with low seed production may have compromise the economic viability of its commercial release. Moreover, evaluating and selecting the trait number of inflorescences in Setaria sphacelata was considered feasible due to the 17% increase in the number of inflorescences in relation to the original population (Jank et al., 2007).
In accordance with analysis of vegetative growth for the studied traits, genotypes with greater growth and regrowth were not in the group with greater seed production. Moreover, the increase in inflorescences mass is a main factor to increase seed production (Bean, 1972). Thus, the selection of individuals that meet market demands for quality forage production combined with proper levels of seed production should be performed during the process of advancing generations.
CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T (2011). Integration of polyamines in the cold acclimation response. Plant Sci. 180(1):31-38. |
|
|
Allard RW (1971). Princípios do melhoramento genético das plantas. 1ª ed. São Paulo: EDGARD BLÜCHER LTDA. 485 p. |
|
|
Ambiel AC, Machado Neto NB, Guaberto LM, Vanderlei T (2010). Brachiaria germplasm dissimilarity as shown by RAPD markers. Crop Breed. Appl. Biotechnol. 10(1):55-64. |
|
|
Araújo SAC, Deminicis BB, Campos PRSS (2008). Melhoramento genético de plantas forrageiras tropicais no Brasil. Archivos de zootecnia 57(1):61-76. |
|
|
Bean EW (1972). Clonal evaluation for increased seed production in two species of forage grasses, Festuca arundinacea Schred. and Phleum pratense L. Euphytica 21(1):377-383. |
|
|
Brazil MAPA (2011). Proteção de cultivares no Brasil. 1ªed. Dourados:UFV. 204 p. |
|
|
Cardoso ED, Sá ME, Haga KI, Binotti FFDS, Nogueira DC, Valério WVF (2014). Desempenho fisiológico e superação de dormência em sementes de Brachiaria brizantha submetidas a tratamento químico e envelhecimento artificial. Semina: Ciênc. Agrár. 35(1):21-38. |
|
|
Coimbra JLM, Carvalho FIF, Oliveira AC (2004). Genetic variability in populations of oat induced by chemical and physical mutagenic agents. Crop Breed. Appl. Biotechnol. 4(1):48-56. |
|
|
Cruz CD (2005). Princípios de genética quantitativa 1ªed. Viçosa: UFV. 394 p. |
|
|
Cruz CD (2013). GENES– a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35(3):271-276. |
|
|
Fuzinatto VA, Pagliarini MS, do Valle CB (2012). Meiotic behavior in apomictic Brachiaria ruziziensis × B. brizantha (Poaceae) progenies. Sci. Agric. 69(1):380-385. |
|
|
Garcia M, Vigna BBZ, Sousa ACB, Jungmann L, Cidade FW, Toledo-Silva G, Francisco PM, Chiari L, Carvalho MA, Karia CT, Faleiro FG, Godoy R, Dall'agnol M, Pagliarini SS, Souza FHD, Souza-Chies TT, Jank L, Resende RMS, do Valle CB, Zucchi MI, Souza AP (2013). Molecular genetic variability, population structure and mating system. Trop. Forages 1(1):25-30. |
|
|
Jank L, Quesenberry KH, Blount ARS, Mislevy P (2002). Selection in Setaria sphacelata for winter survival. New Zealand J. Agric. Res. 45(4):273-281. |
|
|
Jank L, Quesenberry KH, Sollenberger LE, Wofford DS, Lyrene PM (2007). Selection of morphological traits to improve forage characteristics of Setaria sphacelata grown in Florida. New Zealand J. Agric. Res. 50(1):73-83. |
|
|
Lenzi A (2012). Fundamentos do Pastoreio Racional Voisin. Rev. Bras. Agroecol. 7(1):82-94. |
|
|
Muller HJ (1927). Artificial transmutation of the gene. Science 66(1):84-87. |
|
|
Resende RMS, Valle CBdo, Jank L (2008). Melhoramento de forrageiras tropicais 1ªed. Campo Grande: Embrapa Gado de Corte 293 p. |
|
|
Souza FHDde, Matta FdeP, Fávero AP (2013). Construção de ideótipos de gramíneas para usos diversos. 1ªed. Brasília: Embrapa Pecuária Sudeste. 381 p. |
|
|
Souza VQD, Pereira ADS, Koop MM, Coimbra JLM, Carvalho FIFD, Luz VKD, Oliveira ACD (2005). Dissimilaridade genética em mutantes de aveia tolerantes e sensíveis a ácidos orgânicos. Bragantia 64(4):569-575. |
|
|
Taiz L, Zeiger E (2013). Fisiologia vegetal 5ªed. Porto Alegre: Editora Artmed. 954 p. |
|
|
Torres FE, do Valle CB, Lempp B, Teodoro PE, Rigon JPG, Ribeiro LP, Corrêa CCG, Luz Júnior RAAD (2015). Estimativa da divergência entre ecótipos de braquiária baseada em descritores quantitativos e qualitativos. Ciênc. Rural 45(3):485-491. |
|
|
Totti R, Vencovsky R, Batista LAR (2001). Utilização de métodos de agrupamentos hierárquicos em acessos de Paspalum (Graminea (Poaceae)). Semina 22(1):25-35. |
|
|
Valle CBdo, Jank L, Resende RMS (2009). O melhoramento de forrageiras tropicais no Brasil. Rev. Ceres 56(4):460-472. |
|
|
Wang X, Li W, Welti R (2006). Profiling lipid changes in plant response to low temperatures. Physiol. Plant 126(1):90-96. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0